Abstract
Context: Rubia cordifolia Linn. (Rubiaceae) is a medicinal plant used in the ayurvedic system of medicine. It is also known as Indian Madder or Manjistha and is traditionally used as an antiinflammatory, antiseptic, and galactopurifier, but its anticancer propertis are yet not known.
Objective: The ameliorative effect of the Rubia cordifolia methanol extract on N-nitrosodiethylamine-induced experimental hepatocellular carcinogenesis in rats.
Materials and methods: Changes in liver weight, serum markers of liver damage, hydroxyl radicals, lipid peroxidation, levels of enzymic and nonenzymic antioxidants; mitochondrial and respiratory chain enzymes were also investigated using various biochemical parameters and histopathological studies. Male albino rats of Wistar strain were divided into four groups for a study period of 3 months. Animals of group I and group IV served as control and drug control, respectively. Hepatocellular carcinoma was induced in animals of groups II and III with 0.02% N-nitrosodiethylamine.
Results: Upon Rubia cordifolia methanol extract co-treatment (250, 500, and 750 mg/kg bodyweight) in group III alone levels of serum marker enzymes and antioxidants increased significantly in a dose-dependent manner. The levels of hydroxyl radicals and lipid peroxidation decreased. Mitochondrial enzymes and respiratory chain enzymes, which were decreased in N-nitrosodiethylamine-induced rats, increased significantly in RC treated rats. Further histological analysis of liver confirmed the prevention of pathological changes caused by N-nitrosodiethylamine on Rubia cordifolia supplementation.
Discussion and conclusion: These findings demonstrate that Rubia cordifolia can be a source of potent antioxidants for treatment of diseases such as cancer.
Introduction
Hepatocellular carcinoma (HCC) is the most common and lethal of all cancers. A diversity of dietary, endogenous and environmental stimuli mediates hepatocellular carcinogenesis (CitationGuyton & Kensler, 1993). Nitrosamines are a large class of compounds that act as inducers of primary liver cancer due to their environmental presence and high carcinogenic potency. They are found in foods such as meat and dairy products and in alcoholic beverages. The leather tanning, metal working, rubber and tire manufacturing processes produce high concentrations of volatile nitrosamines. It is widely accepted that metabolic activation of nitrosamines by cytochrome P450 enzymes to reactive electrophiles is required for their cytotoxic, mutagenic and carcinogenic activities (CitationArcher, 1989). Because of its relatively simple metabolic pathway and potent carcinogenic activity, N-nitrosodiethylamine (DEN) has found wide spread use as an experimental model in the field of liver carcinogenesis (CitationLee & Lee, 1999). DEN has been suggested to produce oxidative stress and cellular injury due to involvement of reactive oxygen species (ROS) (CitationBansal et al., 2000).
ROS are potentially dangerous by products of cellular metabolism that have direct effect on cell development, growth and survival. Oxidative stress mediated by ROS due to its associated interaction with the membrane causing lipid peroxidation and DNA damage further promotes carcinogenesis. The severity and magnitude of the ROS mediated cancer problem makes it imperative to develop chemopreventive strategies, utilizing synthetic or natural agents to block initiation or to arrest progression in malignant cells (CitationSivaramakrishnan et al., 2008). One such natural herbal agent is Rubia cordifolia Linn. (Rubiaceae) (RC), locally known as manjistha, a perennial herbaceous climber. It grows commonly on higher ghats, Mahabaleswar, Amboli, and Maharastra states of India. It is an important medicinal plant which is used for treatment in an ayurvedic system of medicine (CitationPandey & Sharma, 1994). It possesses antineoplastic (CitationAdwankar et al., 1980), immunomodulatory (CitationJoharapurkar et al., 2003) and antiinflammatory (CitationAntarkar et al., 1983) properties. The major active components of Rubia cordifolia are anthraquinones glycosides and include 1-hydroxy 2-methoxy anthraquinone, 1,4-dihydroxy-2-methyl-5-methoxy anthraquinone, 1,3-dimethoxy 2-carboxy anthraquinone and rubiadin (1,3-dihydroxy-2-methyl anthraquinone) (Mohana CitationRao et al., 2006). Considering these properties of RC, the present work was planned to give a scientific validation to the ameliorative effect of the methanol extract of RC on N-nitrosodiethylamine (DEN) induced experimental hepatocellular carcinoma in rat.
Materials and methods
Preparation of plant extract
Dried roots of RC were purchased from market. The plant material was identified and duly authenticated by Dr. Mathivanan, Center for Advanced Studies in Botany, Guindy Campus, University of Madras, Chennai, India. A voucher specimen of the collected sample was deposited in the departmental herbarium. Roots of RC (1 kg) were treated with 2.5 L of petroleum ether at room temperature in closed container and repeated till 7 days without allowing it to dry. The plant material was removed by vacuum filtration. The plant extract obtained from the above step was subjected to cold percolation using methanol (95%, v/v) as solvent and suitably concentrated by rotary pump vacuum evaporator and dried in a vacuum desiccator and stored at room temperature. It was dissolved in water and administered orally to rats.
Phytochemical screening
The phytochemical examination of the methanol extract was performed by standard methods and showed the presence of various phytochemical constituents (Trease & Evans, 1993).
Chemicals and reagents
N-Nitrosodiethylamine (DEN) was purchased from Sigma Aldrich, USA, and all the other chemicals used here for the study were of analytical reagent grade.
Experimental animals
Male albino rats of Wistar strain (120–150 g) were purchased from Tamil Nadu University of Veterinary Animal Sciences (TANUVAS) and acclimatized to laboratory conditions for 1 week. They were maintained in clean, sterile, polypropylene cages and fed with commercial pellet rat chow (M/s. Hindustan Lever Ltd., Bangalore, India), water ad libitum and kept in a well ventilated room with 12 h light/dark cycles throughout the experimental period. This study has been conducted as per the guidelines of the animal ethical committee of our institution (IAEC No. 01/004/02). In preliminary studies, RC was found to be non-toxic up to a dosage of 3 g/kg/body weight (data not shown) and among the various doses studied, the optimal dosage for its ameliorative efficacy was found to be 500 mg/kg body weight. Animals were divided into four groups, for a total experimental period of 90 days and studied as follows.
Experimental design
Group I: Control rats received normal diet and drinking water.
Group II: Rats were treated with DEN (0.02%) orally in drinking water.
Group III: Rats were given DEN (0.02%) in drinking water along with the methanol extract of RC (250, 500 and 750 mg/kg body weight, respectively) orally.
Group IV: Rats treated with RC alone (500 mg/kg/body weight) orally.
After 90 days, rats were anaesthetized with sodium-pentothal after overnight fasting and euthanized. Serum from control and experimental rats were separated from blood. The liver was excised immediately and kept in ice-cold saline.
Processing of liver tissue
A portion of the liver was homogenized in 0.1 M Tris buffer, pH 7.4 and used for the serum marker enzymes, enzymic and non-enzymic antioxidants, lipid peroxidation, and hydroxyl radical estimation.
Isolation of mitochondria
The mitochondrial isolation procedure used was of CitationJohnson and Lardy (1967). A lobe of the liver was excised into ice cold solution medium (sucrose: 0.25 M; Tris-HCl: 0.005 M, pH 7.4; EDTA: 0.001 M) and were homogenized (10% w/v) using teflon homogenizer. The 10% homogenate was centrifuged at 700 × g for 5 min and the resultant supernatant was centrifuged at 30,000 × g for 10 min. The mitochondrial pellet obtained was resuspended in solution medium (1 mL/g tissue) and used for analyzing mitochondrial and respiratory chain enzymes.
Biochemical assays
The activities of aspartate transaminase (AST), alanine transaminase (ALT) (Mohun & Cook, 1975), alkaline phosphatase (ALP) (CitationKing, 1965a) and lactate dehydrogenase (LDH) (CitationKing, 1965b) were assayed in the serum. The activities of the enzymic and non-enzymic antioxidants superoxide dismutase (SOD) (CitationMisra & Fridovich, 1972), catalase (CAT) (CitationSinha, 1972), glutathione peroxidase (GPx) (CitationRotruck et al., 1973), glutathione S-transferase (GST) (CitationHabig et al., 1974), reduced glutathione (GSH) (CitationMoron et al., 1979) were estimated in liver tissue homogenate. The levels of lipid peroxidation (CitationOhkawa et al., 1979) products were estimated in liver tissue homogenate. The hydroxyl radical (CitationCederbaum & Cohen, 1984) levels were measured in liver tissue homogenate. The levels of mitochondrial enzymes like isocitrate dehydrogenase (ICDH) (CitationBell & Baron, 1960), succinate dehydrogenase (SDH) (CitationSlater & Bonner, 1952), α-ketoglutarate dehydrogenase (α-KGDH) (CitationReed & Mukherjee, 1969) and respiratory chain enzymes like NADH dehydrogenase (CitationMinakami et al., 1962) and cytochrome c oxidase (CitationPearl et al., 1963) were also estimated in liver.
Histological examination
Histological evaluation was performed on a lobe of the liver and a portion of the specimen was fixed in 10% formalin and embedded in paraffin wax. Sections were cut at 4 µm thickness, stained with hematoxylin and eosin and viewed under light microscope for histological changes.
Results
The result of the preliminary phytochemical screening of the methanol extract of RC methanol extract revealed that presence of anthroquinone, glycosides, saponins and absence of tannins and alkaloid ().
Table 1. Phytochemical screening of methanolic extract of Rubia cordifolia.
Rats treated with DEN developed hepatic neoplastic nodules. Liver weight was increased significantly in DEN induced rats when compared to normal rats due to the development of nodules. RC given along with DEN for 90 days significantly prevented the formation of liver tumors and thereby the increase of the liver weight ().
Table 2. Liver weight of control and experimental animals.
The activity of serum marker enzymes of clinical interest-AST, ALT and ALP increased significantly in DEN treated groups when compared to control group. The levels of serum marker enzymes were maintained at near normal level on co-treatment with RC. No significant change in AST, ALT and ALP activities were observed in the group treated with RC alone when compared with control (). There was a significant increase in LDH in DEN induced group, whereas the RC alone group showed normal levels of LDH. Rats subjected to DEN and RC regimen exhibited a significant reduction in LDH activity ().
Table 3. Levels of serum markers of control and experimental groups of rats.
The levels of LPO and hydroxyl radicals in liver in DEN induced group were increased significantly. It was normal in drug (RC) alone group when compared with the control group. RC co-treatment inhibited the accumulation of lipid peroxidation products (TBARS) and hydroxyl radicals significantly when compared with DEN-induced rats ( and ). DEN induced LPO was associated with a decrease in glutathione levels and the effect was found to be statistically significant in liver. RC treatment restored the GSH level to a normal value, thereby maintaining the redox status of the cell ().
Table 4. Antioxidant status in liver of control and experimental groups of rats.
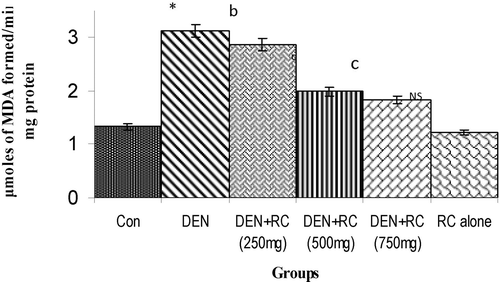
Figure 1. Levels of lipid peroxidation in liver tissue homogenate. Values are expressed as mean ± SD for six animals. LPO was expressed as μmoles of MDA formed/min/mg protein in the liver tissue homogenate of control and experimental groups of rats. Control vs. DEN *P < 0.001, yP < 0.01, xP < 0.05. DEN vs. DEN + RC cP < 0.001, bP < 0.01, aP < 0.05. RC alone vs. Control. NS, Non-significant.
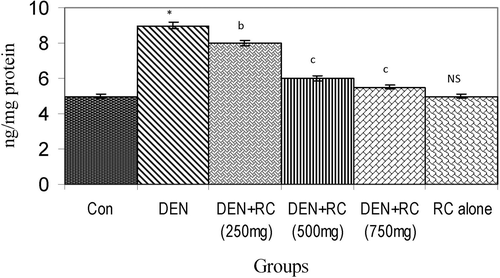
Figure 2. Effect of RC on levels of hydroxyl radicals. Values are expressed as mean ± SD for six animals. Hydroxyl radicals are expressed as ng/mg protein in the liver tissue homogenate of control and experimental groups of rats. Control vs. DEN *P < 0.001, yP < 0.01, xP < 0.05. DEN vs. DEN + RC cP < 0.001, bP < 0.01, aP < 0.05. RC alone vs. Control. NS, Non-significant.
The SOD activity was decreased significantly on treatment with DEN. Co-treatment with RC resulted in higher levels of SOD when compared to DEN induced rats. The activity of CAT, GPx and GST decreased significantly in rats of the group induced with DEN. Co-administration of RC caused significant increase in the activities of CAT, GPx and GST when compared with the group treated with DEN alone ().
Decreased activities of key enzymes of the TCA cycle such as isocitrate dehydrogenase (ICDH), succinate dehydrogenase (SDH), α-ketoglutarate dehydrogenase (α-KGDH) and respiratory chain enzymes (NADH dehydrogenase and cytochrome c oxidase) in liver tissue homogenate were observed in DEN induced rats. DEN induced tumor-bearing animals treated with RC extract showed a significant increase in mitochondrial and respiratory chain enzyme activities. Rats treated with RC extract alone showed no significant variations when compared with control rats ().
Table 5. TCA cycle and respiratory chain enzyme activities in liver of control and experimental groups of rats.
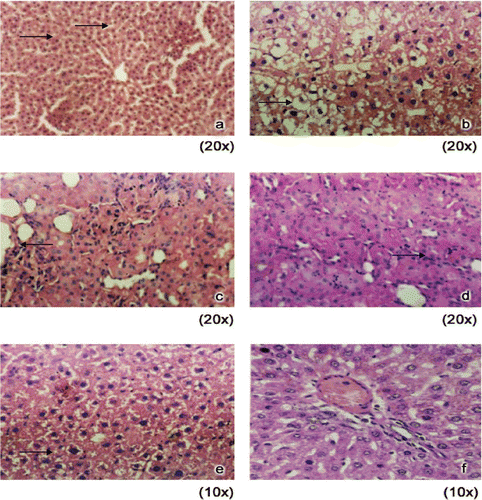
Significant damage was evident to the liver cells in the DEN induced group II compared to control group I animals. On RC supplementation along with DEN caused the amelioration of damage to liver cells as evident from the histology of liver tissue ().
Figure 3. Histology of liver in control and experimental animals. (A) Control rats showing normal architecture. (B) DEN induced rats showing loss of architecture (both nuclear size and shape vary). (C) DEN + RC (250 mg) treated rats showing anaplastic tumor cells with scanty Cytoplasm. (D) DEN + RC (500 mg) treated rats showing scanty strands of tumor cells surrounded by large amount of neutrophils. (E) DEN + RC (750 mg) treated rats showing scanty strands of tumor cells surrounded by large amount of neutrophils. (F) RC alone (500 mg) treated rats showing normal morphology.
Discussion
The preliminary chemical constituents of Rubia cordifolia Linn. was analyzed and the results showed the bioactive properties of medicinal plants are perhaps due to the presence of various secondary metabolites such as anthroquinone, glycosides and saponins (CitationKhandelwal, 2005).
Elevation of serum ALT and AST levels correlate with a high incidence of HCC development due to various risk factors and the prevention of liver injury decreased the incidence of HCC (CitationTarao et al., 1999). ALT and AST are cytosolic enzymes of the hepatocyte, and an increase in the activities of these enzymes in the serum reflects an increase in plasma membrane permeability, which in turn, is associated with liver injury (CitationPlaa & Hewitt, 1982). In this context, we have observed a rise in the level of ALT and AST in DEN-induced rats which were found to be decreased significantly on RC treatment. The present study on the methanol extract of root of RC was found to protect and maintain the structural integrity of hepatic cells by significantly decreasing the levels of serum AST and ALT levels on treatment similar to CitationPradeep et al. (2007) suggesting that its hepatoprotective action might be due to a membrane-stabilizing property. ALP is mainly involved in metabolite transport across cell membranes found in a decreasing order of abundance in placenta, ileal mucosa, kidney, bone, and liver. The elevated level of serum ALP is associated mainly with liver cell damage (CitationMoss, 1997). DEN induced rats showed elevated serum ALP, which confirms the impairment of liver function. Administration of RC along with DEN was found to significantly prevent the elevation of serum ALP levels thereby confirming the hepatoprotective action of RC. LDH is a hydrogen transfer enzyme that catalyzes the oxidation of l-lactate to pyruvate with the mediation of NAD+ as hydrogen acceptor. Increased LDH levels indicate that the energy demands are met by anaerobic respiration through increase in LDH activity. The increased LDH levels, as observed in the present study in the DEN-induced rats, is a direct diagnostic function of liver damage and tumor formation (CitationAl-Attar, 2004). There was a significant decrease in enzyme activity after treatment with RC. The histological observations confirm the amount of liver damage caused by DEN and the corresponding prevention of damage and maintenance of hepatocyte architecture on RC supplementation. This corroborates with results of biochemical assays confirming the protective action of the RC extract against liver damage. The results of the present study indicate that RC acts as a potent hepatoprotective agent against DEN-induced HCC.
Lipid peroxidation is a chain reaction process that involves the participation of free radical species and has been shown to induce alterations in several cellular functions. In the present study, lipid peroxidation products (MDA) were significantly increased following DEN treatment. RC inhibited accumulation of lipid peroxidation products in liver. DEN-induced lipid peroxidation was associated with a decrease in glutathione levels and the effect was found to be statistically significant in liver as similar to Bansal et al. (2007). RC treatment restored the GSH level to a normal value, thereby maintaining the redox status of the cell ().
The level of GSH inside the cell depends primarily on the rate of synthesis and therefore on the pool of cysteine, glycine and glutamate and their assembling enzyme and secondly on the rate of its utilization via glutathione S-transferase and glutathione peroxidase/glutathionereductase system. The remarkably low concentration of one of the most potent antioxidants, GSH on DEN treatment, contributes to impairment in the defense mechanism against oxygen attack (CitationPradeep et al., 2007; CitationSehrawat & Sultana, 2006). An in vitro study has shown that binding of carcinogen metabolites to nuclear DNA is inhibited by conjugation with glutathione (CitationHerse et al., 1980). Therefore, the enzymes may be important in the inhibition of hepatomas (CitationKimihiko & Ichiro, 2002).
The relative deficiency of SOD and catalase could result in decreased detoxification of O2− in tumors (CitationTisdale & Mahmoud, 1983). This could result in cell death from membrane damage arising from peroxidative reaction of polyunsaturated fatty acids (lipid peroxidation) and attack of ROS on protein and nucleic acids. The relatively unreactive O2− is converted by superoxide dismutase (SOD) into superoxide radical which in turn takes part in the “Fenton reaction” using transition metal ions (copper or iron) as catalysts to produce the highly reactive hydroxyl radical. The hydroxyl radical is an extremely reactive oxidizing radical that will react with most biomolecules at diffusion controlled rates. The hydroxyl free radical is several orders of magnitude more reactive towards cellular constituents than superoxide radicals and hydrogen peroxide (CitationHalliwell & Gutteridge, 1992). In the present study, there was a multi-fold increase in the levels of hydroxyl free radicals in DEN-induced animals that was neutralized to near normalcy in RC co-treated ones, thus indicating prevention of increased ROS production by RC by its capacity to scavenge free radicals.
The lower level of GSH-Px, together with that of CAT, might favor accumulation of free radicals, which may induce the neoplastic condition. These reduced levels of SOD, CAT and GPx may permit the accumulation of superoxide or hydrogen peroxide within a tumor, which may facilitate advantageous growth of neoplastic cells. The simultaneous administration of RC with DEN induction maintained the SOD, CAT and GPx, GST activities probably by decreasing the levels of ROS like hydroxyl radicals as evidenced in the case of garlic and silymarin (CitationShaarawy et al., 2009).
GSTs are a group of multifunctional proteins localized mainly in the hepatic cytosol and they participate in detoxification reactions of electrophilic compounds and contribute to the mechanism of cancer chemopreventive agents via induction. A detoxification pathway, which plays a major role in cellular defenses against genetic damage by electrophiles, is the thiol tripeptide glutathione together with glutathione S-transferase (CitationKetterer, 1988) that interact with DEN metabolites and make them less toxic. Individual deficiencies in the protection afforded by phase II detoxification enzymes, including the GSTs, are reported to increase the risk of cancer (CitationStrange et al., 1998). This unique feature of induction of GST activity possibly by RC as evident from our study may also be responsible for its antioxidant, hepatoprotective and anticarcinogenic effect.
DEN-induced tumor-bearing animals treated with RC extract showed a significant increase in mitochondrial enzyme and respiratory chain enzyme activities. Rats treated with RC extract alone showed no significant variations when compared with control rats (). This study confirms that the chemopreventive effect of RC is found to be more effective in the initiation phase of carcinogenesis. Similar results have been observed with other chemopreventive agents. For example, sodium selenite, by maintaining TCA cycle enzymes (CitationThirunavukkarasu et al., 2001) acts as a chemopreventive agent in DEN induced hepatocellular carcinoma. The significant decrease in the activity of liver succinate dehydrogenase suggests that anaerobic metabolism was favored over aerobic oxidation of glucose through Krebs cycle in order to mitigate the energy crisis for survival (CitationArgiles & Bioeto, 1988).
Enhanced lipid peroxidation damaged the membranous structure of mitochondria, which was indicated by the loss of lamellae, and increased oxidation of exogenously added NADH. Loss in membrane integrity altered the activities of the tricarboxylic acid cycle enzymes and levels of cytochromes (CitationJosephine & Keshav, 2002). Similarly, CitationRemus and Firman (1995) reported that the decrease in TCA cycle action might be related to a decline in the activity of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase. Our study confirms that RC was effective in maintaining mitochondrial integrity by increasing the enzymatic activities of TCA cycle, which was initially decreased in DEN-induced animals to normal levels on co-treatment.
Mitochondria carry out a variety of processes of which oxidative phosphorylation is the most important. Detoxification of oxygen via its reduction to water by cytochrome oxidase takes place in the mitochondria. Cytochrome c oxidase and NADH dehydrogenase are enzymes involved in the electron transport chain and are located in the inner mitochondrial membrane. Their role is ultimately linked to the production of useful energy rich compounds such as ATP.
Lipid peroxidation has been reported to decrease the rate of oxygen consumption as well as the activity of cytochrome c oxidase in mitochondria (CitationIshankhodzhaev et al., 1987). The respiratory process involves the transport of electrons via cytochromes to molecular oxygen. Variations in cytochrome concentrations may affect the transport of electrons via the transport chain and thereby alter energy production of mitochondria. It has been reported that reduction in functioning of mitochondrial energy production would impair protein synthesis and energy production. A decrease in mitochondrial cytochrome content could result in the concomitant loss of oxidative phosphorylation capacity (CitationSchultz & Chan, 2001). The close correlation reaction preceding the phase of mitochondrial MDA production between the decline of mitochondrial respiration and the activity of complex III suggests an impairment of respiratory chain in the bc1region, representing one of the functional events in the casual peroxidation reaction (CitationYau-Huei et al., 2001). Upon RC supplementation, the above biochemical changes were reverted in DEN induced animals.
Conclusion
In conclusion, all these observations clearly indicate a significant ameliorative effect of RC extract against DEN-induced carcinogenesis. From our study, it is concluded that RC suppressed the free radical processes by scavenging hydroxyl radicals. Our results underline the capacity of RC to modulate the levels of LPO and significantly increase the endogenous antioxidant defense mechanisms in DEN-induced hepatocellular carcinoma. Our results also show that the significant increase in the levels of serum markers in DEN-induced animals was prevented by RC treatment. From the results obtained, we suggest that RC may be developed as an effective chemotherapeutic agent. Further studies to characterize the active principles and to elucidate the molecular mechanism of action are in progress.
Acknowledgments
The authors wish to thank Dr. V. Mathivanan, Center for Advanced Studies in Botany, University of Madras, Guindy campus, Chennai, India, for authenticating the plant, Rubia cordifolia.
Declaration of interest
The authors declare no conflicts of interest.
References
- Adwankar MK, Chitnis MP, Khandalekar DD, Bhadsavale CG. (1980). Anti-cancer activity of the extracts of Rubia cordifolia Linn. (NSC b668893). Indian J Exp Biol, 18, 102.
- Al-Attar AM. (2004). The influence of dietary grape seed oil on DMBA-induced liver enzymes disturbance in the frog Rana ridibunda. Pak J Nutr, 3, 304–309.
- Antarkar DS, Chinwala T, Bhatt N. (1983). Anti-inflammatory activity of Rubia cordifolia Linn. in rats. Ind J Pharmacol, 15, 185–188.
- Archer MC. (1989). Mechanisms of action of N-nitroso compounds. Cancer Surv, 8, 241–250.
- Argilés JM, Azcón-Bieto J. (1988). The metabolic environment of cancer. Mol Cell Biochem, 81, 3–17.
- Bansal AK, Bansal M, Soni G, Bhatnagar D. (2005). Protective role of vitamin E pre-treatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact, 156, 101–111.
- Bansal AK, Trivedi R, Soni GL, Bhatnagar D. (2000). Hepatic and renal oxidative stress in acute toxicity of N-nitrosodiethylamine in rats. Indian J Exp Biol, 38, 916–920.
- Bell JL, Baron DN. (1960). A colorimetric method for the determination of isocitrate dehydrogenase. Clin Chim Acta, 5, 40–47.
- Cederbaum AI, Cohen G. (1984). Microsomal oxidant radical production and ethanol oxidation. In: Methods in Enzymology, Packer L. (ed.), San Diego, Academic Press, 105, pp. 516–522.
- Corrocher R, Casaril M, Bellisola G, Gabrielli GB, Nicoli N, Guidi GC, De Sandre G. (1986). Severe impairment of antioxidant system in human hepatoma. Cancer, 58, 1658–1662.
- Guyton KZ, Kensler TW. (1993). Oxidative mechanisms in carcinogenesis. Br Med Bull, 49, 523–544.
- Habig WH, Pabst MJ, Jakoby WB. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem, 249, 7130–7139.
- Halliwell B, Gutteridge JM. (1992). Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett, 307, 108–112.
- Herse S, Jerstorm B, Martinez M, Guenthner T, Orrenius. (1980). Inhibition of binding of benzo(a)pyrene metabolites to nuclear DNA by glutathione-S-transferase. Biochem Res Commun, 94, 612–617.
- Ishankhodzhaev TM, Bornikov VT, Zainutdinov BR, Saatov TS. (1987). [Effect of changes in the membrane lipid composition on the activity of respiratory chain enzymes in rat liver mitochondria]. Biokhimiia, 52, 220–224.
- Joharapurkar AA, Deode NM, Zambad SP, Umathe SN. (2003): Immunomodulatory activity of alcoholic extract of Rubia cordifolia Linn. Ind Drugs, 40, 179–181.
- Johnson D, Lardy H. (1967). Isolation of liver or kidney mitochondria. Methods Enzymol, 10, 94–96.
- Josephine SM-N, Keshav KS. (2002). Mitochondria as targets for detection and treatment of cancer. Exp Rev Mol Med, 1–19.
- Kasture VS, Deshmukh VK, Chopde CT. (2000). Anticonvulsant and behavioral actions of triterpene isolated from Rubia cordifolia Linn. Indian J Exp Biol, 38, 675–680.
- Ketterer B. (1988). Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutat Res, 202, 343–361.
- Khandelwal KR. (2005). A Text Book of Practical Pharmacognosy, 27th Edn. Pune. Nirali Prakashan, pp. 151–163.
- Kimihiko S, Ichiro H. (2002). Anomalous elevation of glutathione-S-transferase P-form (GST-P) in the elementary process of epigenetic initiation of chemical hepatocarcinogenesis in rats. Carcinogenesis, 23, 1193–1198.
- King EJ. (1965a). The hydrolases, acid and alkaline phosphatases. In: Practical Clinical Enzymology. Van D (ed.). Nostrand Company Ltd, London, 191–208.
- King EJ. (1965b). The hydrolases, acid and alkaline phosphatases. In: Practical Clinical Enzymology. Van D (Ed). Nostrand Company Ltd, London, pp. 363–367.
- Lee BH, Lee SJ. (1999). Preventive effects of a mixed disulphide from dithiocarbamate and N-acetylcysteine on the genotoxicity of N-nitrosodiethylamine. J Pharm Pharmacol, 51, 105–109.
- Maron MS, Die Fierre JW, Mannervik KB. (1979). Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochem Biophys Acta, 582, 67–78.
- Minakami S, Ringler RL, Singer TP. (1962). Studies on the respiratory chain-linked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. J Biol Chem, 237, 569–576.
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem, 247, 3170–3175.
- Rao GM, Rao CV, Pushpangadan P, Shirwaikar A. (2006). Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J Ethnopharmacol, 103, 484–490.
- Mohun AF, Cook IJ. (1957). Simple methods for measuring serum levels of the glutamic-oxalacetic and glutamic-pyruvic transaminases in routine laboratories. j Clin Pathol, 10, 394–399.
- Moron MS, Depierre JW, Mannervik B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta, 582, 67–78.
- Moss DW. (1997). Physicochemical and pathophysiological factors in the release of membrane-bound alkaline phosphatase from cells. Clin Chim Acta, 257, 133–140.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Pandey S, Sharma M, Chaturvedi P, Tripathi YB. (1994). Protective effect of Rubia cordifolia on lipid peroxide formation in isolated rat liver homogenate. Indian J Exp Biol, 32, 180–183.
- Pearl W, Cascarano J, Zweeifach BW. (1963). Micro-determination of cytochrome oxidase in rat tissues by the oxidation on N-phenyl-p-phenylenediamine or ascorbic acid. J Histochem Cyto, 11, 102–106.
- Plaa GL, Hewitt WR. (1982). Detection and evaluation of chemically induced liver injury. In: Principles and Method of Toxicology. Hayes W (ed.), NewYork: Raven Press, pp. 407–445.
- Pradeep K, Mohan CV, Gobianand K, Karthikeyan S. (2007). Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol, 560, 110–116.
- Reed LJ, Mukherjee BB. (1969). Ketoglutarate dehydrogenase complex from Escherichia coli. In: Methods in Enzymology. (Eds.), Academic Press, New York, 13, 55–61.
- Remus JC, Firman JD. (1995). Effect of thiamin deficiency on energy metabolites in the turkey. J Nutr Biochem, 6, 636–639.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.
- Shaarawy SM, Tohamy AA, Elgendy SM, Elmageed ZY, Bahnasy A, Mohamed MS, Kandil E, Matrougui K. (2009). Protective effects of garlic and silymarin on NDEA-induced rats hepatotoxicity. Int J Biol Sci, 5, 549–557.
- Schultz BE, Chan SI. (2001). Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct, 30, 23–65.
- Sehrawat A, Sultana S. (2006). Evaluation of possible mechanisms of protective role of Tamarix gallica against DEN initiated and 2-AAF promoted hepatocarcinogenesis in male Wistar rats. Life Sci, 79, 1456–1465.
- Sinha AK. (1972). Colorimetric assay of catalase. Anal Biochem, 47, 389–394.
- Sivaramakrishnan V, Shilpa PN, Praveen Kumar VR, Niranjali Devaraj S. (2008). Attenuation of N-nitrosodiethylamine-induced hepatocellular carcinogenesis by a novel flavonol-Morin. Chem Biol Interact, 171, 79–88.
- Slater EC, Bonner WD. (1952). The effect of fluoride on the succinate oxidase system. Biochem J, 111, 375–383.
- Strange RC, Lear JT, Fryer AA. (1998). Glutathione S-transferase polymorphisms: Influence on susceptibility to cancer. Chem Biol Interact, 111-112, 351–364.
- Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N, Totsuka S. (1999). Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer, 86, 589–595.
- Thirunavukkarasu C, Singh JP, Selvendiran K, Sakthisekaran D. (2001). Chemopreventive efficacy of selenium against N-nitrosodiethylamine-induced hepatoma in albino rats. Cell Biochem Funct, 19, 265–271.
- Trease GE, Evans WC. (1986). A Textbook of Pharmacology, 14th Edn. London: Bailliere Tindall Ltd.
- Sabry MS, Amany AT, Saad ME, Zakaria YAE, Abeer B, Maha SM, Emad K, Khalid M. (2009).Protective effects of garlic and silymarin on NDEA-induced rats hepatotoxicity. Int J Biol Sci, 5, 549–557.
- Tisdale MJ, Mahmoud MB. (1983). Activities of free radical metabolizing enzymes in tumours. Br J Cancer, 47, 809–812.
- Yau-Huei W, Ching-You L, Chia-Yu W, Yi-Shing M, Hsin-Chen L. (2001). Oxidative stress in human aging and mitochondrial diseases-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Physiol, 44, 1–11.