Abstract
Context: Sapindus saponaria L. (Sapindaceae) bark, root, and fruits are used as sedatives and to treat gastric ulcer and also demonstrate diuretic and expectorant effects.
Objective: The anti-snake venom properties of callus of S. saponaria are investigated here for the first time.
Materials and methods: In vitro cultivated callus of Sapindus saponaria were lyophilized, and the extracts were prepared with different solvents, before submitting to phytochemical studies and evaluation of the anti-ophidian activity. Crude extracts were fractionated by liquid–liquid partition and the fractions were monitored by thin layer chromatography (TLC). Subsequently, anti-ophidian activities were analyzed toward Bothrops jararacussu Lacerda (Viperidae), B. moojeni Hoge (Viperidae), B. alternates Duméril (Viperidea) and Crotalus durissus terrificus Lineu (Viperidae) venoms and isolated myotoxins and phospholipase A2 (PLA2).
Results: Fractions A1, A2 and the extract in MeOH:H2O (9:1) significantly inhibited the toxic and pharmacological activities induced by snake venoms and toxins, when compared to other extracts and fractions. The lethal, clotting, phospholipase, edema-inducing, hemorrhagic and myotoxic activities were partially inhibited by the different extracts and fractions. TLC profiles of the crude extracts (B and C) and fractions (A1 and A2) showed β-sitosterol and stigmasterol as their main compounds. Stigmasterol exhibited inhibitory effects on enzymatic and myotoxic activities of PLA2.
Discussion and conclusion: Sapindus saponaria extracts and fractions presented anti-ophidian activity and could be used as an adjuvant to serum therapy or for its supplementation, and in addition, as a rich source of potential inhibitors of enzymes involved in several pathophysiological human and animal diseases.
Introduction
Animal venoms, including snake venoms, embody a complex mixture of toxic enzymes. In Brazil, Bothrops and Crotalus snakes are responsible for most envenomations, which mainly induce local tissue damage and systemic effects, including alterations in blood clotting, neurotoxicity, and shock (CitationOwnby, 1990; CitationBraud et al., 2000). The victim’s metabolic response to the venom’s components varies with the species of snake, the volume of venom injected, and the species of the bite recipient. The primary purpose of the venom is not to kill, but rather to immobilize the prey and predigest its tissues. The venom is derived from modified salivary glands. Pit viper venoms are a complex combination of enzymatic and non-enzymatic protein; phosphodiesterase, proteolytic enzymes, thrombin–like enzyme, collagenase, hyaluronidase, phospholipase A2 (PLA2), and l-amino acid oxidase (CitationDa Silva, et al., 2008a; CitationCalgarotto et al., 2008). The toxins of the Bothrops snake venom affects mainly the muscle tissues and blood clotting, and symptoms include; pain, swelling and bleeding (site of the bite, and mucous membranes), and in cases where complications can occur: blisters, gangrene, shock and acute renal failure. In Crotalus envenomation, the poison affects the nervous system (neurotoxic), coagulation and muscles, and in severe cases acute renal failure and death can occur (CitationPeterson, 2006).
Vegetal extracts constitute an alternative for treatment, displaying a large diversity of chemical compounds with several pharmacological activities of medical-scientific interest. A large number of extracts have been prepared and showed excellent anti-venom activities (CitationMelo et al., 1994; CitationIzidoro et al., 2003; CitationSoares et al., 2004; CitationDa Silva et al., 2005, Citation2008a, Citation2009; CitationOliveira et al., 2005; CitationTicli et al., 2005; CitationMarcussi et al., 2007; CitationLomonte et al., 2009). Sapindus saponaria L. (Sapindaceae) bark, root and fruits are used as sedatives, against gastric ulcer and demonstrate diuretic and expectorant effects (CitationMeyer et al., 2002). Other studies show the presence of lipids and flavonoids in this plant, characterizing it as a chemical complex with several scientifically important properties (CitationCastro et al., 1999). Saponins, present in the fruits, are related to several activities such as molluscicide, piscicide, anti-inflammatory, analgesic, expectorant, antioxidant, spermicide, cholesterol-lowering, antibiotic, and antifungal (CitationMurgu, 2002; CitationTsuzuki et al., 2007). CitationCastro et al. (1999) show that extracts of S. saponaria inhibit the hemorrhagic activity induced by B. asper Garman (Viperidae) venom and some compounds such as catechins, flavones, anthocyanins and condensed tannins have been identified by chemical analysis, suggesting their involvement in the inhibitory effect observed, probably acting by the chelation of the zinc ion, which is required for the activity of metalloproteases.
Hundreds of plants are reported to have anti-ophidian activities, although only a few of these have been scientifically studied, with the isolation of components and investigations regarding structure-function relationships (CitationSoares et al., 2004, Citation2005). The wedelolactone isolated from Eclipta prostate Hasskarl (Asteraceae) and the alkaloid, 12-methoxy-4-methylvoachalotine, isolated from Tabernaemontana catharinensis Müll.Arg. (Apocynaceae) (CitationMelo et al., 1994; CitationMelo & Ownby, 1999; CitationBatina et al., 1999), are examples of isolated and characterized anti-ophidian substances. CitationJanuário et al. (2004) isolated a neoclerodane diterpenoid from Baccharis trimera Less (Asteraceae), an inhibitor of snake venom metalloproteases, with anti-proteolytic and anti-hemorrhagic properties.
This study shows the activity of extracts from callus of S. saponaria, and its major active compound (stigmasterol), in the inhibition of pharmacological and toxic effects of snake venoms and isolated proteins from the Crotalus and Bothrops genera.
Materials and methods
Animals, snake venoms and toxins
Male Swiss mice (18–22 g) were used for biological assays. Crude lyophilized B. jararacussu (Bjussu), B. moojeni (Bmooj), B. alternatus (Balter) and C. d. terrificus (Cdt) venoms were obtained from Bioagents serpentarium, Batatais-SP and the Instituto Butantan, São Paulo-SP, Brazil. The isolated toxins, crotoxin and basic PLA2 from Crotalus durissus terrificus (PLA2Cdt or CB) and myotoxins from B. jararacussu (BthTX-I and II) were obtained as previously described by (CitationAndrião-Escarso et al., 2000; CitationSoares et al., 2001). Stigmasterol (B9757) was purchased from Sigma-Aldrich. The experiments described were approved by the Institutional Committee for Ethics in Animal Experimentation of the Universidade de São Paulo (no. 08.1.202.53.1) and were done in accordance with the guidelines of the Brazilian College for Animal Experimentation.
Plant in natura and in vitro
Sapindus saponaria plants were collected in Ribeirão Preto and cultivated in the “Coleção de Plantas Medicinais da Universidade de Ribeirão Preto (UNAERP), Brazil”. Voucher specimen no. HPM 603 is deposited in the “Herbário de Plantas Medicinais (HPM), Unidade de Biotecnologia, UNAERP”. The species was authenticated by Professor Hermógenes de Freitas Leitão Filho from the Instituto de Biologia, UNICAMP, Brazil.
The explants were inoculated in Murashige and Skoog (MS) culture medium (CitationMurashige & Skoog, 1962), supplemented with different combinations of 1-naphthaleneacetic acid (ANA: 0.5, 1.0 and 2.0 mg·L−1) and 6-benzylaminopurine (BAP: 0.5, 1.0 and 2.0 mg·L−1), pH 6.0, and solidified with Phytagel® (2.5 g·L−1) for callus induction. The cultures grew at 25 ± 2°C with a 16 h photoperiod under a white fluorescent lamp, and were then lyophilized.
Phytochemical analysis
For phytochemical studies, several extracts from S. saponaria callus culture were prepared using different solvents. Extract B was prepared by maceration in MeOH:H2O (9:1, v/v; 1 L) for 24 h. Extract C was prepared by maceration in CH2Cl2:MeOH (85:15, v/v; 0.8 L) for 48 h. After the extractions, the solvents were removed under vacuum using an evaporator and extracts were lyophilized.
Lyophilized callus were submitted to extractions with CH2Cl2:MeOH (85:15, v/v; 100 mL) in ultrasound for 30 min. The extract was transferred to a separation funnel, adding 60 mL of water. The organic phase was evaporated (A1) and the aqueous phase had its pH changed to 8.0 (using NH4OH), before being extracted with 30 mL of CH2Cl2 (3 times). The resulting basic organic phases were collected and treated with sodium sulfate (B1), and the neutral aqueous phase (N1) was lyophilized. Extract C was also resuspended in CH2Cl2:MeOH 85:15 (v/v) and subjected to a liquid–liquid partition, giving rise to three fractions denominated as A2 (acid), B2 (basic) and N2 (neutral).
A thin layer chromatography (TLC) procedure was performed with all extracts and fractions at 20 mg/mL. The analysis was carried out using 60G silica gel plates (10 × 10 cm; 0.25 mm; Aldrich®), eluted with Hex:EtOAc (7:3, v/v), CHCl3:MeOH (8:2, v/v) and 1-butanol: acetic acid: water (BAW, 4:1:5, v/v, upper phase) and stained with vanillin-sulfuric acid and ninhydrin reagents.
Neutralization assays of the snake venoms or isolated toxins
Hemorrhagic, myotoxic and phospholipase A2 activities were assayed as previously described (CitationBiondo et al., 2003; CitationDa Silva et al., 2008b, CitationRomero et al., 2010; Citationde Alvarenga et al., 2011).
PLA2 belong to a superfamily of enzymes that cleave phospholipids from cell membrane into fatty acids and lysophospholipids. This reaction is calcium dependent. In this paper we use an experiment that uses phosphatidylcholine from egg yolk in agar plate. It is based on enzymatic breach of these phospholipids with release of soluble fatty acids that form a translucent halo in the agar plate around the point of application of a sample of the enzyme. So, for the venom inhibition assays, phosphate-buffered saline (PBS) solutions containing a fixed amount of venom or isolated enzyme (10–50 μg) were mixed with different amounts of extracts in order to achieve several ratios (w/w) of venom or toxin to extract (or stigmasterol). All mixtures were incubated for 30 min at 37°C and aliquots were assayed in the different test systems. The clotting activity was assayed with 200 μL of citrated human plasma directly incubated with Cdt or Bjussu snake venoms alone (50 μg) or previously incubated with extracts for 30 min, 37°C, at a ratio of 1:5 (w/w) venom:extract.
Both the venom, such as PLA2, causes lysis in muscle cells. The myotoxic activity assay is based on detection of the enzyme creatine kinase (CK) released into the blood of the animal when muscle cells are lysed. The enzyme CK has been widely used as an indirect marker of muscle damage. In this work, plasma CK activity was determined using the CK-UV Kit (Bioclin, Brazil).
The presence of hemorrhage, local and systemic, is common in snake bites. This effect is due principally by the presence in the venom of zinc-dependent metalloproteinases. These enzymes act by damaging the endothelium of capillaries and plasma extravasation. After application of the toxins, animals were euthanized and skinned. The area of hemorrhage observed in the inner skin was measured with precision paquimeter.
Lethality was evaluated for 48 h after the intraperitoneal injection of solutions containing C. d. terrificus (20 μg) or crotoxin (10 μg) with extracts (1:10 and 1:50, w/w) in Swiss mice (18–22 g). Results are presented as mean values ± S.D. obtained with the indicated number of tested animals. The statistical significance of differences between groups was evaluated using Student’s unpaired t-test. A p-value < 0.05 was considered to indicate significance. Edema was evaluated after the subplantar injection of venoms or toxins isolated or preincubated with extracts or fractions obtained from S. saponaria in the right paw of male Swiss mice (18–22 g) using a pachymeter. Inhibition studies were performed after the preincubation of venom/toxins with extracts (1:50 and 1:10, w/w, respectively).
Results and discussion
Callus cultures from leaves of S. saponaria were successfully established, resulting in material with uniform characteristics. The best induction of callus was obtained in the culture medium containing 0.5 mg·L−1 ANA plus 0.5 mg·L−1 BAP, resulting in more intensive growth and friability. The biomass accumulation occurred during the period from day 27 to 30, and the 30th day was selected for the collection of material.
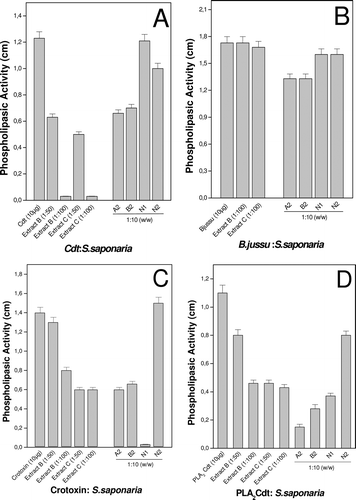
(A, C and D) shows the significant inhibition of the PLA2 activity of C. d. terrificus venom, PLA2Cdt and crotoxin by S. saponaria extracts and obtained fractions. The mechanisms of action of the anti-ophidian substances that have been previously studied are still unknown, but interactions with catalytic sites of specific venom proteins are suggested to be involved. In the experiments of inhibition of PLA2 activity, a greater interaction among the extracts and their fractions with C. d. terrificus venom was observed, with a major inhibition of this activity when compared to the inhibition obtained for B. jararacussu venom (). The differences observed between the inhibition of C. d. terrificus and B. jararacussu venoms can be explained in terms of compositions, biochemical and pharmacological properties of each of the venoms. Variations between the venoms of different genera should be considered. Among other factors that may affect the composition of venoms are geographical location, the animals’ health status, age, type of prey, etc. (CitationFry et al., 2001).
Figure 1. Inhibition of the PLA2 activity of snake venoms (A and B) and isolated toxins (C and D) induced by the extracts and fractions of S. saponaria, preincubated at different ratios (w/w, venoms/proteins: Extracts/fractions) for 30 min at 37°C. Each experiment represents the mean ± S.D. (n = 6).
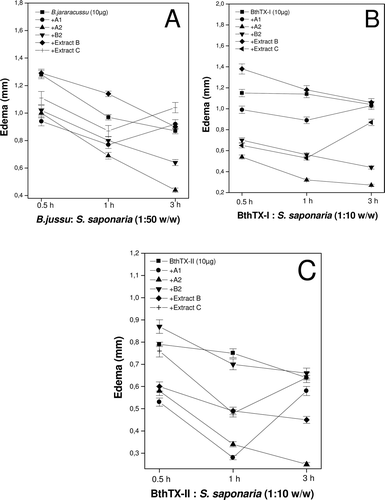
The tested extracts and fractions, in the proportions used, showed no significant inhibition of the edema induced by C. d. terrificus venom (results not shown), but some fractions (A1, A2, B2, extract B and extract C) significantly inhibited the activity induced by B. jararacussu and its isolated myotoxins (BthTX-I and II) (). Inflammatory mediators such as histamine, bradykinin, prostaglandins, leukotrienes, thromboxanes and complement factors may be involved in the formation of edema (CitationZamuner & Teixeira, 2002).
Figure 2. Inhibition of the edema-inducing activity of crude snake venom (A) and isolated toxins (B and C) after incubation for 30 min at 37°C with extracts (B and C) or fractions of S. saponaria (A1, A2, B2) at ratios of 1:50 and 1:10 (w/w) to venom and toxins, respectively. Each denoted value represents the mean ± S.D. (n = 6).
In Bothrops snake envenomations, the presence of both local and systemic hemorrhage is common, mainly due to the presence of zinc-dependent metalloproteases, which act damaging the endothelium of blood vessels (CitationGutiérrez & Rucavado, 2000), causing serious injuries to the skeletal muscle, such as myonecrosis followed by tissue ischemia. The inhibition of the hemorrhagic effect of Bothrops venoms by extracts and fractions of S. saponaria is shown in .
Figure 3. Inhibition of the hemorrhagic activity of crude snake venoms by the extracts and fractions of S. saponaria preincubated at different ratios for 30 min at 37°C. Each denoted value represents the mean ± S.D. (n = 6).
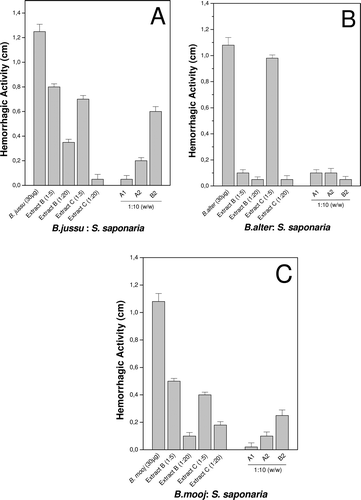
Results suggest the presence of compounds, both in fractions and extracts, able to inhibit the activity of hemorrhagic proteins present in the venoms. These proteins are mostly Zn2+-dependent, and the active compounds of this plant may be interfering in the binding of the proteins to the cofactor, or may be binding to other active sites essential for this activity.
Studies with tropical plant extracts showed that, of the 52 hydroalcoholic extracts tested, ten completely inhibited the hemorrhagic activity of B. asper venom, and eight partially inhibited this activity (CitationCastro et al., 1999). C. sylvestris SW showed an inhibitory effect on B. moojeni, B. neuwiedi Wagler, and B. asper venoms (CitationBorges et al., 2001; Da Silva, 2008). Pithayanukul et al. (2003) showed the inhibitory effects of extracts of Eclipta prostata on the hemorrhagic activity induced by the venom of Calloselasma rhodostoma Kuhl. CitationDiogo et al. (2009) attributed the inhibitory effect of this plant to three of its constituents; wedelolactone, stigmasterol and sitosterol. Some other studies have shown isolated compounds with inhibitory activity, such as ar-turmerone, isolated from the roots of Curcuma longa Linnaeus, which completely inhibited the hemorrhagic activity of B. jararaca snake venom (CitationFerreira et al., 1992).
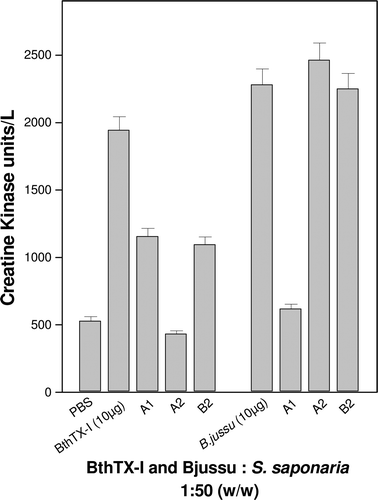
The presence of myotoxins in Bothrops snake venoms is quite common. Various types of anti-venoms studied were not effective for antagonizing the effects of these toxins. CitationBiondo et al. (2003) demonstrated that the aqueous extract of Mandevilla velutina Mart. inhibited 66.5% of the activity of B. jararacussu venom, 75% of BthTX-I and 100% of C. d. terrificus venom and basic phospholipase from crotoxin. CitationOliveira et al. (2003) showed the ability of suramin, a polyanionic compound, to inhibit the myotoxic activity of BthTX-I. In the present study, the myotoxicity induced by BthTX-I was significantly inhibited by fractions A1 (79.09%), B2 (79.44%) and A2 (100%), and for B. jararacussu crude venom, fraction A1 showed an inhibition of 93.18% of this activity ().
Figure 4. Inhibition of the myotoxic activity of B. jararacussu venom and BthTX-I toxin after incubation for 30 min at 37°C with fractions of S. saponaria at a ratio of 1:50 (w/w, venom or toxin/fractions). Each denoted value represents the mean ± S.D. (n = 6).
Extracts B and C and fractions A1 and A2 significantly inhibited the coagulation induced by B. jararacussu venom, while that same activity induced by C. d. terrificus venom was significantly inhibited only by fractions A2 and B2 (). The venom:extract/fraction ratio tested in the clotting activity was 1:5 (w/w), which is considered to be low when compared with ratios used by other authors. A study by CitationMaiorano et al. (2005) showed that the aqueous extract of roots and stems of Mikania glomerata Spreng. inhibited the clotting activity of Bothrops and C. d. terrificus venoms. Results show 100% inhibition, preventing the formation of blood clots, in the proportion of 1:100 (w/w). This inhibition possibly occurred as a result of the interaction of bioactive substances of M. glomerata with serine proteases, responsible for the venom coagulating action. These interactions probably induce irreversible changes in the catalytic site of these toxins, which require conservation of the chemical interactions between amino acids to exert their toxic effects (CitationBorges et al., 2001; CitationGuiguer et al., 2004).
Table 1. Neutralization of the clotting activity induced by B. jararacussu and C. d. terrificus crude venoms preincubated with extracts and fractions of S. saponaria.
Vegetal extracts and natural compounds are proven to be effective at inhibiting the lethal activity of venoms and toxins of several snakes (CitationNúñez et al., 2004). CitationOtero et al. (1999) evaluated the neutralization of the lethal effects of B. asper venom using 74 ethanol extracts of anti-ophidian plants, showing that 7 species completely inhibited this activity. Similarly, CitationMahanta and Mukherjee (2001) showed that aqueous extracts of the dried roots of Mimosa pudica L. inhibited the lethal effect of Naja kaouthia Lesson venom. CitationSoares et al. (2004) demonstrated that compounds such as bredemeyeroside D, a triterpenoid saponin, and ar-turmerone, a phenolic compound, are potent inhibitors of the lethality induced by B. jararaca and C. d. terrificus venoms. Additionally, CitationAdzu et al. (2005) showed that the methanol extract of Annona senegalensis Pers. roots reduced the lethality caused by Naja nigricollis nigricollis Reinhardt venom. In our study, extracts B and C and fractions A1, A2 and B2 of S. saponaria were able to extend the life of animals envenomed by C. d. terrificus venom and crotoxin, with the minor inhibition observed in the presence of extract C ().
Table 2. Survival time of mice injected with C. d. terrificus venom and crotoxin preincubated with extracts and fractions of S. saponaria.
It was concluded that extract B of S. saponaria presented greater inhibitory effects on the activities of snake venoms, while extract C and fractions A2 and B2 showed greater inhibitory effects on the PLA2 activity induced by C. d. terrificus venom and the isolated proteins, crotoxin and PLA2Cdt. The myotoxic activity induced by B. jararacussu venom and BthTX-I was mostly inhibited by fraction A1. Extract C and fraction A1 presented the greatest inhibitory effects on the hemorrhage induced by B. jararacussu venom, while for B. moojeni and B. alternatus venoms, the best results of inhibition were observed with extracts C and B, respectively, and fractions A1, A2 and B2.
With regard to edema, B. jararacussu venom was partially inhibited by fractions A1, A2 and B2. The BthTX-I protein was mostly inhibited by fractions A2 and B2. Fraction A2 presented the greatest inhibition of the coagulation induced by B. jararacussu and C. d. terrificus venoms. Extract B and fractions A1, A2 and B2, incubated with C. d. terrificus venom and crotoxin alone, significantly extended the life of animals.
TLC profiles of the crude extracts (B and C) and fractions (A1 and A2) showed β-sitosterol and stigmasterol as their main compounds (). β-Sitosterol glucoside was identified in all extracts and fractions A1, B1, A2 and B2 (). Using ninhydrin reagent, it was possible to detect nitrogenated compounds in crude extracts and fractions N1 and N2 (). CitationMurgu (2002) detected β-sitosterol, stigmasterol, 3-methoxycinnamyl alcohol and hexadecanoic acid by GC/MS in the callus culture from S. saponaria, where β-sitosterol was found to be the main compound. From extract B, more polar substances were extracted by means of concentration than from extract C, thus this last extract is enriched with β-sitosterol and stigmasterol, while extract B and fractions N1 and N2 are enriched with polyamines.
Figure 5. TLC profiles of extracts and fractions from S. saponaria callus culture and standards. Mobile phase: (A): Hex:EtOAc (7:3); (B): CHCl3:MeOH (8:2); (C): BAW (n-butanol:acetic acid:water, 4:1:5, upper phase). Color reagents: (A) and (B): Vanillin-sulfuric acid; (C): Ninhydrin. Extracts and fractions are represented by their respective nominations (B, C, A1, A2, B1, B2, N1 and N2). Also shown are the standards of β-sitosterol (SI), stigmasterol (ST) and β-sitosterol glucoside (SG).
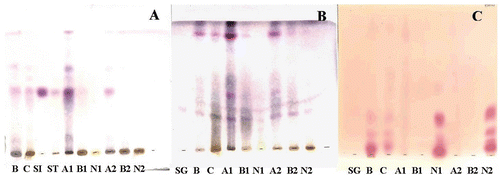
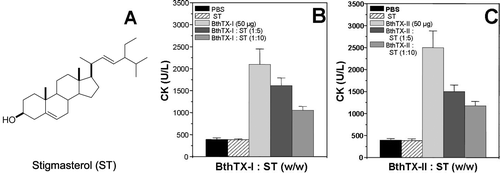
For fractions A1 and A2, a predominance of β-sitosterol, stigmasterol and β-sitosterol glucoside were observed, while in fraction B2, these compounds were found in minor concentrations. shows the anti-myotoxic activity of commercial stigmasterol on myotoxins BthTX-I and II. CitationGomes et al. (2007) reported the neutralization of viper and cobra venom by β-sitosterol and stigmasterol, isolated from the root extract of Pluchea indica. Thus, β-sitosterol, stigmasterol and β-sitosterol glucoside could be responsible for the inhibition of edema, hemorrhagic, myotoxic and PLA2 activities induced by snake venoms and toxins. Moreover, modeling studies on PLA2-inhibitor complexes show favorable interactions with the amino acid residues at the active site of PLA2 from snake venom and stigmasterol, which could indicate the mechanism of action responsible for the inhibition of phospholipase activity (CitationNirmal et al., 2008).
Figure 6. Effect of stigmasterol (A) on myotoxic (B and C) activity induced by isolated myotoxins, BthTX-I and BthTX-II, respectively. Each experiment is represented as mean ± S.D. (n = 6).
In conclusion, Sapindus saponaria L. extracts and fractions presented significant anti-ophidian activity and could be used as an alternative treatment to serum therapy or for its supplementation. Nevertheless, pharmacological studies should still be performed using the isolated active principles responsible for the anti-ophidian activity, thus providing insights to the elucidation of the mechanisms of action of the corresponding toxins and the development of future therapeutic agents for the treatment of ophidian accidents.
Declaration of interest
The authors gratefully acknowledge the financial support of Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade de Ribeirão Preto UNAERP and Faculdade de Ciências Farmacêuticas de Ribeirão Preto-USP.
References
- Adzu B, Abubakar MS, Izebe KS, Akumka DD, Gamaniel KS. (2005). Effect of Annona senegalensis rootbark extracts on Naja nigricotlis nigricotlis venom in rats. J Ethnopharmacol, 96, 507–513.
- Andrião-Escarso SH, Soares AM, Rodrigues VM, Angulo Y, Díaz C, Lomonte B, Gutiérrez JM, Giglio JR. (2000). Myotoxic phospholipase A2 in Bothrops snake venoms: Effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu. Biochimie, 82, 755–763.
- Batina MFC, Cintra ACO, Veronese ELG, Lavrador MAS, Giglio JR, Pereira PS, Dias DA, França SC, Sampaio SV. (1999). Inhibition of the lethal and myotoxic activities of Crotalus durissus terrificus venom by Tabernaemontana catharinensis: Identification of one of the active components. Planta Med, 66, 424–428.
- Biondo R, Pereira AM, Marcussi S, Pereira PS, França SC, Soares AM. (2003). Inhibition of enzymatic and pharmacological activities of some snake venoms and toxins by Mandevilla velutina (Apocynaceae) aqueous extract. Biochimie, 85, 1017–1025.
- Borges MH, Soares AM, Rodrigues VM, Oliveira F, Fransheschi AM, Rucavado A, Giglio JR, Homsi-Brandeburgo MI. (2001). Neutralization of proteases from Bothrops snake venoms by the aqueous extract from Casearia sylvestris (Flacourtiaceae). Toxicon, 39, 1863–1869.
- Braud S, Bon C, Wisner A. (2000). Snake venom proteins acting on hemostasis. Biochimie, 82, 851–859.
- Calgarotto AK, Damico DC, Ponce-Soto LA, Baldasso PA, Da Silva SL, Souza GH, Eberlin MN, Marangoni S. (2008). Biological and biochemical characterization of new basic phospholipase A2 BmTX-I isolated from Bothrops moojeni snake venom. Toxicon, 51, 1509–1519.
- Castro O, Gutiérrez JM, Barrios M, Castro I, Romero M, Umaña E. (1999). Neutralization of the hemorrhagic effect induced by Bothrops asper (Serpentes: Viperidae) venom with tropical plant extracts. Rev Biol Trop, 47, 605–616.
- Da Silva JO, Coppede JS, Fernandes VC, Sant’Ana CD, Ticli FK, Mazzi MV, Giglio JR, Pereira PS, Soares AM, Sampaio SV. (2005). Anti-hemorrhagic, anti-nucleolytic and other anti-ophidian properties of the aqueous extract from Pentaclethra macroloba. J Ethnopharmacol, 100, 145–152.
- Da Silva SL, Calgarotto AK, Chaar JS, Marangoni S. (2008a). Isolation and characterization of ellagic acid derivatives isolated from Casearia sylvestris SW aqueous extract with anti-PLA2 activity. Toxicon, 52, 655–666.
- Da Silva SL, Comar M Jr, Oliveira KMT, Chaar JS, Bezerra ERM, Calgarotto AK, Baldasso PA, Veber CL, Villar JAFP, Oliveira ARM, Marangoni S (2008b). Molecular modeling of the inhibition of enzyme PLA2 from snake venom by dipyrone and 1-phenyl-3-methyl-5-pyrazolone. Int J Quant Chem, 108, 2576–2585.
- Da Silva SL, Chaar Jda S, Yano T. (2009). Chemotherapeutic potential of two gallic acid derivative compounds from leaves of Casearia sylvestris SW (Flacourtiaceae). Eur J Pharmacol, 608, 76–83.
- de Alvarenga ES, Silva SA, Barosa LC, Demuner AJ, Parreira AG, Ribeiro RI, Marcussi S, Ferreira JM, Resende RR, Granjeiro PA, Silva JA, Soares AM, Marangoni S, Da Silva SL. (2011). Synthesis and evaluation of sesquiterpene lactone inhibitors of phospholipase A2 from Bothrops jararacussu. Toxicon, 57, 100–108.
- Diogo LC, Fernandes RS, Marcussi S, Menaldo DL, Roberto PG, Matrangulo PV, Pereira PS, França SC, Giuliatti S, Soares AM, Lourenço MV. (2009). Inhibition of snake venoms and phospholipase A2 by extracts from native and genetically modified Eclipta alba: Isolation of active coumestans. Basic Clin Pharmacol Toxicol, 104, 293–299.
- Ferreira LA, Henriques OB, Andreoni AA, Vital GR, Campos MM, Habermehl GG, de Moraes VL. (1992). Anti-venom and biological effects of ar-turmerone isolated from Curcuma longa (Zingiberaceae). Toxicon, 30, 1211–1218.
- Fry BG, Wickramaratna JC, Jones A, Alewood PF, Hodgson WC. (2001). Species and regional variations in the effectiveness of anti-venom against the in vitro neurotoxicity of death adder (Acanthophis) venoms. Toxicol Appl Pharmacol, 175, 140–148.
- Gomes A, Saha A, Chatterjee I, Chakravarty AK. (2007). Viper and cobra venom neutralization by β-sitosterol and stigmasterol isolated from the root extract of Pluchea indica Less. (Asteraceae). Phytomedicine, 14, 637–643.
- Guiguer EL, Ticli FK, Cintra ACO, Giglio JR, Sampaio SV. (2004). Inhibition of the activity of some snake venoms a peptide in the aqueous extract from Tabernaemontana catharinensis A.D.C. (Apocynaceae). J Venom Anim Toxins, 10, 497–515.
- Gutiérrez JM, Rucavado A. (2000). Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie, 82, 841–850.
- Izidoro LF, Rodrigues VM, Rodrigues RS, Ferro EV, Hamaguchi A, Giglio JR, Homsi-Brandeburgo MI. (2003). Neutralization of some hematological and hemostatic alterations induced by neuwiedase, a metalloproteinase isolated from Bothrops neuwiedi pauloensis snake venom, by the aqueous extract from Casearia mariquitensis (Flacourtiaceae). Biochimie, 85, 669–675.
- Januário AH, Santos SL, Marcussi S, Mazzi MV, Pietro RC, Sato DN, Ellena J, Sampaio SV, França SC, Soares AM. (2004). Neo-clerodane diterpenoid, a new metalloprotease snake venom inhibitor from Baccharis trimera (Asteraceae): Anti-proteolytic and anti-hemorrhagic properties. Chem Biol Interact, 150, 243–251.
- Lomonte B, León G, Angulo Y, Rucavado A, Núñez V. (2009). Neutralization of Bothrops asper venom by antibodies, natural products and synthetic drugs: Contributions to understanding snakebite envenomings and their treatment. Toxicon, 54, 1012–1028.
- Mahanta M, Mukherjee AK. (2001). Neutralisation of lethality, myotoxicity and toxic enzymes of Naja kaouthia venom by Mimosa pudica root extracts. J Ethnopharmacol, 75, 55–60.
- Maiorano VA, Marcussi S, Daher MA, Oliveira CZ, Couto LB, Gomes OA, França SC, Soares AM, Pereira PS. (2005). Anti-ophidian properties of the aqueous extract of Mikania glomerata. J Ethnopharmacol, 102, 364–370.
- Marcussi S, Sant’Ana CD, Oliveira CZ, Rueda AQ, Menaldo DL, Beleboni RO, Stabeli RG, Giglio JR, Fontes MR, Soares AM. (2007). Snake venom phospholipase A2 inhibitors: Medicinal chemistry and therapeutic potential. Curr Top Med Chem, 7, 743–756.
- Melo PA, do Nascimento MC, Mors WB, Suarez-Kurtz G. (1994). Inhibition of the myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrata (Asteraceae) extracts and constituents. Toxicon, 32, 595–603.
- Melo PA, Ownby CL. (1999). Ability of wedelolactone, heparin, and para-bromophenacyl bromide to antagonize the myotoxic effects of two crotaline venoms and their PLA2 myotoxins. Toxicon, 37, 199–215.
- Meyer Albiero AL, Aboin Sertié JA, Bacchi EM. (2002). Anti-ulcer activity of Sapindus saponaria L. in the rat. J Ethnopharmacol, 82, 41–44.
- Murashige T, Skoog F. (1962). A revised medium for rapid growth and bioassay with tabacco tissue culture. Physiology Plantarum, 15, 473–497.
- Murgu M. (2002). Saponinas e glicosídeos de Sapindus saponaria: Metodologia e análise por espectometria de massa e relação com fungos endofíticos. Universidade Federal de São Carlos, UFSCar, PhD thesis, Brazil.
- Nirmal N, Praba GO, Velmurugan D. (2008). Modeling studies on phospholipase A2-inhibitor complexes. Indian J Biochem Biophys, 45, 256–262.
- Núñez V, Otero R, Barona J, Fonnegra R, Jimenéz S, Osório RG, Quintana JC, Díaz A. (2004). Inhibition of the toxic effects of Lachesis muta, Crotalus durissus cumanensis and Micrurus mipartitus snake venoms by plant extracts. Pharm Biol, 42, 49–54.
- Oliveira CZ, Marcussi S, Maiorano VA, Januário AH, Lourenço MV, França SC, Pereira PS, Soares AM. (2005). Anti-coagulant properties of the aqueous extract from Bauhinia forficata against Bothrops jararacussu snake venom. J Ethnopharmacol, 98, 213–216.
- Oliveira M, Calvalcante WLG, Arruda EZ, Melo PA, Silva MD, Gallacci M. (2003). Antagonism of myotoxic and paralyzing activities of bothropstoxin-I by suramin. Toxicon, 42, 373–379.
- Otero R, Gutiérrez JM, Rojas G, Núñez V, Díaz A, Miranda E, Uribe AF, Silva JF, Ospina JG, Medina Y, Toro MF, García ME, León G, García M, Lizano S, De La Torre J, Márquez J, Mena Y, González N, Arenas LC, Puzón A, Blanco N, Sierra A, Espinal ME, Lozano R. (1999). A randomized blinded clinical trial of two anti-venoms, prepared by caprylic acid or ammonium sulphate fractionation of IgG, in Bothrops and Porthidium snake bites in Colombia: Correlation between safety and biochemical characteristics of anti-venoms. Toxicon, 37, 895–908.
- Ownby CL. (1990). Locally acting agents: Myotoxins, hemorrhagic toxins and dermonecrotic factors. In: Lee C, ed. Handbook of Toxicology. New York: Springer-Verlag, 601–654.
- Peterson ME. (2006). Snake bite: Pit vipers. Clin Tech Small Anim Pract, 21, 174–182.
- Pithayanukul P, Laovachirasuwan S, Bavovada R, Pakmanee N, Suttisri R. (2004). Anti-venom potential of butanolic extract of Eclipta prostrata against Malayan pit viper venom. J Ethnopharmacol, 90, 347–352.
- Romero L, Marcussi S, Marchi-Salvador DP, Silva FP Jr, Fuly AL, Stábeli RG, da Silva SL, González J, Monte AD, Soares AM. (2010). Enzymatic and structural characterization of a basic phospholipase A2 from the sea anemone Condylactis gigantea. Biochimie, 92, 1063–1071.
- Soares AM, Mancin AC, Cecchini AL, Arantes EC, França SC, Gutiérrez JM, Giglio JR. (2001). Effects of chemical modifications of crotoxin B, the phospholipase A2 subunit of crotoxin from Crotalus durissus terrificus snake venom, on its enzymatic and pharmacological activities. Int J Biochem Cell Biol, 33, 877–888.
- Soares AM, Januário AH, Lourenço MV, Pereira AMS, Pereira PS. (2004). Neutralizing effects of Brazilian plants against snake venoms. Drugs Future, 29, 1105–1117.
- Soares AM, Ticli FK, Marcussi S, Lourenço MV, Januário AH, Sampaio SV, Giglio JR, Lomonte B, Pereira PS. (2005). Medicinal plants with inhibitory properties against snake venoms. Curr Med Chem, 12, 2625–2641.
- Ticli FK, Hage LI, Cambraia RS, Pereira PS, Magro AJ, Fontes MR, Stábeli RG, Giglio JR, França SC, Soares AM, Sampaio SV. (2005). Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): Anti-serum action potentiation and molecular interaction. Toxicon, 46, 318–327.
- Tsuzuki JK, Svidzinski TI, Shinobu CS, Silva LF, Rodrigues-Filho E, Cortez DA, Ferreira IC. (2007). Anti-fungal activity of the extracts and saponins from Sapindus saponaria L. An Acad Bras Cienc, 79, 577–583.
- Zamuner SR, Teixeira CF. (2002). Cell adhesion molecules involved in the leukocyte recruitment induced by venom of the snake Bothrops jararaca. Mediators Inflamm, 11, 351–357.