Abstract
Context: Berberis aristata DC (Berberidaceae) is an important medicinal plant with claims of widespread medicinal value in indigenous medicine. It is used by herbal healers to treat oral cancers.
Objective: To evaluate the antineoplastic activity of the extracts of Berberis aristata in Ehrlich ascites carcinoma (EAC)-bearing mice with cisplatin as positive control in the advanced stage of tumorigenesis.
Materials and methods: Brine shrimp lethality bioassay (BSL) of extracts and effect on the tumor cell viability in vitro were carried out. EAC was induced in Swiss albino mice by injecting 106 cell/mL of tumor cell suspension i.p. Antineoplastic activity of the aqueous and ethanol extracts (100 and 6.5 mg/kg i.p., respectively) was compared with that of cisplatin (3.5 mg/kg i.p.) on the parameters such as percentage increase in weight, median survival time, and hematology.
Results: Ethanol extract attenuated percentage increase in weight gain (−6.86 ± 1.50) due to tumor cell proliferation and increased the survival time (19.5 days) when compared to control group (19.10 ± 2.31 and 16 days, respectively). However, the effect was less than that of cisplatin. In vitro cytotoxicity assay as well as BSL test showed the cytotoxic effect of the extracts. Cisplatin and the extracts reversed the tumor-induced alterations in total white blood cell count, differential leukocyte counts, total red blood cell count, and hemoglobin contents.
Discussion and conclusion: Of the two extracts, the ethanol extract was observed to be more efficient and the presence of alkaloids and flavonoids may be responsible for the observed anticancer effects.
Introduction
Despite substantial progress in the understanding of the molecular basis, diagnosis, and treatment, cancer is still a major health concern. It is the second leading cause of death in the world after the cardiac diseases. According to the recent available information, globally in the year 2002, excluding the non-melanoma skin cancers, there were more than 10 million new cases of cancer recorded, with nearly 7 million cancer deaths (CitationWCRF/AICR, 2007). Projections are that by the year 2020, these figures will increase to over 16 million new cases, with 10 million deaths, and that in 2030 there may be more than 20 million new cases of cancer, with 70% of cancer deaths in the low income countries, which have minimal resources to treat (CitationWCRF/AICR, 2007).
Irrespective of the cause, localized malignant tumors are best managed by surgical removal or radiotherapy, or both, while the treatment options for advanced and metastasized tumors is mostly chemotherapy (CitationSikora & Halnan, 1990). However, most of the clinically used synthetic chemotherapeutic agents exhibit severe normal tissue toxicity, and cause undesirable side effects in the patients receiving them (CitationDorr & Fritz, 1980). Therefore, there is a need is felt to find alternative drugs, which at non-toxic doses are highly effective, inexpensive, and affordable to the common man. One of the best approaches in searching novel anticancer agents from plant resources is identifying time tested ethnomedical practices and evaluating their actual efficacy and safety through established methods of modern science (CitationJain, 1994; CitationCragg et al., 1997; CitationMann, 2002). The use of ethnomedical knowledge has contributed to health care and systematic studies have shown that the evaluation of traditionally used medicines may lead towards effective drug discovery (CitationMann, 2002).
Since time immemorial, vast ethnobotanical knowledge exists in India and most people use traditional medicine as an alternative treatment for diseases, including cancer, due to lower toxicity (CitationJain, 1994). These herbal systems are still in place today because of their organizational strengths, based on the principles of Ayurveda, the traditional system of Indian Medicine. The herbal drugs used by traditional healers primarily focus on multicomponent mixtures and are mostly prepared by boiling the plants in water (CitationJain, 1994).
Berberis aristata DC (Berberidaceae), an erect spinous shrub, is an important medicinal plant, as almost every part has some medicinal value in the various Ayurvedic preparations. Commonly known as the Indian Barberry tree turmeric, this shrub is found growing wild in the sub-Himalayan tract at an altitude ranging from 850 to 2500 m and also in the Nilgiris and Sri Lanka (CitationParmar & Kaushal, 1982). The dried stem, root bark, and wood are alterative, antiperiodic, deobstruent, diaphoretic, laxative, ophthalmic, and tonic (bitter) medicine (CitationParmar & Kaushal, 1982; Chopra et al., 1986).
Infusion prepared from the stem is used in the treatment of malaria, eye complaints, skin diseases, menorrhagia, diarrhea, and jaundice (CitationParmar & Kaushal, 1982; Chopra et al., 1986). The stem is said to be diaphoretic and laxative and useful in rheumatism. The dried extract of the roots is used as an application in ophthalmia and is an excellent medication in the case of sun-blindness. The fruits are given as a cooling laxative to children. The root bark is a valuable medicine in intermittent and remittent fevers. A watery solution of this preparation is also used for washing piles, Oriental sores, and glandular swellings (Chopra et al., 1986).
A very valuable preparation called rasaut is prepared from this plant and is used in curing many human ailments. For preparing rasaut, the root bark and lower part of the stem bark are boiled in water, strained, and evaporated until a semi-solid mass (rasaut) is obtained. It is mixed with butter and alum, or with opium and lime-juice and is applied externally to the eyelids to cure ophthalmia and other eye diseases (CitationJain, 1994). It is also reported to be a mild laxative, a tonic and is useful in curing ulcers and fevers (CitationParmar & Kaushal, 1982). Rasaut is used as a purgative for children and as a blood-purifier, a tonic, and a febrifuge. It is also given in diarrhea, jaundice, and skin diseases (Chopra et al., 1986).
Ethnobotanical documentation studies on the traditional medical practices in remote villages of Uttar Pradesh and in parts of Himalayas, India by CitationUniyal and Tewari (1991) have shown that Berberis aristata is used by herbal healers to treat cancers to good effect, especially the oral cancers. These observations are in agreement for its documented use in treating Arbuda (cancer) in the ancient system of Indian medicine, the Ayurveda (CitationNadkarni, 1976; Chemexcil, 1992; Jain, 1994Citation). In spite of the strong ethnobotanical evidence and documentation, a detailed scientific literature study showed (Medline) that no experimental studies with regard to the antitumor properties of Berberis aristata have ever been performed. However, berberine, which is one of the principle compounds present in it as well as in other plants such as Tinospora cordifolia (Willd.) Miers ex Hook. F. & Thoms, Hydrastis canadensis L., Coptis chinensis Franch etc. has been reported to possess antineoplastic activities in various cell lines and also in the tumor bearing animals (CitationJagetia & Baliga, 2004). Studies have also shown that berberine was effective in preventing chemical carcinogenesis in rats and mice (CitationAnis et al., 2001).
The wide usage of Berberis aristata in Ayurveda, the cancer curative observations of CitationUniyal and Tewari (1991), and the lack of scientific study were the three main factors that encouraged us to investigate for the antitumor activity of whole extract of Berberis aristata in mice bearing Ehrlich ascites carcinoma. Further, as cancer is mostly detected in late stages, the antineoplastic effects in this study was particularly carried out in the advanced stage of tumor growth with Ehrlich ascites carcinoma (10th day post tumor inoculation) to obtain a meaningful validation of observed ethnobotanical practices.
The study was strictly followed as per the standard protocol recommended by the National Service Center of Cancer Chemotherapy, CCNSC, USA, with cisplatin as a positive control (CitationGeran et al., 1972) and care was taken to follow both, accepted scientific protocols and the traditional principles that are followed in ethnomedicinal practices in the Ayurvedic system of medicine (CitationJain, 1994).
Materials and methods
Collection and extraction of the plant
The stem with bark of Berberis aristata was obtained from Yucca enterprises, Mumbai. The drug was authenticated by comparing with sample provided by National Botanical Research Institute, Lucknow. The crude drug was shade dried for 20 days and then coarse powdered. Soxhlet extraction method was used for extraction as described by CitationSuffness and Douros (1979). The coarse powder was loaded in thimble made of Whatman filter paper no. 1 and extracted in the Soxhlet extraction column with 1–2 L of 95% alcohol (95% alcohol was obtained by distillation of rectified spirit) at 55°C. Each batch was extracted for 30 cycles in the Soxhlet extraction column. The extract obtained was thick and syrupy with a characteristic odor.
The excess alcohol was back distilled and the remaining was evaporated on a water bath to obtain a yield of 5% and was stored in desiccators. The aqueous extract was prepared by boiling the dried powder (100 g) in 1000 mL of distilled water for 3–4 h. The solution was cooled and decanted into a beaker and then concentrated on a water bath to obtain a yield of 4% and stored as described earlier.
Animal care and handling
Six-week old female Swiss albino mice (25 ± 5 g body weight) were selected from Central Animal Facility, Manipal University, Manipal, Karnataka, India. The animals were acclimatized to the experimental room having a temperature 23 ± 2°C, controlled humidity conditions, and 12:12 hour light and dark cycle. The mice were housed in sterile polypropylene cages containing sterile paddy husk as bedding material with a maximum of four animals in each cage. The mice were fed autoclaved standard mice food pellets (Hindustan Lever) and water ad libitum. The animal experiments were performed according to the rules and regulations of the Institutional Animal Ethics Committee (IAEC).
Tumor model
The Ehrlich ascites carcinoma, which was originally obtained from Dr. Ramdasan Kutan (Director, Amala Cancer Research Center, Amala Nagar, Thrissur, Kerala, India), was maintained and propagated by serial intraperitoneal transplantation of EAC cells in an aseptic environment. For experiments, 106 viable EAC cells were injected intraperitoneal into each animal in a volume of 250 µL of sterile serum free RPMI in an aseptic condition and the day of tumor inoculation was considered as day 0.
Preparation of drug and mode of administration
Both aqueous and alcoholic extracts were prepared by dissolving in water under slight heat. These solutions were cooled and administered, once daily, in a volume of 0.1 mL/10g mouse through intraperitoneal route for five consecutive days from day 11 to 15 post tumor inoculation.
Acute toxicity studies
Acute toxicity of Berberis aristata was determined according to CitationPrieur et al. (1973) and CitationGhosh, (1984). Briefly, the animals (nontumor bearing) were allowed to fast by withdrawing the food and water for 18 h. The acute toxicity studies were conducted to determine the safe dose by an up and down staircase method (CitationGhosh 1984). The animals were provided with food and water immediately after the drug administration.
The animals were observed continuously for the first 2 h, followed by once every hour up to 6 h for any changes in behavior and manifestations of the toxic symptoms like increased motor activity, tremors, clonic convulsions, tonic extensions, Straub reaction, pilo-erection, muscle spasm, catatonia, spasticity, opisthotonus, hyperesthesia, loss of right reflex, decreased motor activity, ataxia, sedation, muscle relaxation, hypnosis, analgesia, anesthesia, arching, and rolling, ptosis, lacrimation, exophthalmoses, salivation, viscid, watery diarrhea, writhing, respiration, depression, stimulation, failure, blanching, skin coloration, cyanosis, and vasodilatation. The mortality of the animals was observed up to 30 days post drug treatment as described earlier (CitationGhosh, 1984).
Antineoplastic effects in Ehrlich ascites carcinoma-bearing mice
The antineoplastic activities of both ethanol and aqueous extracts were evaluated following the standard protocol recommended by the National Service Center of Cancer Chemotherapy, CCNSC, USA (CitationGeran et al., 1972). Ehrlich Ascites Carcinoma is one of the well characterized and the oldest transplantable tumor models for anticancer studies (CitationHenry, 1974). Female Swiss albino mice were divided into four groups of 12 animals each. Twenty-four hours after the tumor inoculation, the animals were divided into the following groups:
DDW control
The animals of this group received 0.23 to 0.26 mL of sterile water once daily, consecutively for 5 days from day 11 to day 15 post tumor inoculation.
Cisplatin group
Cisplatin was used as a positive control and was dissolved in sterile saline in the dark. The animals assigned for this group were injected once with 3.5 mg/kg body weight. on day 11 post tumor inoculation. The dose of 3.5 mg/kg body weight was extrapolated for mice from human dose that is used in clinics in cancer treatment (75 mg/m2) (CitationDorr & Fritz, 1980).
Berberis aristata aqueous
The animals of this group were injected with 100 mg/kg body weight of the extract once daily, consecutively for 5 days from day 11 to day 15 post tumor inoculation as per the pharmacological principles (CitationGhosh, 1984).
Berberis aristata ethanol
The animals of this group were injected with 6.5 mg/kg body weight (one-tenth of the LD0/30) of the extract once daily, consecutively for 5 days from day 11 to day 15 post tumor inoculation as per the pharmacological principles (CitationGhosh, 1984).
The animals of all the experiments were monitored regularly for alteration in body weight, signs of toxicity, and mortality. The weights of animals were recorded every third day after tumor inoculation in all the groups.
The percentage increase in weight was calculated the formula: %increase in weight = [(animal weight on respective day/animal weigh on day 0)− 1] × 100 (CitationEchardt et al., 1982). The median survival time (MST) was calculated as described earlier by CitationUma Devi et al. (1994) using the formula: [first death + last death in the group]/2. The increase in median life span (% IMLS) was as described earlier by CitationUma Devi et al. (1994), using the formula: (MST of treated mice − MST of control) × 100]/ MST of control.
Effect on the hematological parameters
A separate experiment was carried out to evaluate the effect of cisplatin as well as the extracts on the hematological parameters using six animals per group. The blood was drawn from each mouse by retro orbital puncture with the anticoagulant heparin. The total white blood cell count (WBC), differential leukocyte counts, total red blood cell count, and hemoglobin content were measured as described earlier (CitationD’Amour et al., 1965).
Effect on the tumor cell viability in vitro
A separate experiment was planned to study the cytotoxic effect of the test agents as described earlier by CitationMazumdar et al. (1997) using six animals per group. On day 15 post tumor cell inoculation, 0.1 mL of ascitic fluid was aspirated under aseptic conditions. Equal volumes of cell suspension and tryphan blue were mixed thoroughly. The diluted suspension was charged into hemocytometer. The viable cells were (unstained) counted in WBC chamber under microscope and mean number of cells in four chambers was calculated as follows: total number of cells = mean number of cells × dilution factor × 104.
Brine shrimp lethality bioassay of extracts
Since its introduction by CitationMeyer et al. (1982), this in vivo lethality test has been successively employed for testing variety of toxic substances, toxicological studies, validate ethnobotanical claims for cytotoxic activities of plants and chemicals and for bioassay-guide fractionation of active cytotoxic agents.
The experiments were carried our as described by CitationMayer et al. (1982), briefly, the brine shrimp (Artemia salina) eggs were purchased from Brine Shrimp Direct, Ogden, UT, USA and hatched in artificial sea water at room temperature under constant aeration for 48 h. The hatched larvae (10 per group) were placed in a vial containing 5 mL of artificial sea water and a drop of dry yeast suspension 3 mg in 5 mL was added to each vial as food.
Test substances in different concentrations 10, 100, and 1000 ppm were added to the vial before making the final volume to 5 mL with sea water. The shrimp in artificial sea water alone served as control. The vials were maintained under illumination. The experiments were set in triplicate and the mean of three readings was taken as the final result. After 24 h, survivors were counted by using 3× magnifying glass and the percent deaths and LC50 values were calculated using the Finney Computer program.
Phytochemical investigation
The phytochemical investigations for detecting the nature of the active constituents like alkaloids, carbohydrates, phytosterols, fixed oils and fats, saponins, phenolic compounds, tannins, proteins, free amino acids, gums, mucilages, and flavonoids in the plant extracts were conducted as described by CitationKokate (1994).
Statistical analysis
Results were analyzed by one-way ANOVA followed by Scheffe’s post hoc test using SPSS computer package. A p value < 0.05 was considered statistically significant. For all in vivo experiments six animals, were used per group.
Results
Acute toxicity studies
In the acute toxicity studies, it was observed that the aqueous extract was safe at 3000 mg/kg i.p., clearly suggesting it to be nontoxic in nature. The ethanol extract was safe at 60 mg/kg. However, a further increase in the drug concentration caused akinetic movements leading to hypokinesia, drowsiness, sedation, anesthesia, and death in mice. At 100 mg/kg i.p., all the animals succumbed to the drug-induced toxic effects.
Antineoplastic effects in Ehrlich ascites carcinoma-bearing mice
There was no spontaneous regression of tumors in the mice transplanted with the EAC cells and the animals exhibited a constant weight gain and increase in the volume due to tumor cell multiplication and growth. The aqueous extract of Berberis aristata did not have any influence on weight gain, while the ethanol extract significantly (p < 0.05) reduced the weight gain on day 15 when compared with control. Administration of the alcoholic extract of Berberis aristata was comparable to the Cisplatin treatment group and showed significant (p < 0.05) reduction in the weight on day 12 and day 15 ().
Table 1. Effect of drugs on body weight changes in tumor-induced mice.
The administration of DDW (control group) did not affect the cancer cell proliferation of animals and a mean survival time (MST) of 16 days was observed. A reliable criterion for judging the value of any anticancer drug is prolongation of lifespan (CitationClarkson & Burchenal, 1965). Cisplatin showed the highest increase of 50% in life span of tumor-bearing animals. Cisplatin significantly (p < 0.05) increased the MST to 24 days. Neither the aqueous extract nor the ethanol extract was as effective as cisplatin and increased the MST to only 18 days and 19.5 days, thereby resulting in a % IMLS of 112.5 and 121.87, respectively ().
Table 2. Effects of drugs on survival time in tumor-induced mice.
Effect on the hematological parameters
Tumor induction increased the total number of WBC by nearly four fold and was significant (p < 0.05). Administration of cisplatin and the extracts significantly (p < 0.05) reversed the tumor-induced rise in total counts of WBC. However, the extracts were not as efficacious as cisplatin in reversing the tumor-induced total counts. Tumor progression affected the differential leukocyte counts. A reduction in lymphocytes count and increase in neutrophil count was observed in the tumor-bearing control animals and was statistically significant (p < 0.05).
Cisplatin as well as the extracts reversed these changes significantly (p < 0.05). However, the extracts were less efficacious than cisplatin in their effects (). Tumor development in the animals caused significant anemia (decrease in hemoglobin content), while cisplatin treatment reversed this significantly (p < 0.05). The treatment with extracts failed to alter the anemia (). Tumor induction caused a significant decrease in RBC to almost half of that of the normal animals. None of the treatments including cisplatin could reverse this effect ().
Table 3. Effect of different treatment on hematological parameters.
Effect on the tumor cell viability in vitro
In the cytotoxic assay (using the trypan blue dye), it was observed that the mean number of viable tumor cells in ascitic fluids of control animals was 19.82 millions/mm3. Administration of cisplatin caused a significant (p < 0.05) 58% reduction (8.36 millions/mm3) in the number of viable cells. Administration of the extracts caused a significant decline in the number of viable cells by 43.87 (11.12 million/mm3) and 47.67% (10.37 million/mm3), respectively, for the aqueous and ethanol extracts when compared to that of control ().
Table 4. Influence of drugs on tumor living cell number in ascitic fluid of tumor-induced mice.
Brine shrimp lethality bioassay of extracts
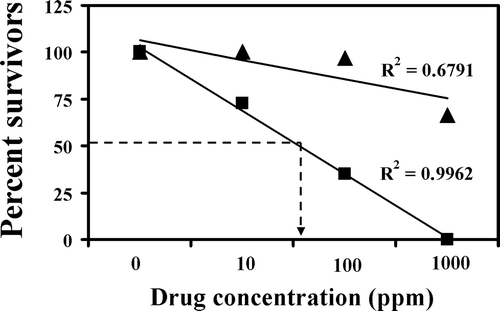
The results of this experiment show that the aqueous extract was not as toxic as the ethanol extract. At the lowest concentration of 10 ppm, the aqueous extract did not cause any mortality, while at the same concentration the ethanol caused 27.5% lethality. At the next concentration of 100 ppm, ethanol extract was 19.52 fold more effective than the aqueous extract in causing lethality as 3.33 and 65% mortality were observed, respectively, in aqueous and the ethanol extracts. At the highest concentration of 1000 ppm, no survivors were found in the ethanol extract group, while 66.66% survival was observed in the aqueous extract group. The LC50 was calculated and was found to be 3322 and 59 ppm for the aqueous and ethanol extract, respectively (; ).
Table 5. Effect of drugs on brine shrimp lethality bioassay.
Phytochemical investigation
As there was a considerable difference in the cytotoxic and antineoplastic effects with the aqueous and ethanol extracts, we deemed it important to perform a preliminary generic phytochemical assay. The analysis showed that aqueous extract and alcoholic extract contained alkaloids, carbohydrates, flavonoids, proteins, and tannins.
Discussion
India has a rich source of medicinal plants and many of them are an integral part of the medications being used in the various systems of medicine such as Ayurveda, Unani, and Siddha; and also in the traditional and folk practices in villages. However, only a few of them have been scientifically studied in accordance to the internationally accepted scientific procedures and assays. In the present study, for the first time an attempt is made to validate the antitumoral efficacy of Berberis aristata, a plant reported to possess significant anticancer effects in Ayurveda and in the ethnobotanical folk practices in parts of Northern India (CitationUniyal & Tewari, 1991) using the Ehrlich ascites carcinoma-bearing mice in controlled conditions. The antitumor effects were performed specifically in stages (10 day post tumor inoculation), representing the advanced stages to obtain consequential translational observations for possible human studies in future. Cisplatin a clinical used synthetic drug was used as a positive control.
Inoculation of the Ehrlich ascites carcinoma in the peritoneal cavity caused a rapid increase in ascites tumor volume in all mice. This caused gain of body weight, and by day 10, there was an approximate 15–24% increase. These results are in agreement to the earlier observations with Ehrlich ascites carcinoma (CitationJagetia & Baliga, 2004). Treatment with cisplatin caused a marked decrease in the body weight gain as well as increased the survival of the tumor bearing mice. When comparing with cisplatin, the antineoplastic effect of the 6.5 mg/kg body weight. (which corresponds to one-tenth of LD0/30) of the alcoholic extract was not effective. However, the most important fact that need to be considered is that the concentration of cisplatin used (3.5 mg/kg body weight) is almost one-fifth of the LD50/30 dose in our mice colony and that at this concentration it causes genotoxic and systemic toxicity in the recipient animals (CitationSrilatha, 2003). However, no such effects were seen with Berberis aristata, suggesting it was safe (CitationSrilatha, 2003).
Of the two extracts it was observed that the alcoholic extract was more potent than the aqueous extract, clearly suggesting that the type and concentration of the phytochemicals present in the extract are responsible for the effect. Preliminary phytochemical investigation showed that Berberis aristata possessed alkaloids and flavonoids as major constituents. It is observed that the alkaloids are more soluble in organic solvents (ethanol) than in the aqueous, while the reverse is true with most flavonoids (Kokate, 1992; CitationGraham et al., 2000). Generally, it is observed that the alkaloids are more cytotoxic (CitationGraham et al., 2000) and this might have been the reason for the pronounced cytotoxic effect of the ethanol extract.
In vitro studies with the Ehrlich ascites carcinoma cells have shown that all the treatments increased the percentage of dead cells (Tryphan blue-positive stained) indicating that they had cytotoxic effects. This fact was further substantiated for the extracts in the brine shrimp lethality assay, which in most cases correlates reasonably well with cytotoxic activity to antitumor properties (CitationMeyer et al., 1982). As the extract is introduced in to the tumor, growing in the peritoneal cavity of mice, a similar mechanism might be occurring and slows the tumor weight gain, with a concomitant increase in the survival.
Berberine, an isoquinoline alkaloid present in the plants belonging to the family of Berberidaceae (including Berberis aristata) has been reported to possess anticancer activities in both in vitro and in vivo systems of studies (CitationKuo et al., 1995; CitationLetasiova et al., 2005, Citation2006). From previous experience on the antineoplastic studies with berberine (CitationJagetia & Baliga, 2004), the authors are of the view that the antineoplastic activities of the extracts of Berberis aristata in the Ehrlich ascites carcinoma-bearing mice may not be solely due to the presence of berberine.
Phytochemical fractionation and isolation studies with the ethanol extract have shown that the extract contains approximately 12.8% (128 mg for 1 g of the extract) of berberine. The optimal effect with the extract was observed at 6.5 mg/kg body weight. of the ethanol extract which when calculated for the berberine concentration suggests that the extract contains approximately about 0.832 mg of berberine. In a previous study (CitationJagetia & Baliga, 2004), it was observed that administration of 8 and 10 mg/kg body weight of berberine to Ehrlich ascites carcinoma-bearing mice (one-fifth and one-fourth, respectively, of the LD50/14 dose of 40 mg/kg body weight) once daily for nine consecutive days (from day 1 to day 9 post tumor inoculation) was effective in increasing the survival of the tumor-bearing mice, while at lower concentrations (1, 2, 4, and 6 mg/kg body weight) was infective (CitationJagetia & Baliga, 2004).
In the present study, the extracts of Berberis aristata were administered from day 11 to day 15 post tumor inoculation (advanced stages) and were observed to be effective in controlling the tumor growth and increased the lifespan of mice. However, berberine when administered at 10 mg/kg body weight. from day 9 to 17 days post tumor inoculation time, daily for nine consecutive days was not effective (CitationJagetia & Baliga, 2004). These results clearly indicate that berberine alone may not be responsible for the antineoplastic activity of the extracts and other minor components might contribute synergistically to berberine.
In addition to berberine, Berberis aristata also contains berbamine, aromaline, karachine, pamatine, oxyacanthine, oxyberberine, taxilamine, chelidonic acid, palmitine, jastorrhizine, columbamine, tetrahydropalmitine, berbamine, oxyberberine, and oxyacanthine (CitationChakravarti et al., 1950; CitationRahman & Ansari, 1983) and these compounds might have had a role in controlling the tumor growth. To substantiate this view, recently, CitationXu et al. (2006) observed that berbamine, the other important constituent of Berberis aristata, was selectively anti-proliferative in activity to primary leukemia cells from CML patients. The authors also observed that it was a novel bcr/abl inhibitor and down-regulates p210bcr/abl oncoprotein level, and induces apoptosis of bcr/abl+ cells through caspase-3-dependent pathway (CitationXu et al., 2006).
The growth of tumor also results in hematological and immunological changes and all these changes contributed to the death of the animals. With conventional cancer chemotherapy, the major problems encountered are the drug-induced myelosuppression and anemia (CitationPrice &Greenfield, 1958; CitationHogland, 1982). The anemia observed in tumor-bearing mice in this study is mainly due to reduction in RBC or hemoglobin percentage, and this may occur due to iron deficiency or hemolytic or myelopathic conditions (CitationFenninger & Mider, 1954).
Treatment with cisplatin as well as the plant extracts reversed the tumor-induced changes in the hematological parameters like total and DLC of WBC, total RBC, and hemoglobin content. When compared with the nontumor-bearing animal, the hematological parameters were altered. In the tumor controls (without any treatments), there was a 360.62, 482, and 150 fold increase in the WBC, neutrophils, and monocytes, with nearly 57.8, 30, and 48.4% decrease in RBC, Hb, and lymphocytes, respectively, clearly suggesting that the tumor caused anemia. Treatment with Berberis aristata partially restored the hemoglobin content, RBC, and WBC count indicating that Berberis aristata possesses protective action on the hemopoietic system.
The lower toxicity of the extracts, when compared to berberine (LD50/14 40 mg/kg body weight.) may also lie in the composite nature of the extract, where the presence of several chemicals in it, could counteract the toxic implications of berberine without compromising on the anticancer effect and is in agreement with the documented ethnobotanical evidence of herbal practice seen in parts of India (CitationSathyavathi et al., 1987; CitationWarrier et al., 1996).
Conclusions
The present finding that the alcoholic extract of Berberis aristata possessed tumor growth inhibitory activity in late stage of cancer is very encouraging and may be due to its composite nature. Certain minor compounds may enhance the potency of the active compounds (berberine in this case) and thereby result in an additive or synergistic effect, reduce the toxic implications of treatment, reverse the tumor associated alterations in the hematological parameters, and afford immense benefit. Studies are being planned with the ethanol extract of Berberis aristata in more meaningful tumor models (like the refractory B16F1 melanoma, the metastatic B16F10 melanoma, refractory fibrosarcoma, and the highly metastatic 4T1 breast cancer model) to evaluate its overall potentiality for clinics. Studies are also underway to know which active principle/s are involved in the antineoplastic effects and their mode of action.
Acknowledgments
We thank Prof. N Udupa, Principal, Manipal College of Pharmaceutical Sciences, Manipal University, Manipal, for providing us with the necessary facilities to carry out this study. We are also grateful to Dr. Ramdasan Kutan, Director, Amala Cancer Research Center, Amala Nagar, Thrissur, Kerala, India for providing us with the Ehrlich ascites carcinoma cells for this study.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anis KV, Rajeshkumar NV, Kuttan R. (2001). Inhibition of chemical carcinogenesis by berberine in rats and mice. J Pharm Pharmacol, 53, 763–768.
- Chakravarti KK, Dhar DC, Siddiqui S. (1950). Alkaloidal consistuents of the bark of Berberis aristata. J Sci Indus Res, 9B, 161–164.
- Chemexcil. (1992). Selected medicinal plants of India. Basic Chemicals, Pharmaceutical and Cosmetic Export Promotion Council, Bombay, India, 205–207.
- Chopra RN. (1965). Problems and prospects of a pharmacological career in India. Annu Rev Pharmacol, 10, 1–8.
- Clarkson BD, Burchenal JH. (1965). Progress in leukemias. Prog Clin Cancer, 10, 625–663.
- CPCSEA. (2003). CPCSEA Guidelines for laboratory animal facility. Indian J Pharmacol, 35, 257–274.
- Cragg GM, Newman DJ, Weiss RB. (1997). Coral reefs, forests, and thermal vents: The worldwide exploration of nature for novel antitumor agents. Semin Oncol, 24, 156–163.
- D’Amour FE, Blood FR, Belden DA. (1965). Manual for Laboratory Work in Mammalian Physiology, 3 Ed, The University of Chicago, Chicago Press, 4–6.
- Dorr RT, Fritz WL. (1980). Cellular Chemotherapy Consideration in Cancer Chemotherapy Hand Book. London: Klimpton Publishers, 3–11.
- Eckhardt AE, Malone BN, Goldstein IJ. (1982). Inhibition of Ehrlich ascites tumor cell growth by Griffonia simplicifolia I lectin in vivo. Cancer Res, 42, 2977–2979.
- Fenninger LD, Mider GB. (1954). Energy and nitrogen metabolism in cancer. Adv Cancer Res, 2, 229–253.
- Geran RI, Greenberg NH, Mac Donald MM, Schumacher AM, Abbot BJ. (1972). Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother Rep, 3, 1–86.
- Ghosh MN. (1984). Fundamentals of Experimental Pharmacology. Scientific Book Agency, Calcutta, 153.
- Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. (2000). Plants used against cancer - an extension of the work of Jonathan Hartwell. J Ethnopharmacol, 73, 347–377.
- Henry DW. (1974). Adriamycin (NSC-123127) and its analogs. Cancer Chemother Rep, 2, 4, 5–9.
- Hogland HC. (1982). Hematological complications of cancer chemotherapy. Semin Oncol, 9, 95–102.
- Jagetia GC, Baliga MS. (2004). Effect of Alstonia scholaris in enhancing the anticancer activity of berberine in the Ehrlich ascites carcinoma-bearing mice. J Med Food, 7, 235–244.
- Jain SK. (1994). Ethnobotany and research on medicinal plants in India. Ciba Found Symp, 185, 153–64; discussion 164.
- Kokate CK. (1994). Phytochemical Screening. In Practical Pharmacognosy. New Delhi, Vallabha Prakshan, 107–111.
- Kuo CL, Chou CC, Yung BY. (1995). Berberine complexes with DNA in the berberine-induced apoptosis in human leukemic HL-60 cells. Cancer Lett, 93, 193–200.
- Letasiová S, Jantová S, Miko M, Ovádeková R, Horváthová M. (2006). Effect of berberine on proliferation, biosynthesis of macromolecules, cell cycle and induction of intercalation with DNA, dsDNA damage and apoptosis in Ehrlich ascites carcinoma cells. J Pharm Pharmacol, 58, 263–270.
- Letasiová S, Jantová S, Múcková M, Theiszová M. (2005). Antiproliferative activity of berberine in vitro and in vivo. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 149, 461–463.
- Mann J. (2002). Natural products in cancer chemotherapy: Past, present and future. Nat Rev Cancer, 2, 143–148.
- Mayer BN, Forrigni NR, Mc Laughlin JC. (1982). A convienent general bioassay for active plant constituents. Planta Medica, 45, 31–34.
- Mazumdar UK, Gupta M, Maiti S, Mukherjee D. (1997). Antitumor activity of Hygrophila spinosa on Ehrlich ascites carcinoma and sarcoma-180 induced mice. Indian J Exp Biol, 35, 473–477.
- Nadkarni AK. (1976). Indian Materia Medica, 3rd edition, Mumbai, India: Popular Press Ltd, 1308.
- Parmar C, Kaushal MK. (1982). Berberis aristata. Wild Fruits of the Sub-Himalayan Region. New Delhi: Kalyani Publishers, 10–14.
- Price VE, Greenfield RE. (1958). Anemia in cancer. Adv Cancer Res, 5, 199–290.
- Prieur DJ, Young DM, Davis RD, Cooney DA, Homan ER, Dixon RL, Guarino AM. (1973). Procedures for preclinical toxicologic evaluation of cancer chemotherapeutic agents: Protocols of the laboratory of toxicology. Cancer Chemother Rep 3, 4, 1–39.
- Rahman Attar-Ur Ansari, AA. (1983). Alkoloids of Berberis aristata-isolation of aromoline and oxyberberine. J Chem Soc Pakis, 5, 282–285.
- Sikora K, Halnan KE.(1990). Treatment of Cancer. London: Chapman and Hall.
- Sathyavathi GV, Gupta A, Tandon N. (1987). Medicinal plants of India. Spec Rep Ser Indian Counc Med Res, 2: 230–239.
- Srilatha P. (2003). Preliminary Studies to assess the Possible Antitumor Activity of Berberis aristata DC and Impatiens kleinii. A dissertation submitted to Manipal Academy of Higher Education, Manipal.
- Suffness M, Douros J. (1979). Drugs of plant origin. In: Devita VT and Busch H (Ed.), Methods in Cancer Research. New York: Academic Press, XVI: 79.
- Uma Devi P, Emerson Soloman F, Sharada AC. (1994). In vivo tumor inhibitory and radiosensitizing effects of an Indian medicinal plant, Plumbago rosea on experimental mouse tumors. Indian J Exp Biol, 32, 523–528.
- Uniyal MR, Tewari LC. (1991). Anti-cancer drugs from U.P. Himalaya. Ancient Sci Life, XI (1 & 2), 50–55.
- Warrier PK, Nambiar VPK, Ramankutty C. (1996). Indian Medicinal Plants. Hyderabad: Orient Longman Ltd, 1, 225–228
- WCRF/ AICR. (2007) World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington DC: AICR.
- Xu R, Dong Q, Yu Y, Zhao X, Gan X, Wu D, Lu Q, Xu X, Yu XF. (2006). Berbamine: A novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk Res, 30, 17–23.