Abstract
Context: Clerodendrum infortunatum Linn. is a widely used plant in the Indian indigenous system of medicine for the treatment of tumors.
Objective: The present study evaluated the anticancer activity of methanol extract of C. infortunatum (MECI) against Ehrlich’s ascites carcinoma (EAC) bearing Swiss albino mice and isolation of bioactive terpenoids from it.
Methods: HPLC analysis of the methanol extract showed the presence of three major components. Out of those, two compounds were isolated and characterized as oleanolic acid and clerodinin A. The anticancer activity of MECI was assessed by measuring the tumor growth response, percentage increase of life span, study of hematological parameters, lipid peroxidation, antioxidant enzyme activity like glutathion and CAT. In vitro cytotoxicity assay was also performed using EAC cell lines.
Result and conclusion: Treatment with MECI causes significant decrease in the tumor cell volume and increase in the life span. The median survival time (MST) of EAC control group was found as 19.42 ± 0.91 d, whereas the MST was increased to 23.44 ± 2.69 d and 27.57 ± 2.57 d for the groups treated with MECI at 100 and 200 mg/kg, respectively. All the hematological parameters, malonaldehyde content and antioxidant enzymes’ activity were restored towards the normal level. IC50 value of MECI was found as 498.33 µg/mL in cytotoxicity study. The experimental results suggested that MECI has significant anticancer activity, which can be attributed to the presence of oleanolic acid and clerodinin A.
Introduction
Clerodendrum infortunatum Linn. is a terrestrial shrub having square, blackish stem and simple, opposite, decussate, petiolate, exstipulate, coriaceous, hairy leaves with a disagreeable odor (CitationNadkarni & Nadkarni, 2002). Different species of Clerodendrum genus have been traditionally used over centuries and their antioxidant and hepatoprotective potential have already been proved (CitationGopal & Sengottuvelu, 2008; CitationVidya et al., 2007; CitationChae et al., 2005). C. infortunatum is very common throughout the plains of India and found widely in West Bengal. Various parts of the plant are used by tribes in colic, scorpion sting and snake bite, tumors and certain skin diseases (CitationNadkarni & Nadkarni, 2002). The leaves are slightly bitter, cure inflammation, skin diseases and good in small pox (CitationChopra et al., 1992). The plant was found to contain triterpenes, steroids and flavonoids (CitationJoshi et al., 1977; CitationAkihisa et al., 1988; CitationSinha et al., 1981; CitationManzoor-Khuda & Sarela, 1965). The antioxidant (CitationSannigrahi et al., 2009), antimicrobial (CitationRajakaruna et al., 2002), antimalarial (CitationGoswami et al., 1998) and analgesic (CitationPal et al., 2009) activities of the plant has further created an upsurge in investigations on the plant.
Cancer, considered as one of the most common causes of morbidity and mortality worldwide, is caused by the sequential acquisition of mutations in genes implicated in cell proliferation and cell death. In the recent times, the trend in the research in the field of cancer is once again shifting towards identifying new medicines for the treatment of cancer from natural sources. Wide varieties of compounds are in use in current cancer therapeutic practices.
The present study evaluated the isolation of two bioactive terpenoids and anticancer activity of the methanol extract of C. infortunatum (MECI) in Ehrlich’s ascites carcinoma (EAC) in mice.
Materials and methods
Plant material and extraction
Fresh leaves of the plant were collected locally (Bankura district of West Bengal, India) in the month of December, 2005 and identified by the botanist of Botanical Survey of India, West Bengal, India. The voucher specimen (DKP 02/2005) has been deposited in the laboratory for further reference. After collection, the leaves were washed properly and fungal leaves were picked out. Air-dried and powdered leaves (1.5 kg) were extracted successively with petroleum ether and methanol using Soxhlet apparatus. Solvent was evaporated under reduced pressure to obtain the crude methanol extract (95 g). Weighed amount of MECI was suspended in Tween 80 prior to administration.
Reagents and chemicals
EAC cells were obtained from Chittaranjan National Cancer Institute, Kolkata, India. All the chemicals were of analytical grade and commercially available including thiobarbituric acid, nitroblue tetrazolium chloride (NBT) (Loba Chemie, Bombay India), 5,5′-dithio bis-2-nitrobenzoic acid (DTNB) (SICCO Research Laboratory, Bombay).
HPLC analysis of methanol extract
HPLC analysis of MECI was performed in a HPLC system consisting of Agilent 1200 series with quaternary pump and PDA detector. A reverse phase Zorbax SB C-18 column (50 mm × 4.6 mm diameter; particle size 1.8 µm) was used for the study. The data were recorded in a computer using ChemStation software. A linear gradient elution method using water and acetonitrile as mobile phase was used for the analysis. The flow rate was kept constant at 0.3 mL/min at room temperature and 2–5 µL sample volume was used for the analysis.
Isolation of terpenoids
MECI was subjected to column chromatographic analysis and eluted with n-hexane and chloroform in gradient fashion. Fractions were collected and monitored by TLC analysis. Based on the Rf value, the similar fractions were combined. The combined fraction of 16–20 obtained from n-hexane–chloroform (9:1) gave single spot on TLC, which was further purified with preparative TLC using n-hexane–ethyl acetate (8:2) to afford compound 1 (C1). Another combined fraction of 32–35 obtained from n-hexane–chloroform (8:2) was purified by PTLC using CH2Cl2–acetone (9:1) to afford compound 2 (C2).
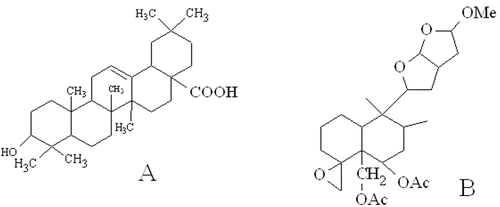
Compound 1(22 mg): UV λmax in MeOH 205 nm. IR (KBr) Vmax cm−1: 3472 (OH stretching); 3074 (carboxyl); 2872 (C–H stretching); 1687 (C=O stretching); 1645 (olefinic carbon). TOF-MS: m/z 456.19 [M]+; 438 [M−H2O(18)]+; 423 [438-CH3(15)]+; 248; 245; 207; 203; 148; 133. 1H NMR data in CDCl3 were found similar with oleanolic acid (CitationGangwal et al., 2010).
Compound 2 (26 mg): UV λmax in CHCl3 214 nm. IR (KBr) Vmax cm−1: 3429 (OAc); 3028 (oxiran ring); 1695 (ester). TOF-MS: m/z 466; 375; 315; 297; 253; 236; 217; 173; 143; 129; 111. 1H NMR data in CDCl3 were found similar with clerodinin A (CitationLin et al., 1989).
Acute toxicity study
The LD50 value of the MECI was calculated according to the methods of CitationLitchfield and Wilcoxon (1949). Animals were divided into different groups (10 in each group) and treated with aliquot doses of the extracts orally (200,500, 1000, 1200, 1400, 1500 and 1600 mg/kg). The mortality and symptoms of toxicity referred to as CNS behavioral activities were observed and recorded.
Experiment protocol
Swiss albino mice weighing 20–25 g were used for the study. Animals were divided into five groups, with 12 animals in each group. Group I was treated as the normal group, which received normal saline (5 mL/kg, i.p.). EAC cells (2 × 106) were injected intraperitoneally (i.p.) to each mouse of groups II–V. After 24 h of tumor inoculation, MECI at doses of 100 (MECI 100) and 200 mg/kg, b.w. (MECI 200) were administered i.p. daily for 9 consecutive days to groups III–IV, respectively. Group V received 5-flurouracil (5-FU) at a dose of 20 mg/kg as a standard drug for 9 days (CitationGupta et al., 2004). The studies were approved by Jadavpur University Animal Ethical Committee, Kolkata, India. After administration of the last dose, followed by fasting for 24 h, six mice from each group were sacrificed for assessment of anticancer activity by measuring different hematological and biochemical parameters. Liver tissue was collected for evaluation of in vivo antioxidant status. The remaining animals in each group were kept for lifespan study.
Effect of MECI on survival time
Median survival time (MST) of each group was calculated from the formula (CitationGupta et al., 2000)
The mortality rate was noted in all the groups and the percentage increase in life span (ILS) was calculated using the following formula:
where T and C are MST of treated and control group, respectively.
Effect of MECI on tumor growth response and on hematological parameters
The effect of MECI on tumor growth was examined by studying tumor volume, tumor cell count and the percentage of the viable and non-viable cell count (CitationMazumdar et al., 1997). Red blood cell count (RBC), white blood cell count (WBC) and hemoglobin content were measured using the conventional procedure from blood obtained intracardially (CitationD’Amour et al., 1965).
In vivo antioxidant status
Lipid peroxidation (CitationOkhawa et al., 1979) reduced glutathione (GSH) content (CitationEllman, 1959) and catalase (CitationAebi, 1984) activity in the liver homogenate were determined using the described procedures.
Assay for in vitro cytotoxicity
The in vitro short-term cytotoxicity of MECI was assayed using EAC cell lines. Briefly, 1 × 106 viable cells of the cell line suspended in 0.1 mL of phosphate buffered saline (0.2 M, pH 7.4) containing various concentrations of the test compound (50–500 µg/mL) and phosphate buffer in a final volume of 1 mL were incubated at 37°C for 3 h (CitationSheeja et al., 1997). The percentage of cytotoxicity was determined by calculating the IC50 value.
Statistical analysis
The statistical significance was assessed by means of variance followed by Tukey’s multiple comparison tests. Values are expressed as mean ± SD and p values less than 0.05 were considered as significant.
Results
Preliminary phytochemical analysis of methanol extract of C. infortunatum showed the presence of saponins, terpenoids and flavonoids. The acute toxicity of MECI was found to have the LD50 value of 1015.63 mg/kg.
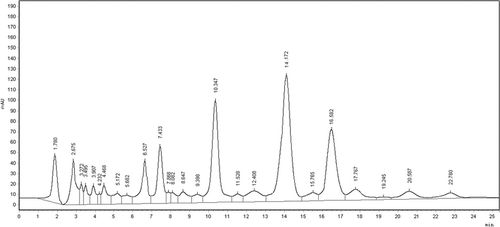
HPLC analysis of crude methanol extract revealed the presence of more than 20 compounds in the extract (). Three major compounds were indicated to present in the extract. Chromatographic separation of MECI resulted in the isolation of two bioactive terpenoids which were characterized by spectroscopic analysis as oleanolic acid and clerodinin A ().
Figure 1. HPLC chromatogram of methanol extract of C. infortunatum performed in HPLC system consisting of Agilent 1200 series with quaternary pump, PDA detector and reverse phase Zorbax SB C-18 column (50 mm × 4.6 mm diameter; particle size 1.8 µm).
Effect of MECI on survival time on EAC bearing mice is shown in . The MST of EAC control group was found as 19.42 ± 0.91 d, where as the MST was increased to 23.44 ± 2.69 d and 27.57 ± 2.57 d for the groups treated with MECI at 100 and 200 mg/kg, respectively. In EAC control group, there was an increase in tumor volume compared to normal group. Treatment with MECI significantly reduced the tumor volume and viable cell count compared to those of EAC control mice, whereas non-viable cell count was found to be significantly high in the treated groups ().
Table 1. Effect of methanol extract of C. infortunatum (MECI) on survival time on Ehrlich’s ascites carcinoma (EAC) bearing mice.
Table 2. Effects of MECI on tumor growth of EAC cells bearing mice in vivo.
Changes of hematological parameters on MECI treated group are shown in . There were significant (p < 0.001) decrease in hemoglobin and RBC count and increase in WBC count in EAC bearing mice in comparison to normal group. On treatment with MECI at a dose of 200 mg/kg, not only significantly increased the hemoglobin (p < 0.001) and RBC (p < 0.05) count but also lowered the WBC count almost towards normal level. At the dose of 100 mg/kg, only WBC count was significantly (p < 0.001) reduced, but the other parameters were not significantly changed.
Table 3. Effect of MECI on hematological parameters of EAC bearing mice.
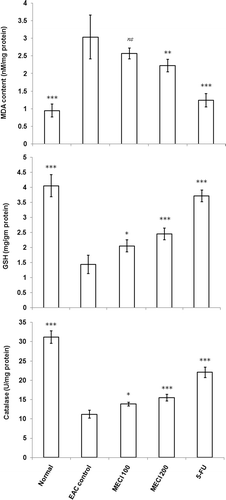
The effect of MECI on lipid peroxidation, GSH content and catalase activity is shown in . The content of malondialdehyde (MDA) was significantly (p < 0.001) higher in EAC control group (3.043 nM/mg of protein) compared to normal group (0.955 nM/mg of protein). MDA content was reduced on treatment with MECI at 100 mg/kg (2.573 nM/mg of protein) and 200 mg/kg (2.228 nM/mg of protein). The significant (p < 0.001) difference of GSH content was found between normal group (4.062 mg/g of tissue) and EAC control group (1.442 mg/g of tissue). Treatment with tested compounds increased GSH content significantly. Catalase activity in EAC control group was also significantly (p < 0.001) reduced compared to normal group, which was restored by treatment with MECI. MECI showed concentration dependant cytotoxicity effect on EAC cell line. IC50 value of MECI was found as 498.33 µg/mL in cytotoxicity study.
Discussion
EAC cell is the frequently used in vivo model to screen anticancer activity. In the present study, the anticancer activity of MECI was studied in EAC bearing mice.
Ascites fluid of EAC cell bearing mice is the direct nutritional source for tumor cells and a rapid increase in ascitic fluid with tumor growth would be a means to meet the nutritional requirement of tumor cells. Treatment with MECI decreased the tumor volume resulting decrease in nutritional fluid volume and arresting tumor growth and increased the lifespan of EAC bearing mice.
Hematological parameters are considered as markers for screening of EAC-induced anticancer study (CitationRoy et al., 1981). A significant increase in the WBC count along with the decrease in the hemoglobin and RBC count were found in EAC treated mice. Anemia is associated with cancer patients. The anemia encountered in tumor bearing mice is mainly due to the reduction in RBC or hemoglobin percentage, and this may occur either due to iron deficiency or due to hemolytic or myelopathic conditions (CitationFenninger & Mider, 1954). Treatment with MECI decreased the WBC count but increased the hemoglobin and RBC towards the normal. This result indicates that MECI possess protective action on the hematopoietic system.
MDA, the end product of lipid peroxidation, was reported to be higher in cancer tissues than in non-diseased organ (CitationYagi, 1987). It has been shown that MDA is mutagenic to human cells (CitationNiedernhofer et al., 2003) and plays a significant role in DNA damage, sister-chromatid exchanges (SCEs) and carcinogenesis (CitationRay et al., 2001). In EAC control group, higher concentration of MDA was found compared to normal group due to excess lipid peroxidation in vivo (CitationSinclair et al., 1990). MECI treatment significantly reduced the elevated level of MDA content that indicates less damage of macromolecules. Glutathione and catalase are the important contents of antioxidant enzymatic defense mechanism. Significant decrease in the GSH and CAT was observed in EAC control group but on treatment with MECI these parameters were increased towards the normal. Modulation of lipid peroxidation and increase in antioxidant enzyme content is considered as an important mechanism for oncostatic activity against oxidative stress induced by EAC cells (CitationNoaman et al., 2008). Result of in vitro cytotoxicity study indicates the toxic effect on EAC cell.
Terpenoids are also considered as plant antioxidant (CitationPetronelli et al., 2009) and promising anticancer agents (CitationGrassmann, 2005). Terpenoids obtained from the plant and green algae exhibit potent anticancer activity against classical and atypical multidrug resistant cancer cells (CitationLage et al., 2010). The anticancer effects of MECI may be due to the suppression of lipid peroxidation and increase in the content of the enzymatic defense system.
Conclusion
Based on experimental data, it can be concluded that MECI exhibits significant anticancer effects against EAC cell which may be due to presence of oleanolic acid and clerodinin A in MECI. Inhibition of lipid peroxidation and increase in the content of enzymatic defense system can be attributed to its probable mechanism for anticancer activity.
Declaration of interest
The authors are declaring no potential conflict of interest.
References
- Aebi H. (1984). Catalase in vitro. Meth Enzymol, 105, 121–126.
- Akihisa T, Matsubara Y, Ghosh P, Thakur S, Shimizu N, Tamura T, Matsumoto T. (1988). The 24α- and 24β-epimers of 24-ethylcholesta-5,22-dien-3β-ol in two Clerodendrum species. Phytochemistry, 27, 1169–1172.
- Chae S, Kim JS, Kang KA, Bu HD, Lee Y, Seo YR, Hyun JW, Kang SS. (2005). Antioxidant activity of isoacteoside from Clerodendron trichotomum. J Toxicol Environ Health Part A, 68, 389–400.
- Chopra RN, Nayer SL, Chopra IC. (1992). Glossay of Indian Medicinal Plant, Publication and Information Directorate, CSIR, New Delhi, p 71.
- D’Amour FE, Blood FR, Belden DA. (1965). Manual for Laboratory Work in Mammalian Physiology, 3rd Ed. The University of Chicago Press, Chicago, IL, p 4–6.
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys, 82, 70–77.
- Fenninger LD, Mider GB. (1954). Energy and nitrogen metabolism in cancer. Adv Cancer Res, 2, 229–253.
- Gangwal A, Parmar SK, Sheth NR. (2010). Triterpenids, flavonoids and sterols from Lagenaria siceraria fruits. Der Pharmacia Lett, 2, 307–317.
- Gopal N, Sengottuvelu S. (2008). Hepatoprotective activity of Clerodendrum inerme against CCl4 induced hepatic injury in rats. Fitoterapia, 79, 24–26.
- Goswami A, Dixit VK, Srivastava BK. (1998). Anti-malarial activity of aqueous extract of Clerodendrum infortunatum. Bionature, 48, 45–48.
- Grassmann J. (2005). Terpenoids as plant antioxidants. Vitam Horm, 72, 505–535.
- Gupta M, Mazumder UK, Kumar RS, Kumar TS. (2004). Antitumor activity and antioxidant role of Bauhinia racemosa against Ehrlich ascites carcinoma in Swiss albino mice [corrected]. Acta Pharmacol Sin, 25, 1070–1076.
- Gupta M, Mazumder UK, Rath N, Mukhopadhyay DK. (2000). Antitumor activity of methanolic extract of Cassia fistula L. seed against Ehrlich ascites carcinoma. J Ethnopharmacol, 72, 151–156.
- Joshi KC, Prakash L, Shah RK. (1977). Chemical constituents of Clerodendrum infortunatum and Ficus racemosa. J Indian Chem Soc, 54, 1104–1106.
- Lage H, Duarte N, Coburger C, Hilgeroth A, Ferreira MJ. (2010). Antitumor activity of terpenoids against classical and atypical multidrug resistant cancer cells. Phytomedicine, 17, 441–448.
- Lin YL, Kuo YH, Chen YL. (1989). Two new clerodane type diterpenoids, Clerodinin A and B from Clerodendrum brachyanthum SCHAUER. Chem Pharm Bull, 37, 2191–2193.
- Litchfield JT Jr, Wilcoxon F. (1949). A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther, 96, 99–113.
- Manzoor-Khuda M, Sarela S. (1965). Constituents of Clerodendron infortunatum (bhat)-I. Isolation of clerodolone, clerodone, clerodol and clerosterol. Tetrahedron, 21, 797–802.
- Mazumdar UK, Gupta M, Maiti S, Mukherjee D. (1997). Antitumor activity of Hygrophila spinosa on Ehrlich ascites carcinoma and sarcoma-180 induced mice. Indian J Exp Biol, 35, 473–477.
- Nadkarni KM, Nadkarni AK. (2002). Indian Materia Medica, Popular Publications, Mumbai, p 353.
- Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. (2003). Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem, 278, 31426–31433.
- Noaman E, Badr El-Din NK, Bibars MA, Abou Mossallam AA, Ghoneum M. (2008). Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett, 268, 348–359.
- Okhawa H, Oishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Pal D, Sannigrahi S, Mazumder UK. (2009). Analgesic and anticonvulsant effects of saponin isolated from the leaves of Clerodendrum infortunatum Linn. in mice. Indian J Exp Biol, 47, 743–747.
- Petronelli A, Pannitteri G, Testa U. (2009). Triterpenoids as new promising anticancer drugs. Anticancer Drugs, 20, 880–892.
- Rajakaruna N, Harris CS, Towers GHN. (2002). Antimicrobial activity of plants collected from serpentine outcrops in Srilanka. Pharm Biol, 40, 235–244.
- Ray GN, Shahid M, Husain SA. (2001). Effect of nitric oxide and malondialdehyde on sister-chromatid exchanges in breast cancer. Br J Biomed Sci, 58, 169–176.
- Roy MR, Guhathakurta S, Roychowdhury J. (1981). Hematological changes in experimental tumours. Indian J Med Res, 74, 896–903.
- Sannigrahi S, Mazumder UK, Pal DK, Parida S. (2009). In vitro antioxidant activity of methanol extract of Clerodendrum infortunatum Linn. Oriental Pharm Exp Med, 9, 128–134.
- Sheeja KR, Kuttan G, Kuttan R. (1997). Cytotoxic and antitumor activity of barberin. Amala Res Bull, 17, 73–76.
- Sinclair AJ, Barnett AH, Lunec J. (1990). Free radicals and antioxidant systems in health and disease. Br J Hosp Med, 43, 334–344.
- Sinha NK, Seth KK, Pandey VB, Dasgupta B, Shah AH. (1981). Flavonoids from the flowers of Clerodendron infortunatum. Planta Med, 42, 296–298.
- Vidya SM, Krishna V, Manjunatha BK, Mankani KL, Ahmed M, Singh SD. (2007). Evaluation of hepatoprotective activity of Clerodendrum serratum L. Indian J Exp Biol, 45, 538–542.
- Yagi K. (1987). Lipid peroxides and human diseases. Chem Phys Lipids, 45, 337–351.