Abstract
Context: Polygonum multiflorum is known as a medicinal plant. It has been used as a folk medicine which showed antioxidative property.
Objective: Protective effects of the water extracts (w/v:1/10) from fresh P. multiflorum (WEP) on carbon tetrachloride (CCl4)-induced liver damage in rats were investigated.
Materials and methods: CCl4 was used for inducing liver damage of SD rats, and WEP and emodin were fed for eight consecutive weeks.
Results: We found that emodin levels in fresh WEP was higher than that in ripening WEP. Rats were administered WEP and emodin, the main active compound, for 56 consecutive days. WEP significantly lowered the serum levels of hepatic enzyme markers, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) and reduced the generation of malonaldehyde. Treatment with WEP recovered glutathione S-transferase and catalase activity in rats as compared to treatment with CCl4 alone. In addition, serum tumor necrosis factor-α, an inflammatory marker, was found to decrease in rats treated with WEP. In histopathological evaluation, fatty degeneration and necrosis were found to be significantly decreased in the CCl4 plus WEP treatment group.
Discussion and conclusion: WEP may be effective in attenuating liver damage by reducing lipid peroxidation as well as by positively modulating inflammation.
Introduction
There is numerous evidence that shows an excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) may lead to oxidative stress, loss of cell function as a result of an increased risk of various diseases and certain forms of cancer (CitationHalliwell & Gutteridge, 1998). Oxidative stress is a phenomenon of imbalance between the status of antioxidant defense system and production of oxygen derived species. Under physiological conditions, non-enzymatic antioxidants (e.g., vitamin E and glutathione) and antioxidant enzymes such as glutathione peroxidase (GPx), glutathione reductase (GR), glutathione S-transferase (GST) and catalase (CAT) clear these reactive oxygen species and thereby protect cells from oxidative damage (CitationAnderson, 1996). On the contrary, a deficient cellular antioxidant system makes genetic and other damage worse (CitationVan Remmeu et al., 2004). For example, the long-term exposure to ROS causes severe hepatic tumors (CitationToyokuni et al., 1996). For liver, it regulates many important metabolic functions and is very active in the metabolism of exogenous chemicals. That is why the liver is a target for toxic substances (CitationKim et al., 2007). Despite many therapeutic treatments for liver injuries have been developed, up to now, there are not many drugs available for therapy for liver diseases (CitationChaterrjee, 2000). Currently, there is a strong interest in the study of natural compounds with therapeutic effect on numerous diseases including liver disorder.
Polygonum multiflorum, named as he-so-wow in Taiwan, is known as a medicinal plant. It has been used as a folk medicine for the treatment of arthritis, rheumatitis, cough influenza and nephritis for many certuries (CitationKuo et al., 1996). In our preliminary test, P. multiflorum showed marked scavenging free radical capacity in vitro. Also, P. multiflorum showed antioxidative properties in vitro and in vivo (CitationIp et al., 1997; CitationLv et al., 2007). In addition, biological compounds have been identified (CitationKuo et al., 1996). The emodin level of WEP was evaluated in our previous study (CitationLin et al., 2010), and we found that the emodin level in fresh WEP was higher than ripening WEP. Now that fresh P. multiflorum has been proven to possess marked antioxidant activity (CitationLin et al., 2010), it is possible that this herb possesses hepatoprotective activity. However, whether it has any hepatoprotection in vivo was unclear. Thus, the aim of this study was to explore the protection of P. multiflorum against CCl4-induced liver injury of rats.
Materials and methods
Preparation of water extracts
P. multiflorum (root) was supplied from the abi-er farm (Tainan, Taiwan). The root of P. multiflorum was cut into 4–8 mm thickness and freeze-dried. Approximately 25 g of the sample was mixed with 250 mL of distilled water and extracted by ultrasonication (Ultrasonic Delta DC600H, Tainan, Taiwan) for 40 min. After extraction, the extracts were freeze-dried to powder. The water extracts of root of P. multiflorum were named WEP and stored at −20°C until used.
Cell treatment
Clone 9 cell is a normal liver cell of rat (BCRC 60201) cultured in Ham’s F-12K medium. Media was supplemented with 10% heat-inactivated fetal bovine serum (FBS), 0.1 mM non-essential amino acids, 2 mM l-glutamine, 1.0 mM sodium pyruvate and antibiotics (100 U/mL of penicillin and 100 μg/mL of streptomycin). Cells were cultured at 37°C in a humidified atmosphere of 5% CO2. Clone 9 cells were treated with WEP or emodin with CCl4 (0.4%; v/v) dissolved in 0.25% DMSO for 24 h. Cell viability was determined by MTT assay, and hepatic damage markers (AST and ALT) were determined by the assay kit (Randox Lab. Crumlin, UK). On the other hand, the antioxidase and GSH were further assayed.
Animal treatment
Male Sprague-Dawley rats (4-week-old) (BioLASCO, Taiwan Co., Ltd.) were used in this study. The animals were cared and used after the experimental protocols approved by institutional animal ethics committee, Chiayi University, Chiayi, Taiwan, R.O.C. The rats were randomly divided into five groups (six rats/group) and provided with food and water ad libitum. The animals were maintained in a controlled environment at 21 ± 2°C, 50 ± 5% relatively humidity and a cycle of 12 h dark/light, however, they were acclimatized for 1 week prior to use. To study the protective effect against CCl4-induced acute hepatic damage, the CCl4 was dissolved in olive oil [CCl4/olive oil = 1:4 (v/v)] thereby administering to the rats per-oral (0.5 mL/kg of bw). High dose (400 mg/kg of bw) and low dose (200 mg/kg of bw) of WEP were fed for eight consecutive weeks. The control group was fed a basal diet and olive oil, the positive control was fed silymarin (200 mg/kg of bw), and the emodin administration group was fed (3 mg/kg of bw). We have determined the emodin level in WEP (CitationLin et al., 2010). At the end of 8 weeks of treatment, rats were decapitated and then blood was collected.
To study the protective effect against CCl4-induced acute hepatic damage, high dose (400 mg/kg of bw) and low dose (200 mg/kg of bw) of WEP were fed for eight consecutive weeks. The control group was fed a basal diet, but the positive control was fed silymarin (200 mg/kg of bw) and CCl4 (20% CCl4/0.5 mL/kg of bw), the negative control, was fed CCl4 (20% CCl4, 0.5 mL/kg of bw). At the end of 8 weeks of treatment, rats were decapitated after CCl4 administering and then blood was collected. Blood was centrifuged at 1000 g for 10 min and serum obtained was frozen at −20°C until analysis. For each rat, the liver was removed, rinsed with PBS, frozen quickly with liquid nitrogen, and stored at −80°C until used.
Histopathologic study
Liver tissues, which were previously trimmed into 2 mm thickness, were fixed with buffered formaldehyde for 24 h. The fixed tissues were processed including embedded in paraffin, sectioned and rehydrated. The histological examination by above conventional methods evaluated the index of CCl4-induced necrosis by assessing the morphological changes in the liver sections stained with hematoxylin and eosin (H&E) (CitationGray, 1964).
Assay for serum biochemical values
The serum biochemical levels of AST, ALT, BUN and CRE of rat serum were assayed with biochemical parameters by the method of CitationReitman and Frankel (1957).
Measurement of lipid peroxidation products
Liver tissues were homogenized in cold Tris-HCl (pH 7.4) (1:10, w/v) of 20 mM. The homogenate was centrifuged for 30 min at 2500 g and under 40°C. The homogenate was stored at −80°C for the following experiments. MDA, which is one of the lipid peroxidation products, was determined by the method of CitationBuege and Aus (1978). Briefly, 1 mL of the homogenate was mixed with 1 mL of cold trichloroacetic acid (TCA) (75 mg/mL) to precipitate proteins and then centrifuged at 1500 rpm. The supernatant was reacted with 1 mL of TBA (8 mg/mL) TBA in boiling water for 45 min. Lipid peroxidation products were estimated by measuring the concentration of thiobarbituric acid reaction substances (TBARS) in fluorescence at 530 nm ex/552 nm.
Assay of glutathione (GSH)
The reduced GSH content of liver homogenate was determined as previously described (CitationVan Dam et al., 1999). Liver homogenate was mixed with TCA (50 mg/mL) mixture and incubated for 5 min, centrifuged at 8000 g for 10 min under 4°C. The homogenate was reacted with DTNB for 5 min under 4°C. The absorbance was measured at 412 nm, and the concentration of GSH was calculated using the absorbance expressed by μmole/mg protein.
Assay for antioxidant enzyme
GPx activity was determined as previously described (CitationMohandas et al., 1984). Briefly, 0.1 mL of homogenate was mixed with 0.8 mL of 100 mM potassium phosphate buffer (1 mM EDTA, 1 mM NaN3, 0.2 mM NADPH, 1 unit/mL GR, and 1 mM GSH, pH 7.0) and incubated for 5 min at room temperature. Thereafter, the reaction was initiated after adding of 0.1 mL of 2.5 mM hydrogen peroxide (H2O2). GPx activity was calculated by the change of the absorbance at 340 nm for 5 min. In another reaction containing 0.1 M phosphate buffer (1 mM MgCl2-6H2O, 50 mM GSSG, and 0.1 mM NADPH, pH 7.0), 0.1 mL of liver homogenate was added for GR activity determination (CitationBellomo et al., 1987). The decreased absorbance at 340 nm was measured for 3 min. The catalase (CAT) activity was determined by the method of CitationAebi (1984). The 50 µl of homogenate mixed with 950 µl 0.02 M H2O2 was incubated at room temperature for 2 min. The CAT activity was calculated by the change of the absorbance at 240 nm for 3 min. The glutathione S-transferase (GST) activity was determined as previously described (CitationHabig et al., 1974). 100 µl of homogenate was mixed with 20 µl of 50 mM CDNB (1-chloro-2,4-dinitrobenzene) and 880 µl of 100 mM phosphate buffer (pH 6.5) that contained 1 mM GSH. The GST activity was calculated by the change of the absorbance at 340 nm for 5 min.
Assay for inflammation factor
TNF-α level of serum was determined by immunoassay kit (Catalog Number MT100; Quantikine, USA).
Statistical analysis
Above data are expressed as means ± SD. The software of ANOVA was used to evaluate the difference between multiple groups. If significance was observed between each group, Duncan’s multiple range was used to compare the means of two specific groups, and P < 0.05 was considered to be significant.
Results
Effects of WEP on cell viability and antioxidation
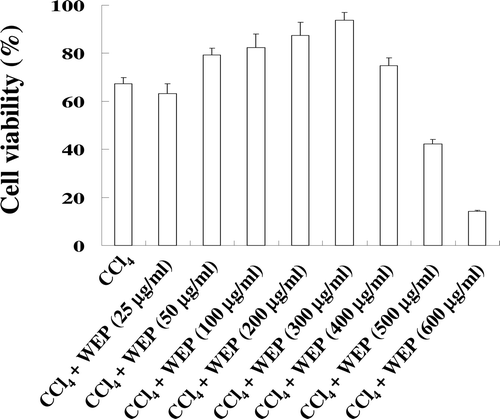
Cell viability of clone 9 cells treated with WEP for 24 h was decreased to 63% in the CCl4-treated group. However, cell viability was recovered on WEP treatment (50–300 μg/mL) that attenuated CCl4 cytotoxicity (). Furthermore, AST and ALT levels in the culture medium were significantly increased in Clone 9 cells treated with CCl4 as compared to the control group. WEP treatment effectively decreased the release of AST and ALT from Clone 9 cells (). Finally, the antioxidant enzyme activity of Clone 9 cells was investigated by determining the levels of GPx, GR, GST, and SOD. As shown in , CCl4 induced the loss of these antioxidant enzymes in a 24 h treatment. However, the activity of these enzymes was recovered by WEP (50–300 μg/mL) treatment. These results demonstrate that WEP exerts hepatoprotection against CCl4-induced liver damage. The levels of active compound in WEP were investigated in our previous study, and we found that emodin was the main antioxidant in WEP (CitationLin et al., 2010). We further investigated the hepatoprotective effects of WEP and emodin against liver damage in CCl4-inducing rats. In addition, the level (7.5 mg in 100 mg of WEP) of emodin had been measured, and therefore, 30 mg/kg bw of emodin was administered in the CCl4-treated rats in the present study.
Table 1. The inhibitory effects of WEP on GOT and GPT levels in clone 9 cells.
Table 2. Effects of WEP on antioxidase activity in Clone 9 cells.
Effects of WEP on organ weight
When toxins infiltrate organisms, they may generate a variation in physiological behaviors that can influence organ and tissue weight. Therefore, variations in organ or tissue weight can be used as an index for toxicity (CitationDe et al., 1982). Treatment with CCl4 significantly elevated liver weight compared to the control group. WEP at 400 mg/kg per bw showed significant decrease relative to the liver weight, as compared to the rats treated with CCl4 alone. No other significant differences in body weights and relative heart and kidney weights of CCl4-induced rats with or without WEP treatment compared with the control were found (data not shown).
Effects of emodin and WEP on glutathione and lipid peroxidation products
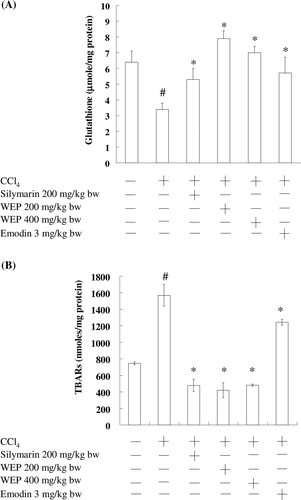
shows the effects of emodin and WEP on GSH contents in CCl4-induced liver damage in rats. The CCl4-treated group showed significantly reduced GSH levels. Pretreatment with emodin and WEP significantly protected GSH depletion and almost recovered GSH levels, indicating that WEP protects GSH depletion induced by CCl4. To further evaluate the possible mechanism of the hepatoprotective effect of WEP, MDA generation in CCl4-induced liver damage in rats was investigated. As shown in , MDA generation rapidly increased on CCl4 administration; however, emodin and WEP (200 and 400 mg/kg of bw, respectively) significantly decreased the concentration of MDA, indicating that WEP may prevent oxidative damage in livers induced by CCl4. This result suggests that WEP displays antioxidant defense and protects liver cells against oxidative damage.
Effects of emodin and WEP on CCl4-induced hepatic damage
AST, ALT, BUN, and CRE are indexes of liver and kidney function in clinical assessments. As shown in , no significant differences were found in the levels of BUN and CRE of CCl4-induced rats with or without 8 consecutive weeks of WEP or silymarin treatment when compared with the control. However, CCl4 exerted toxication in normal rats and thereby elevated the levels of AST and ALT as compared to the control. Administration of emodin and WEP significantly decreased the elevated levels of AST and ALT.
Table 3. Effects of emodin and WEP on serum BUN, CRE, GOT, and GPT in CCl4-induced rats.
Effects of emodin and WEP on antioxidant enzyme activity
shows the protective effects of WEP on the activities of hepatic antioxidant enzymes in rats with CCl4-induced liver damage. Treatment with CCl4 reduced the activities of hepatic antioxidant enzymes (GPx, GR, GST, and SOD) as compared with the control. WEP (200 mg/kg bw) did not elevate GR and SOD, as compared to the rats treated with CCl4. However, pretreatment with emodin and WEP (400 mg/kg bw) resulted in a significant protection against the CCl4-induced decrease in antioxidant enzyme activity.
Table 4. Effects of emodin and WEP on hepatic antioxidase activity in CCl4-induced rats.
Pathological histology
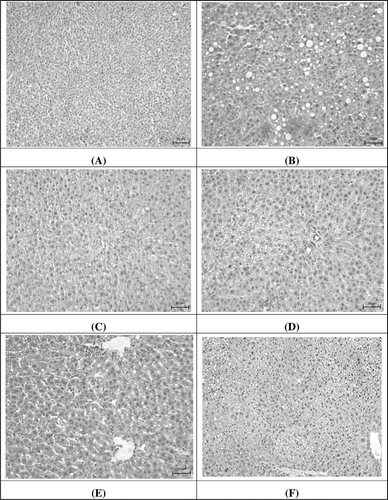
CCl4-induced liver injury caused permeability of the cell membrane, a concave liver surface, and lymphocyte infiltration in the central vein, leading to cell necrosis or acute inflammation (CitationHalliwell & Gutteridge, 1998). shows that the hepatic cells were found to have fatty degeneration, necrosis, and cytoplasmic vacuolization in the CCl4-treatment group. Emodin and WEP may beneficially decrease these effects caused by CCl4. Histological examination showed the preventive effects of WEP on CCl4-induced hepatotoxicity. The grade of histopathological scores is shown in . These results indicate that administration of emodin and WEP effectively improve vacuole formation and inflammation in rats with CCl4-induced liver damage.
Table 5. Effects of emodin and WEP on hepatic histopathology scores of liver damage in rat treated with CCl4.
Figure 3. Effect of emodin and WEP on CCl4-induced liver damage in rats: (A) control group; (B) treated with CCl4 (20% CCl4, 0.5mL/kg bw); (C) treated with CCl4 (20% CCl4, 0.5mL/kg bw) and silymarin (200 mg/kg bw); (D) treated with CCl4 (20% CCl4, 0.5mL/kg bw) and WEP (200 mg/kg bw); (E) treated with CCl4 (20% CCl4, 0.5mL/kg bw) and WEP (400 mg/kg bw); (F) treated with CCl4 (20% CCl4, 0.5mL/kg bw) and emodin (3 mg/kg bw). Scale bars: 20 µm.
Effect of WEP on inflammation
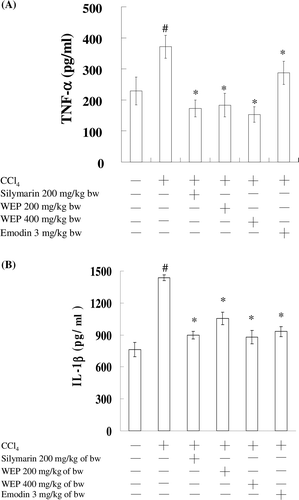
In , the effects of WEP on the release of TNF-α and IL-1β in CCl4-treated rats are shown. Serum TNF-α level was drastically increased in rats treated with CCl4 (378 pg/mL) as compared to the control (230 pg/mL). WEP and silymarin resulted in significant decreases in serum TNF-α level. No significant difference was found between WEP (200 and 400 mg/kg of bw) and silymarin in repressing serum TNF-α levels. In addition, the TNF-α inhibitory activity of WEP was more than that of emodin. However, we found that the IL-1β inhibitory activity of emodin was similar to WEP in the CCl4-treated rats. This observation implies that treatment with emodin and WEP produced a marked decrease in the TNF-α level due to an overwhelmed defense system in WEP.
Discussion
CCl4 exposure can damage the liver due to cytochrome p450-dependent reduction of CCl4 to the reactive trichloromethyl radical (CitationZangar et al., 2000), which in turn leads to CCl4 hepatotoxicity by initiating lipid peroxidation in the cell membranes (CitationBahcecioglu et al., 1999). Many published studies demonstrate that oxidative damage can be limited by naturally occurring substances in plants. The present work shows that CCl4 increases the liver lipid peroxide level as a result of elevation of MDA concentration, which induces protein cross-linking (CitationBahcecioglu et al., 1999). Hepatic lipid peroxide levels dropped in emodin and WEP-treated rats, suggesting that emodin may effectively scavenge the intermediates derived from CCl4 metabolism. Bioactive compounds such as anthraquinones, emodin, emodin 1-O-β-d-glucoside, physcion, and physcion 1-O-β-d-glucoside were found in WEP (CitationKuo et al., 1996). Several compounds such as emodin and anthraquinones have been demonstrated to display biological effects (CitationYen et al., 2007). Therefore, these compounds and other biological constituents may participate in the series of scavenging reactions and consequently suppress liver lipid peroxidation. In other words, bioactive compounds (emodin) present in WEP could be responsible for the plant’s biological effects. In addition, the increases in serum AST and ALT activities were significantly inhibited by emodin- and WEP-treated rats, an effect probably related to the low levels of liver lipid peroxidation in emodin- and WEP-treated rats (CitationHasegawa et al., 1995).
GSH plays a central role in protecting against toxicity and can participate in the elimination of reactive intermediates by conjugation and hydroperoxide reduction as well as by scavenging free radicals (CitationWang et al., 2000). Oxidative stress in tissues generally involves the GSH system. Many reports indicate that tissue injury induced by various stimuli is coupled with GSH depletion. In other words, emodin and WEP play a role in maintaining adequate levels of GSH. This effect may be responsible for the elimination of reactive intermediates produced by CCl4, leading to protection against liver damage. In the present study, no significant differences were found between the control group and CCl4-treated rats. This observation requires further investigation.
Several antioxidant enzymes such as GPx, GR, and SOD exist in cells and convert ROS to less noxious compounds. These antioxidant enzymes provide cellular protection against oxidative damage in cells. GST is a soluble protein, which plays an important role in detoxification and excretion of xenobiotics. GST catalyzes the conjugation of the thiol functional groups of GSH to electrophilic xenobiotics and leads to increased solubility of hydrophobic substances, thereby reducing liver damage (Ahmad, 1995; CitationKim et al., 2000; CitationYu et al., 2008). According to the data in , WEP and emodin, by elevating GST and CAT activity, may positively regulate metabolism of toxic substances to nontoxic ones as well as reduce ROS levels.
Several lines of evidence indicate that the activation of pro-inflammatory cascades involving the release of cytokines such as TNF-α and IL-1β plays an essential role in the pathogenesis of many types of nerve-injury related pain (CitationCata et al., 2008). TNF-α is a key pro-inflammatory cytokine, which has been shown to contribute to cancer development through a series of potential mechanisms (Umannova et al., 2008). Thus, much attention has recently been focused on the roles of naturally occurring substances in modulating cell signaling and inflammation. In this study, WEP and emodin significantly decreased the gross lesion scores and TNF-α production. We hypothesize that the effect of WEP on TNF-α and IL-1β may be mediated either by suppressing the activation of this pro-inflammatory mediator and its transcriptional regulator (Frongoza et al., 2004; CitationEl-Abhar et al., 2008), and/or by inhibiting its production from macrophages (CitationTripathi et al., 2007). To the best of our knowledge, this is the first study to report that emodin and WEP may influence the generation of TNF-α and IL-1β WEP and emodin significantly reduced the levels of MDA, AST, ALT, TNF-α, and IL-1β in rats treated with CCl4, suggesting that WEP and emodin may prevent liver damage. These findings were confirmed by histological observations of the liver, wherein emodin was found to protect from fatty degeneration, necrosis, and other pathological changes and maintain the hepatic architecture.
Conclusions
In conclusion, our results suggest that WEP and emodin have the ability to prevent oxidative liver damage induced by CCl4. The mechanism of the protective action of WEP may be related to the modulation of antioxidant enzymes as well as the suppression of lipid peroxidation. In addition, the positive modulation of inflammatory processes by emodin and WEP may be valuable for the protection of liver injury induced by CCl4. Thus, WEP and emodin could be beneficial as complementary agents in therapies for liver diseases.
Declaration of interest
This research work was supported by the Council of Agriculture, Republic of China, under Grant 96AS-3.1.3-FD-Z1(15).
References
- Aebi H. (1984). Catalase in vitro. Meth Enzymol, 105, 121–126.
- Ahamd S. (1995). Antioxidant mechanisms of enzymes and proteins. In Oxidative Stress and Antioxidant Defenses in Biology. New York, Thomaon: Publisher and Distributor.
- Anderson D. (1996). Antioxidant defences against reactive oxygen species causing genetic and other damage. Mutat Res, 350, 103–108.
- Bahcecioglu IH, Ustundag B, Ozescan I, Ercel E, Baydas G, Akdere T. (1999). Protective effect of Ginkgo biloba extract on CCl4-induced liver damage. Hepatol Res, 15, 215–224.
- Bellomo G, Mirabelli F, DiMonte D, Richelmi P, Thor H, Orrenius C, Orrenius S. (1987). Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. A study with isolated hepatocytes and menadione (2-methyl-1,4-naphthoquinone). Biochem Pharmacol, 36, 1313–1320.
- Buege AJ, Aus SD. (1978). Microsomal lipid peroxidation. Meth Enzymol, 52, 302–310.
- Cata JP, Weng HR, Dougherty PM. (2008). The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res, 1229, 100–110.
- Chaterrjee TK. (2000). Medicinal plants with hepatoprotective properties. Herbal options books and allied (p) Ltd., Calcutta.
- De LIF, Sturgess JM, Feuer G. (1982). New approaches for assessment of hepatotoxicity by means of quantitative functional-morphological interrelationship In: Toxicity of the Liver. Raven Press 47–102.
- El-Abhar HS, Hammead LNA, Gawad HAS. (2008). Modulating effect of ginger extract on rats with ulcerative colitis. J Ethnopharmacol, 640, 162–169.
- Frondoza CG, Sohrabi A, Polotsky A, Phan PV, Hungerford DS, Lindmark L. (2004). An in vitro screening assay for inhibitors of pro-inflammatory mediators in herbal extracts using human synoviocyte cultures. In Vitro Cell Dev Biol Anim, 40, 95–101.
- Gray P. (1964). Handbook of Basic Microtechnique, third ed. McGraw-Hill, New York, 85–145.
- Habig WH, Pabst MJ, Jakoby WB. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem, 249, 7130–7139.
- Halliwell B, Gutteridge JMC. (1998). Free Radicals in Biology and Medicine. Oxford: University Press: New York, 27–30.
- Hasegawa R, Chujo T, Sai-Kato K, Umemura T, Tanimura A, Kurokawa Y. (1995). Preventive effects of green tea against liver oxidative DNA damage and hepatotoxicity in rats treated with 2-nitropropane. Food Chem Toxicol, 33, 961–970.
- Ip SP, Tse ASM, Poon MKT, Kow KM. (1997). Antioxidant activities of Polygonum multiflorum Thunb in vivo and in vitro. Phytother Res, 11, 42–44.
- Kim MK, Lee HS, Kim EJ, Won NH, Chi YM, Kim BC, Lee KW. (2007). Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem Toxicol, 45, 1738–1744.
- Kim HS, Lim HK, Chung MW, Kim YC. (2000). Antihepatotoxic activity of bergenin, the major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated hepatocytes. J Ethnopharmacol, 69, 79–83.
- Kuo TC, Ou JC, Tsai J, Wu CL, Sun CM. (1996). Evaluation of Chinese herbs that affect the cell-mediated immunity (II). J Chin Med, 7, 119–131.
- Lin HT, Nan SL, Huang YY, Wu SC. (2010). Potential antioxidant components and characteristics of fresh Polygonum multiflorum. J Food Drug Anal, 18, 120–127.
- Lv SL, Xiao HG, Jian T, Ho CT. (2007). Antioxidant activity of stilbene glycoside from Polygonum multiflorum Thunb in vivo. Food Chem, 104, 1678–1681.
- Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. (1984). Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res, 44, 5086–5091.
- Reitman S, Frankel S. (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol, 28, 56–63.
- Toyokuni S. (1996). Iron-induced carcinogenesis: The role of redox regulation. Free Radic Biol Med, 20, 553–566.
- Tripathi S, Maier KG, Bruch D, Kittur DS. (2007). Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J Surg Res, 138, 209–213.
- Umannová L, Machala M, Topinka J, Nováková Z, Milcová A, Kozubík A, Vondrácek J. (2008). Tumor necrosis factor-alpha potentiates genotoxic effects of benzo[a]pyrene in rat liver epithelial cells through upregulation of cytochrome P450 1B1 expression. Mutat Res, 640, 162–169.
- Van Dam PS, Van Asbeck BS, Bravenboer B, Van Oirschot JF, Marx JJ, Gispen WH. (1999). Nerve conduction and antioxidant levels in experimentally diabetic rats: effects of streptozotocin dose and diabetes duration. Metab Clin Exp, 48, 442–447.
- Van Remmen H, Qi W, Sabia M, Freeman G, Estlack L, Yang H, Mao Guo Z, Huang TT, Strong R, Lee S, Epstein CJ, Richardson A. (2004). Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med, 36, 1625–1634.
- Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. (2000). Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol, 38, 411–416.
- Yen GC, Duh PD, Chuang PD. (2007). Antioxidant activity of anthraquinones and anthrone. Food Chem, 70, 437–441.
- Yu HM, Wang BS, Chu HL, Chang LW, Yen WJ, Lin CJ. (2008). Napiergrass (Pennisetum purpureum S.) protects oxidative damage of biomolecules and modulates antioxidant enzyme activity. Food Chem, 105, 1364–1374.
- Zangar RC, Benson JM, Burnett VL, Springer DL. (2000). Cytochrome P450 2E1 is the primary enzyme responsible for low-dose carbon tetrachloride metabolism in human liver microsomes. Chem Biol Interact, 125, 233–243.