Abstract
Context: Olive [Olea europaea L. (Oleaceae)] is a long-lived evergreen tree that is widespread in different parts of the world.
Objective: Olive oil has been reported to relieve pain; however, there is still insufficient data in the literature on the subject. Thus, it is considered worthwhile investigating the antinociceptive and anti-inflammatory effects of olive oil in adult male Balb/C mice.
Materials and methods: The antinociceptive effects were studied using formalin, hot plate and writhing tests. The acute anti-inflammatory effects of olive oil in mice were studied using xylene ear edema test. Olive oil (1, 5 and 10 ml/kg body wt.) was injected intraperitoneally. Intact animals served as controls.
Results: Our results showed that the olive oil only decreased the second phase of formalin-induced pain. In the hot plate test, olive oil did not raise the pain threshold over the 60 min duration of the test. Olive oil exhibited antinociceptive activity against writhing-induced pain by acetic acid. In the xylene ear edema test, olive oil showed significant anti-inflammatory activity in the mice.
Discussion and conclusion: The present data indicated that olive oil has antinociceptive and anti-inflammatory effects in mice but further investigation of these effects is required to elucidate the mechanism(s) involved in analgesic and anti-inflammatory effects of Olea europaea oil.
Keywords::
Introduction
Pain is a sensorial modality, which in many cases represents the only presenting symptom of disease. It often has a protective function. Throughout history, man has used many different therapies for pain relief. Medicinal herbs are highlighted due to their popular use. The analgesic effects of opiates are generally considered to be mediated through the central nervous system by acting at three opioid receptors (µ, κ and δ). Opiates are commonly used for the treatment of chronic pain. Although morphine has reigned for centuries as the king of painkillers, its rule has not been totally benign. There are serious concerns about its addictive properties and side effects, which include respiratory depression, drowsiness, decreased gastrointestinal motility, nausea and several alterations of the endocrine and autonomic nervous systems (CitationAlmeida, 2001).
Traditionally, medicinal plants are used throughout the world for a wide range of pain complications. Plant drugs are frequently considered to be less toxic and free of side effects compared to the synthetic counterparts. The study of such medicines might offer a natural key to pain management in the future.
The olive tree [Olea europaea L. (Oleaceae)] has been cultivated in various parts of the world. Not only the olive oil, but also the leaves have been used for medical purposes. Extra olive oil is characterized by various volatile compounds that include carbonyl compounds, alcohols, esters and hydrocarbons (CitationFlath et al., 1973). It has beneficial effects on cardiovascular and metabolic diseases, inflammatory and autoimmune diseases; and also has been shown to be instrumental in the prevention of breast and colon cancers (CitationAlarcón et al., 2001). Olive oil is an integral ingredient in the Mediterranean diet. There is growing evidence that it may have great health benefits including the reduction in coronary heart disease risk, the prevention of some cancers and the modification of immune and inflammatory responses (CitationVisioli & Galli, 2002; CitationKeys, 1995; CitationStark, 2002). In addition, olive oil is traditionally used to alleviate pain. Olive oil phenolic compounds are considered to possess anti-inflammatory properties, and therefore, were proposed as an alternative natural approach to prevent or treat chronic inflammatory diseases (CitationSergent et al., 2010). Olive oil contains omega-3 fatty acid. It has been suggested that omega-3 fatty acid may block pain neuron voltage-gated sodium channels (CitationShapiro, 2003). This study has been designed based on the reported use of olive oil in folk medicine for pain relief and the treatment of inflammation (CitationZargari, 1996). In the present study, we have examined the possible antinociceptive and anti-inflammatory effects of the olive oil in male Balb/C mice. A comparison was made between the action of olive oil, morphine or indomethacin (with known analgesic properties) and dexamethasone (with known anti-inflammatory effects).
Materials and methods
Materials
Extra virgin olive oil was purchased from local market in Roodbar area. Gas Chromatography (GC) was used for determination of the fatty acid composition of olive oil. The materials used in the study included acetic acid (Merck, Germany), formalin (Merck, Germany), xylene (Merck, Germany). The other drugs used in the study included morphine sulfate (Temad, Iran), indomethacin (Sigma, Poole, UK) and dexamethasone (Sigma, Poole, UK). All the drugs were dissolved in saline.
Animals
Male Balb/C mice, weighing 25–30 g, were housed in clean Plexiglass cages with temperature (22–24°C), 12-h light/12-h dark cycle and relative air humidity 40–60%. Mice had continuous access to standard laboratory chow (35% carbohydrates, 25% proteins, 7% lipids, and 3% vitamins) and to tap water. The diet was purchased from Pars-Dam food service, Tehran, Iran. Experimental procedures involving the animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national and international laws and Guidelines for Care and Use of Laboratory Animals in Biomedical Research as adopted and promulgated by the World Health Organization, and United States National Institutes of Health (1985).
Phytochemical analysis
Olive oil was analyzed for determination of its fatty acids composition using a GC method. In our analysis, fatty acids were converted to their methyl ester using boron trifloride as methylating reagent according to United States Pharmacopia-National Formulary method under Fats and Fixed Oils instruction (CitationUnited States Pharmacopeia, 2007). GC analyses were carried out using a Younglin Acm6000 with HP-5 capillary column [phenyl methyl siloxane, 25 m × 0.25 mm × 0.25 μm]. The oven temperature was programmed as follows: initial oven temperature was 150°C staying for 2 min, increasing up to 300°C at 6°C/min; injector temperature, 290°C; detector temperature 260°C; carrier gas He (0.8 ml/min); splitless mode with FID detector was used.
Analgesic activity
Formalin test
Morphine sulfate (10 mg/kg) was dissolved in saline solution. Olive oil was administered at dose levels of 1 ml/kg (0.03 ml/mouse), 5 ml/kg (0.15 ml/mouse) and 10 ml/kg (0.3 ml/mouse). The initial dose was 10 ml/kg (0.3 ml/mouse) (CitationNermine & Hanan, 2011) and it showed significant decrease so we reduced the doses to 5 ml/kg and then 1 ml/kg. An insulin syringe was used for all injections. The analgesic activity of the drugs was determined using the formalin test described by CitationDubuisson and Dennis (1977). One hour before testing, the animal was placed in a standard cage (30 × 12 × 13 cm) that served as an observation chamber. Then 2.5% formalin (50 µl) was injected to the dorsal surface of the right hind paw. The mouse was observed for 45 min after the injection of the formalin, and the pain scores in the injected hind paw were recorded. The initial nociceptive scores from 0–5 min (first phase) and 15–45 min (second phase) were counted after injection of formalin. The drugs were administrated 30 min before the injection of formalin intraperitoneally. Intact animals served as controls. A modified Dubuisson method was used to evaluate the pain response as follows: Score 0: walking as usual; Score 1: limping, not moving with the injected paw at the floor; Score 2: raising the injected paw; Score 3: paw licking or gnawing. The duration of the different behaviors described above was recorded separately (CitationHunskaar et al., 1985; CitationMoore et al., 1991).
Hot plate test
Mice were placed on an aluminum hot plate kept at a temperature of 55 ± 0.5°C for a maximum time of 30 s (CitationFranzotti et al., 2000). Reaction time was recorded when the animals licked their fore and hind paws and jumped; at before (0) and 15, 30, 45 and 60 min after intraperitoneal administration of olive oil (1, 5 and 10 ml/kg body wt.) to different groups of eight animals each. Morphine (10 mg/kg) was used as the reference drug. Intact animals served as controls.
Acetic acid-induced abdominal writhing
The test was performed as described by CitationCollier et al. (1968). Nociception was induced by an intraperitoneal (i.p.) injection of acetic acid 1.0%, 0.1 ml/kg body weight. Mice (eight per group) were intraperitoneal pretreated with the olive oil (1, 5 and 10 ml/kg body wt.) or indomethacin (10 mg/kg) 30 min before acetic acid injection. Intact animals served as controls. The number of abdominal writhes (full extension of both hind paws) was cumulatively counted every 5 min over a period of 20 min immediately after the acetic acid injection. The analgesic activity was expressed as inhibition percentage of abdominal writhes.
Anti-inflammatory activity
Xylene-induced ear edema
Thirty minutes after intraperitoneal injection of the olive oil (1, 5 and 10 ml/kg body wt.) or dexamethasone (10 mg/kg), 0.03 ml of xylene was applied to the anterior and posterior surfaces of the right ear. The left ear was considered as control. Two hours after xylene application, mice were sacrificed and both ears removed. Circular sections were excised, using a cork borer with a diameter of 7 mm, and weighed. The increase in ear weight caused by the irritant was measured by subtracting the weight of the untreated left ear section from that of the treated right ear section (CitationHosseinzadeh et al., 2000). Intact animals served as controls.
Statistical analysis
The data were expressed as mean values ± SEM and tested, using analysis of one-way ANOVA followed by Tukey post hoc test. The criterion for statistical significance was p < 0.05.
Results
Analysis of the oil
GC analysis showed that the oil was rich in oleic acid (61.5% w/w) and linoleic acid (18.7% w/w), which are unsaturated compounds. Other major fatty acid components were arachidonic acid (9.1% w/w), palmitic acid (7.2% w/w) and palmitoleic acid (2.2% w/w).
Formalin test
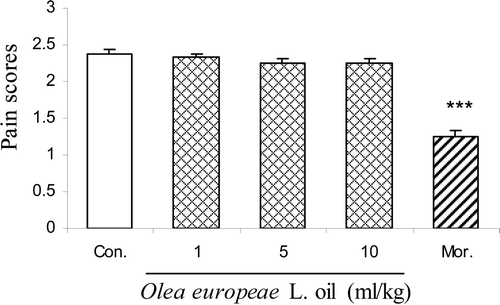
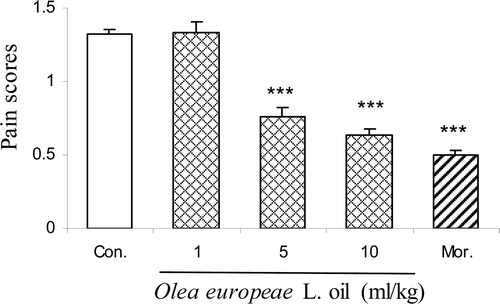
Intraplantar injection of 2.5% formalin evoked a characteristic biphasic nociceptive response. As shown in and , pretreatment (30 min) with different doses of the olive oil (at doses 5 and 10 ml/kg body wt.) produced a marked reduction in the duration of nociceptive activity only in the second phase [F(4,24) = 55.98, p < 0.0001]. Morphine (10 mg/kg) produced a marked reduction in the duration of nociceptive activity in both the first and second phases. The maximal inhibition of the nociceptive response was achieved at 10 ml/kg.
Hot plate test
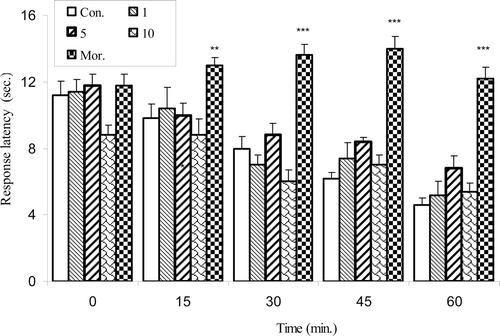
In the hot plate test, the administration of olive oil (at doses 1, 5 and 10 ml/kg body wt.) did not produce significant effects [F(4,24) = 18.07, p < 0.0001]. Morphine (10 ml/kg) was capable of increasing the latency period of pain induced by heating of the plate ().
Figure 3. Effect of the intraperitoneally administration of morphine (Mor.) and olive oil at doses of 1, 5 and 10 on hot plate test. Thermal antinociceptive latency before, and at 15, 30, 45, and 60 min after the treatment was measured. Each column represents mean ± SEM for eight mice. **p < 0.01, ***p < 0.001 different from control group. Intact animals served as controls.
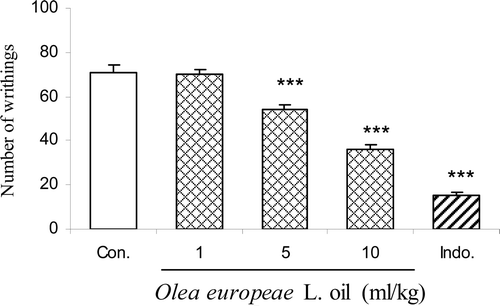
Acetic acid-induced writhing
Olive oil (at doses 5 and 10 ml/kg body wt.) and indomethacin (10 mg/kg body wt.) showed an inhibitory effect on the writhing response induced by acetic acid [F(4,24) = 122.21, p < 0.0001]. The maximal inhibition of the nociceptive response was achieved at a dose of 10 ml/kg ().
Figure 4. Effect of intraperitoneally administration of indomethacin (Indo.) and olive oil at doses of 1, 5 and 10 ml/kg body wt. on acetic acid-induced writhing response in mice. Each column represents mean ± SEM for eight mice. ***p < 0.001 different from control group. Intact animals served as controls.
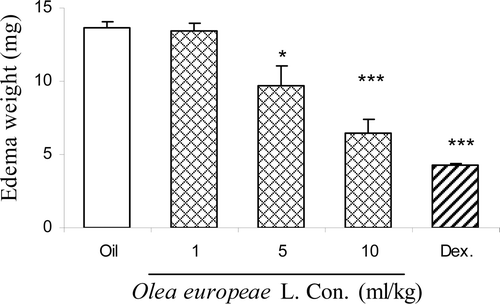
Xylene-induced ear edema
Olive oil (at doses 5 and 10 ml/kg body wt.) and dexamethasone (10 mg/kg body wt.) significantly reduced the ear edema induced by xylene [F(4,24) = 29.86, p < 0.0001] ().
Figure 5. Effect of intraperitoneally administration of dexamethasone (Dex.) and olive oil at doses of 1, 5 and 10 ml/kg body wt. on weight ear edema induced by xylene in mice. Each column represents mean ± SEM for eight mice. *p < 0.05, ***p < 0.001 different from control group. Intact animals served as controls.
Discussion
For centuries, cultures around the world have utilized medicinal plants to relieve pain successfully. In the last decades, the essential oils and various extracts of plants have been of great interest as they have been the sources of natural products. Traditionally, plant medicines are used throughout the world for a wide range of pain complications. The study of such medicines might offer a natural key to pain management in the future.
The present results indicate that olive oil has marked peripheral analgesic activity. The olive oil also showed anti-inflammation effects. The analgesic effects were assessed by three different models: formalin test, hot plate test and acetic acid-induced writhing test in mice, whereas the anti-inflammatory effects were examined by ear edema model.
The formalin test is a valid and reliable model of nociception and is sensitive for various classes of analgesic drugs. Subcutaneous formalin injection elicits biphasic nociceptive behaviors, and central sensitization induced by nociceptive inputs arriving during the first phase largely contributes to the second phase (CitationTanabe et al., 2008). Therefore, the test can be used to clarify the possible mechanism of antinociceptive effects of a proposed medication (CitationTjolsen et al., 1992). Centrally acting drugs such as opioids inhibit both phases equally (CitationShibata et al., 1989). Recent studies have shown that the earlier phase of formalin-induced pain reflects the direct effect of formalin on nociceptors, whereas the late phase reflects inflammatory pain, which appears to be attributable to prostaglandin synthesis (CitationHong & Abbott, 1995). The effect produced in the first phase may be due to immediate and direct effects on sensory receptors, bradykinin receptors or glutamatergic way, whereas for the last phase the analgesic effect is related to the inflammatory responses induced by arachidonic acid cascade (CitationDubuisson & Dennis, 1977; CitationBodi & Nodine, 1964; CitationSouza et al., 1998). Olive oil in dose dependent manner only inhibited the second phase of formalin induced pain. This result on the formalin test indicates that the analgesic effect of olive oil may be peripherally mediated.
The hot plate test is a method to evaluate supraspinal analgesic effects, and it reflects activity in thermally sensitive afferent fibers and activity of Aδ and C fibers (CitationChiba et al., 2009). The hot plate test measures the response to a brief, noxious stimulus; the formalin test, on the other hand, measures the response to a long-lasting nociceptive stimulus, and thus, may bear a closer resemblance to clinical pain (CitationRosland et al., 1990). The hot plate test with its short stimulation properties showed the action on somatic rather than visceral sites (CitationKesim et al., 2005). Our results showed that administration of olive oil did not raise the pain threshold in comparison with control. Morphine, used as a reference drug, produced a significant antinociceptive effect during all the observation times when compared with control values (CitationHiruma-Lima et al., 2000). These results on the hot plate test confirm that olive oil analgesic effect may be mediated via peripheral and not central mechanisms.
In the present study, acetic acid injection was demonstrated to induce a characteristic writhing response in the mice. Acetic acid-induced writhing is related to the increase in the peritoneal fluid levels of PGE2 and PGF2α (CitationDeraedt et al., 1980). The abdominal-constriction response is thought to involve, in part, local peritoneal receptors (CitationJais et al., 1997). The chemical stimulus induced by acetic acid provokes continuous and unavoidable pain causing abdominal contractions, movement of whole body, twisting of dorsoabdominal muscles, and a reduction in motor activities, which is evidence of visceral but not somatic pain (CitationKesim et al., 2005). It is, therefore, possible that the olive oil exerts an analgesic effect probably by inhibiting synthesis or action of prostaglandins. It was reported that prostaglandin biosynthesis plays an important role in the nociceptive mechanism in this pain model (CitationFranzotti et al., 2000). In addition to prostaglandins, several other inflammatory mediators, including sympathomimetic amines, tumour necrosis factor-α, interleukin-1β and interleukin-8, have been reported to be associated with the nociceptive response to acetic acid in mice (CitationDuarte et al., 1988; CitationFerreira et al., 1988; CitationFerreira et al., 1993a,Citationb; CitationRibeiro et al., 2000a). It is reported that writhing response induced by acetic acid is highly dependent on both peritoneal macrophages and mast cells (CitationRibeiro et al., 2000b). The olive oil produced a significant analgesic effect on the number of writhes induced by acetic acid, suggesting that the olive oil might have a role to inhibit the synthesis of prostaglandins.
The ear edema model permits the evaluation of anti-inflammatory steroids and is less sensitive to non-steroidal anti-inflammatory agents. In xylene-induced ear edema test, mediators of inflammation are released following stimulation. This leads to dilation of arterioles and venules and to increased vascular permeability (CitationVogel & Vogel, 1997). The olive oil had significant anti-inflammatory effects in this test, thus it may have a membrane-stabilizing effect that reduces capillary permeability and/or has inhibitory effects on mediators. Intraperitoneal administration of the olive oil, 30 min before topical application of xylene, dose dependently inhibited the development of ear edema. The inhibition produced by 10 ml/kg of the olive oil was comparable to that produced by 10 mg/kg dexamethasone. The effect of the olive oil in this model suggests inhibition of phospholipase A2.
Olive oil contains monounsaturated (oleic) and polyunsaturated fatty acids, α-tocopherol, phenol compounds, carotenoids, squalene, phytosterols, and chlorophyll (CitationViola & Viola, 2009). Antioxidant and anti-inflammatory properties have been reported for dietary polyphenols (CitationZern & Fernandez, 2005). It has been shown that oleocanthal, a phenolic compound, exhibited analgesic effects (CitationBeauchamp et al., 2005). There are reports on the role of vitamin E (α-tocopherol) and oleic acid in the treatment of inflammation (CitationDevaraj et al., 2007; CitationVassiliou et al., 2009). Thus, the antinociceptive and anti-inflammatory effects of the olive oil may be due to their phenolic compound, α-tocopherol and oleic acid.
The mechanism by which olive oil exerts analgesic activity still remains undetermined, but our results suggest that analgesic effects of the olive oil may be mediated via peripheral and not central mechanisms. It is concluded that olive oil can serve as a good adjuvant in the present armamentarium of analgesic drugs. Further studies are needed to clarify the exact mechanism (s) and active compound (s) involved in these pharmacological effects.
Acknowledgments
We would like to thank Deputy Research, Science and Research Branch, Islamic Azad University, for financial support of the project.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alarcón de la Lastra C, Barranco MD, Motilva V, Herrerias JM. (2001). Mediterranean diet and health: Biological importance of olive oil. Curr Pharm Des, 7, 933–950.
- Almeida RN, Navarro DS, Barbosa-Filho JM. (2001). Plants with central analgesic activity. Phytomedicine, 8, 310–322.
- Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, Lee CH, Smith AB, Breslin PA. (2005). Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature, 437, 45–46.
- Bodi T, Nodine JH. (1964). Clinical techniques for evaluation of anti-anxiety drugs. In: Nodine JH, Siegler PE, ed. Animal and Clinical Pharmacologic Techniques in Drug Evaluation, 1st ed. Yearbook Medical, 325–329.
- Chiba S, Nishiyama T, Yoshikawa M, Yamada Y. (2009). The antinociceptive effects of midazolam on three different types of nociception in mice. J Pharmacol Sci, 109, 71–77.
- Collier HDJ, Dinnin LC, Johnson CA, Schneider C. (1968). The abdominal response and its suppression by analgesic drugs in the mouse. BR. J Pharmacol Chemother, 32, 295–310.
- Deraedt R, Jouquey S, Delevallée F, Flahaut M. (1980). Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol, 61, 17–24.
- Devaraj S, Tang R, Adams-Huet B, Harris A, Seenivasan T, de Lemos JA, Jialal I. (2007). Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr, 86, 1392–1398.
- Duarte ID, Nakamura M, Ferreira SH. (1988). Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz J Med Biol Res, 21, 341–343.
- Dubuisson D, Dennis SG. (1977). The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain, 4, 161–174.
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. (1988). Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature, 334, 698–700.
- Ferreira SH, Lorenzetti BB, Cunha FQ, Poole S. (1993a). Bradykinin release of TNF-alpha plays a key role in the development of inflammatory hyperalgesia. Agents Actions, 38 Spec No, C7–C9.
- Ferreira SH, Lorenzetti BB, Poole S. (1993b). Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol, 110, 1227–1231.
- Flath RA, Forrey RR, Guadagni DG. (1973). Aroma components of olive oil. J Agric Food Chem, 21, 948–952.
- Franzotti EM, Santos CVF, Rodrigues HMSL, Mourao RHV, Andrade MR, Antoniolli AR. (2000). Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca). J Ethnopharmacol, 72, 273–277.
- Hiruma-Lima CA, Gracioso JS, Bighetti EJ, Germonsén Robineou L, Souza Brito AR. (2000). The juice of fresh leaves of Boerhaavia diffusa L. (Nyctaginaceae) markedly reduces pain in mice. J Ethnopharmacol, 71, 267–274.
- Hong Y, Abbott FV. (1995). Peripheral opioid modulation of pain and inflammation in the formalin test. Eur J Pharmacol, 277, 21–28.
- Hosseinzadeh H, Ramezani M, Salmani G. (2000). Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol, 73, 379–385.
- Hunskaar S, Fasmer OB, Hole K. (1985). Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods, 14, 69–76.
- Kesim M, Duman EN, Kadioglu M, Yaris E, Kalyoncu NI, Erciyes N. (2005). The different roles of 5-HT(2) and 5-HT(3) receptors on antinociceptive effect of paroxetine in chemical stimuli in mice. J Pharmacol Sci, 97, 61–66.
- Keys A. (1995). Mediterranean diet and public health: Personal reflections. Am J Clin Nutr, 61, 1321S–1323S.
- Mat Jais AM, Dambisya YM, Lee TL. (1997). Antinociceptive activity of Channa striatus (haruan) extracts in mice. J Ethnopharmacol, 57, 125–130.
- Moore PK, Oluyomi AO, Babbedge RC, Wallace P, Hart SL. (1991). l-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br J Pharmacol, 102, 198–202.
- Nermine KS, Hanan AS. (2011). Olive oil effectively mitigates ovariectomy-induced osteoporosis in rats. BMC Complement Altern Med, 4, 1–11.
- Ribeiro RA, Vale ML, Ferreira SH, Cunha FQ. (2000a). Analgesic effect of thalidomide on inflammatory pain. Eur J Pharmacol, 391, 97–103.
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. (2000b). Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol, 387, 111–118.
- Rosland JH, Tjølsen A, Maehle B, Hole K. (1990). The formalin test in mice: Effect of formalin concentration. Pain, 42, 235–242.
- Sergent T, Piront N, Meurice J, Toussaint O, Schneider YJ. (2010). Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem Biol Interact, 188, 659–667.
- Shapiro H. (2003). Could n-3 polyunsaturated fatty acids reduce pathological pain by direct actions on the nervous system? Prostaglandins Leukot Essent Fatty Acids, 68, 219–224.
- Shibata M, Ohkubo T, Takahashi H, Inoki R. (1989). Modified formalin test: Characteristic biphasic pain response. Pain, 38, 347–352.
- Souza MM, De Jesus RAP, Cechinel Filho V, Schlemper V. (1998). Analgesic profile of hydroalcoholic extract obtained from Marrubium vulgare. Phytomedicine, 5, 103–107.
- Stark AH, Madar Z. (2002). Olive oil as a functional food: Epidemiology and nutritional approaches. Nutr Rev, 60, 170–176.
- Tanabe M, Murakami T, Ono H. (2008). Zonisamide suppresses pain symptoms of formalin-induced inflammatory and streptozotocin-induced diabetic neuropathy. J Pharmacol Sci, 107, 213–220.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. (1992). The formalin test: An evaluation of the method. Pain, 51, 5–17.
- United States Pharmacopeia. (2007). National Formulary (USP 30 NF 25), Fats and Fixed Oils; Capture 401, US: Rockville.
- Vassiliou EV, Gonzalez A, Garcia C, Tadros JH. (2009). Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF both in vitro and in vivo systems. Lipids Health Dis, 8–25.
- Visioli F, Galli C. (2002). Biological properties of olive oil phytochemicals. Crit Rev Food Sci Nutr, 42, 209–221.
- Viola P, Viola M. (2009). Virgin olive oil as a fundamental nutritional component and skin protector. Clin Dermatol, 27, 159–165.
- Vogel HG, Vogel WH.(1997). Drug Discovery and Evaluation, Pharmacological Assays. Springer: Berlin, 402–403.
- Zargari A. (1996). Medicinal Plants. 6th ed. Tehran: Tehran University Publication. pp. 319–326.
- Zern TL, Fernandez ML. (2005). Cardioprotective effects of dietary polyphenols. J Nutr, 135, 2291–2294.