Abstract
Context: There has been enormous interest in the development of alternative medicines for the control of diabetes. Use of carbohydrate-hydrolyzing enzyme inhibitors proved to be an important strategy for the management of postprandial hyperglycemia by delaying the process of carbohydrate hydrolysis and absorption.
Objective: Three common traditional herbs, namely, stem bark of Terminalia arjuna (Combretaceae), seeds of Eugenia cumini (Myrtaceae), and leaves of Aegle marmelos (Rutaceae), were tested for their α-amylase inhibitory activities to establish antidiabetic potential.
Materials and methods: The plant extracts (aqueous, 50%, and 100% methanol) obtained were subjected to an in vitro amylase inhibitory assay using starch as a substrate and pancreatic amylase as the enzyme. Statistical differences and linear regression analysis were performed using GraphPad prism 5 software.
Results: The 50% methanol extracts of T. arjuna, E. cumini, and A. marmelos at a concentrations 50–500 μg/mL showed maximum percentage inhibition on amylase activity with IC50 values of 302 ± 0.55, 632 ± 0.21, and 503 ± 0.28 μg/mL, respectively. However, the 100% methanol extracts of all the three plants showed the least inhibitory activity.
Discussion and conclusion: The results show that T. arjuna > E. cumini > A. marmelos have excellent inhibitory activity and, therefore, might be effective in lowering postprandial hyperglycemia.
Introduction
Diabetes is one of the most challenging diseases. Diabetes has been estimated to affect 177 million people worldwide, and this figure is projected to increase to 300 million by 2025 (CitationPorter & Barrett, 2005). Diabetes is caused by decreased secretion or increased resistance of insulin resulting in high postprandial glucose levels. Inhibition of enzymes involved in the digestion of carbohydrates can significantly decrease the postprandial hyperglycemia (CitationAli et al., 2006). Hence, the attractive targets like in vitro inhibition of α-glucosidases are currently in vogue. Synthetic enzyme inhibitors such as carbose, miglitol, and voglibose are prescribed to type 2 diabetic patients, but these drugs have certain adverse effects such as hyperglycemia at higher doses, liver problems, lactic acidosis, and diarrhea. Since medicinal plants have long been used for the ethnomedical treatment of diabetes in various systems of medicine, therefore, natural enzyme inhibitors from plant sources can be an important strategy in the management of postprandial blood glucose level.
Terminalia arjuna Roxb. (Combretaceae), commonly known as Arjuna, is traditionally used for several medicinal purposes in India. The bark is antidysenteric, antipyretic, astringent, cardiotonic, lithotriptic, and tonic, while the powder of the bark acts as a diuretic in cirrhosis of liver and gives relief in symptomatic hypertension (CitationChatterjee, 1994). The bark powder is also given with honey in fractures and contusions with echymosis. Furthermore, the extract of the bark, as an astringent, is used for cleaning sores, ulcers, and cancers, and so on. The active constituents of T. arjuna include tannins, triterpenoid saponins (arjunolic acid, arjunic acid, arjungenin, arjunglycosides), flavonoids (arjunone, arjunolone, luteolin), gallic acid, oligomeric proanthocyanidins, polyphenols, calcium, magnesium, zinc, and copper (CitationMiller, 1998).
Eugenia cumini Skeels (Myrtaceae) is a large evergreen tree valued in Ayurveda and Unani systems of medication for possessing variety of therapeutic properties. E. cumini is reported to have hypolipidemic effect; it reduces blood cholesterol, triglycerides, and free fatty acids (CitationSagrawat et al., 2006). This effect was reported to be due to presence of flavonoids, saponins, and glycoside in the extract, which in turn decreased the activity of enzyme 3-HMG Co-A reductase in liver, responsible for cholesterol biosynthesis (CitationRavi et al., 2005). E. cumini seed apart from hypoglycemic activity has been reported to have anti-inflammatory (CitationChaudhuri et al., 1990), neuropsycho-pharmacological (Chakrabarty et al., 1985), antibacterial (CitationBhuiyan et al., 1996), and anti-HIV (CitationKusumoto et al., 1995) effects. EJ seed kernel decreased the oxidative stress in diabetic rats, which in turn may be due to its hypoglycemic property. Most of the plant parts of E. cumini have various phytoconstituents such as glycosides (jamboline), ellagic acid, gallic acid, tannins, fatty oil, steroids, flavonoids, triterpenes, phenolics, monoterpenoids, minerals, proteins, calcium, and vitamins (CitationKamble et al., 2009).
Aegle marmelos (Rutaceae), an indigenous plant of India, is commonly known as bael in Hindi. Several studies on different parts of A. marmelos showed that the plant possess antidiarrheal (CitationShobha, 2001), antidiabetic (CitationKamalakkannan & Prince, 2003), anticancer (CitationLotufu et al., 2005), radioprotective (CitationJagetia et al., 2004), antifungal (CitationRana & Jain, 1997), antimicrobial (CitationMazumder et al., 2006), antimicrofilarial (CitationSahare et al., 1981), anti-inflammatory, antipyretic, and analgesic activities (CitationVeerappan et al., 2005). Phytochemical analyses of leaves have shown to contain several bioactive compounds including essential oils (e.g., marmenol, β-caryophyllene, α-humulene), triterpenoids (e.g., lupeol, β- and γ-sitosterol), flavonoid (e.g., rutin), alkaloids (e.g. rutacin, aegeline, aegelinine), coumarins (e.g., marmesinin, umbeliferone), condensed tannins, anthocyanins, and flavonoid glycosides (CitationVeerappan et al., 2005).
Therefore, the aim of this study was to investigate the anti-α-amylase potential of aqueous, 50%, and 100% methanol extracts of bark of T. arjuna, seeds of E. cumini, and leaves of A. marmelos.
Materials and methods
Chemicals and reagents
Pancreatic α-amylase and 3,5 dinitrosalisylic acid were purchased from Sisco Research Laboratory, Mumbai, India. All the other chemicals and reagents used in this study were of extra pure analytical grade.
Plant material
The plant materials were collected from local market of Ahmedabad, India in the month of August 2009 and were identified as bark of T. arjuna, seeds of E. cumini, and leaves of A. marmelos; voucher specimens were deposited at Department of Botany, Gujarat University, Ahmedabad, India.
Extract preparation
The extract was prepared according to World Health Organization protocol CG-06 (1983). The powdered sample (5 g) was extracted with 100 mL of distilled water (aqueous), 50% hydro-alcoholic, and 100% alcoholic solvent for 3 h. The extract was then twice filtered with Whatman filter paper No. 1 and then dried in incubator and stored in an airtight container at 4°C untill use. The yields (w/w) of the dried extracts were calculated.
Assay of α-amylase inhibitory activity
The α-amylase inhibitory activity for each extract was determined based on the spectrophotometric assay (CitationNickavar et al., 2008). The starch solution (0.5% w/v) was prepared in distilled water. The enzyme solution (amylase) was prepared in 20mM sodium phosphate buffer (pH 6.9) containing 6.7mM sodium chloride. The aqueous and 50% methanol extracts were dissolved in distilled water and 100% methanol extracts were dissolved in DMSO to give concentrations from 50 to 500 μg/mL (50, 100, 200, 300, 400, and 500 μg/mL). The plant extract (1 mL) and 1 mL of enzyme solution were incubated with 1 mL of starch solution at 25°C for 30 min. Then, 1 mL of coloring agent (3,5 dinitrosalicylic acid) was added and the reaction mixture was incubated into an 85°C water bath for 15 min. The absorbance was recorded at 540 nm spectrophotometrically. Plant extract was replaced in control tubes with distilled water or DMSO. Percent inhibition was calculated as follows:
Statistical analysis
The results are expressed as mean ± standard error of the mean (SEM). Statistical analysis and linear regression analysis were performed using GraphPad Instat, software, version 5.0. The values were analyzed by one-way Analysis of Variance followed by Tukey multiple comparison test at a significance level of p < 0.05.
Results
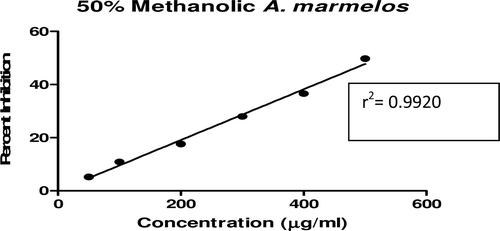
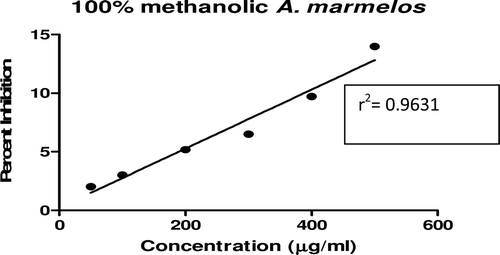
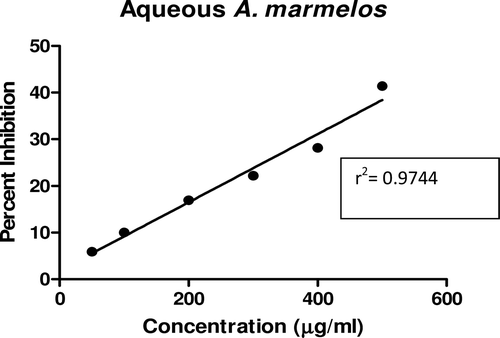
In this study, an in vitro inhibitory effect of different extracts of T. arjuna, E. cumini, and A. marmelos at graded concentrations of 50, 100, 200, 300, 400, and 500 µg/mL on pancreatic amylase activities were evaluated. The percentage yield of each extract and IC50 displayed by each extract are shown in . In the case of T. arjuna, the maximum yield are shown by 50% methanol extract followed by aqueous and minimum by 100% methanol extract, whereas in the case of A. marmelos and E. cumini aqueous extracts, the maximum yield followed by 50% and 100% methanol extracts. The aqueous extract of T. arjuna and A. marmelos showed IC50 values of 592 ± 0.34 and 648 ± 0.18 μg/mL, respectively, followed by E. cumini aqueous extract with an IC50 value of 898 ± 0.46 μg/mL (). The aqueous extracts showed significant dose-dependent inhibition (p < 0.01) of the enzyme with the inhibitory activities ranging from 6%−47%, 2%−30%, and 5%−41%, respectively, for T. arjuna, E. cumini, and A. marmelos. As compared with control, the 50% methanol extract of T. arjuna showed a significant increase (p < 0.001) in amylase inhibitory activity with an IC50 value of 302 ± 0.55 μg/mL (), followed by 50% methanol extracts of A. marmelos with an IC50 value of 503 ± 0.28 μg/mL (), and comparatively less potent E. cumini with an IC50 value of 632 ± 0.21 μg/mL (), again in a dose-dependent manner. The inhibitory activities of 50% methanol extracts of T. arjuna, E. cumini, and A. marmelos ranged between 15%–86%, 4%–41%, and 5%–50%, respectively. However, 100% methanol extracts produced a weak enzyme inhibition and they did not achieve 50% inhibition of the enzyme activity. The IC50 values of 100% methanol extracts of T. arjuna, E. cumini, and A. marmelos are 1503 ± 0.7, 1680 ± 0.48, and 2168 ± 0.87 μg/mL () and inhibitory activities ranged from 1%–18%, 2%–13%, and 2%–14% (). The coefficient of regression r2 was obtained by linear regression. All results exhibited coefficient of regression r2 > 0.9 (p < 0.01) (). An enzyme is usually protein in nature and needs nonprotein components or co-factors, which may affect the enzyme in several ways.
Table 1. Percentage yields of extracts (%W/W) and IC50 values of plant species investigated.
Figure 1. Effect of T. arjuna extracts on α-amylase activity. Values are means ± SEM. Values are significantly different from control as well as within the groups at p < 0.001.
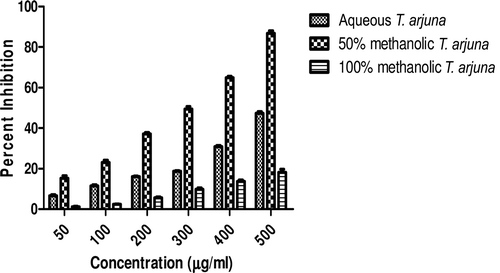
Figure 2. Effect of E. cumini extracts on α-amylase activity. Values are means ± SEM. Values are significantly different from control as well as within the groups at p < 0.01.
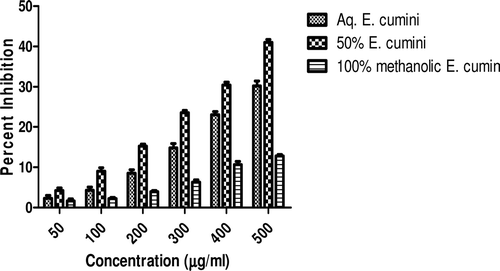
Figure 3. Effect of A. marmelos extracts on α-amylase activity. Values are means ± SEM. Values are significantly different from control as well as within the groups at p < 0.01.
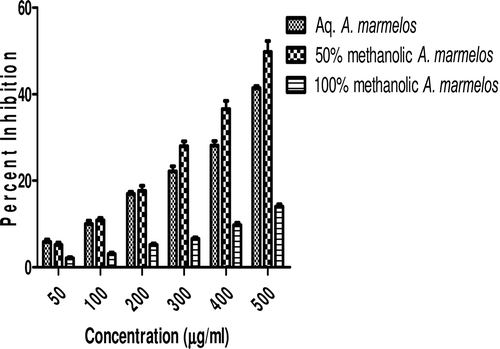
Figure 4. Comparative effect of most potent 50% methanolic extracts on α-amylase activity. Values are means ± SEM. Values are significantly different from control as well as within the groups at p < 0.01.
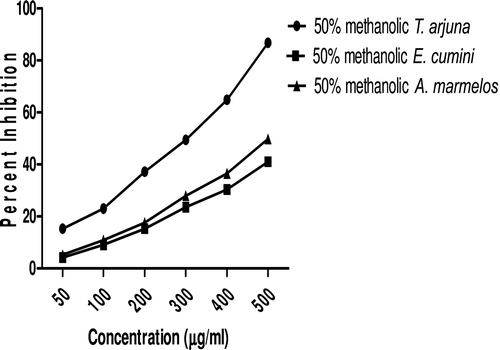
Figure 5. Linear regression curve of percent inhibition of α-amylase at concentrations of aqueous T. arjuna extract.
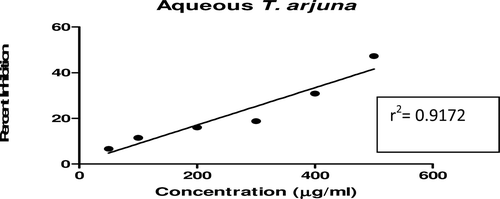
Figure 6. Linear regression curve of percent inhibition of α-amylase at concentrations of 50% methanolic T. arjuna extract.
Figure 7. Linear regression curve of percent inhibition of α-amylase at concentrations of 100% methanolic T. arjuna extract.
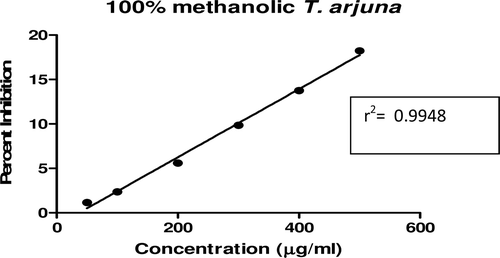
Figure 8. Linear regression curve of percent inhibition of α-amylase at concentrations of aqueous E. cumini extract.
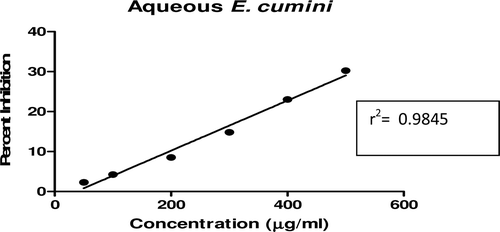
Figure 9. Linear regression curve of percent inhibition of α-amylase at concentrations of 50% methanolic E. cumini extract.
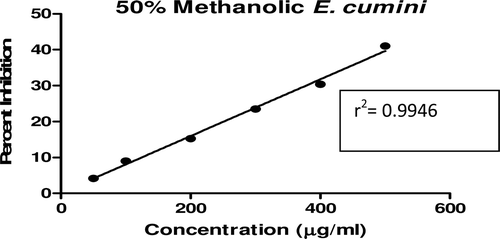
Figure 10. Linear regression curve of percent inhibition of α-amylase at concentrations of 100% methanolic E. cumini extract.
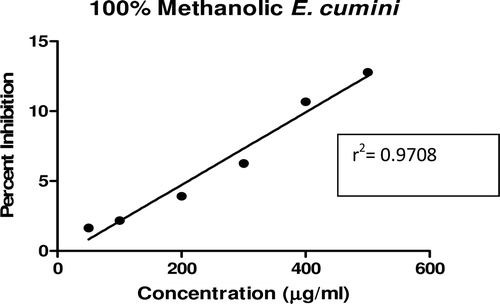
Figure 11. Linear regression curve of percent inhibition of α-amylase at concentrations of aqueous A. marmelos extract.
Discussion
Many herbal extracts have been reported to have antidiabetic activities and are used in Ayurveda for the treatment of diabetes. Natural α-amylase inhibitors from food-grade herbal sources offer an attractive therapeutic approach to the treatment of postprandial hyperglycemia by decreasing glucose release from starch and may have potential for use in the treatment of diabetes mellitus and obesity. Many natural products have been investigated with respect to suppression of glucose production from carbohydrates in the gut or glucose absorption from the intestine (CitationMatsui et al., 2007). α-Amylase catalyses the rate-limiting hydrolysis of 1,4-glucosidic linkages of starch, glycogen, and various oligosaccharides into simple sugars, which can be readily available for the intestinal absorption. Postprandial glucose peaks may be attenuated by delayed glucose absorption. Inhibition of α-amylase enzyme in the digestive tract of human is being considered to be effective in controlling diabetes by decreasing the absorption of glucose from starch (CitationHara & Honda, 1990). In this study of all the extracts of the plants tested, 50% methanol extracts of all the three plants found to possess favorable inhibitory effects on starch breakdown in vitro. A dose-dependent inhibition of pancreatic amylase was observed by all the three plants. In the presence of 50% methanol extract of T. arjuna, the α-(1,4) linkage breakdown was reduced significantly, which might be due to the presence of flavonoids that are known to inhibit glucose transporter of small intestinal epithelial cells (CitationKim et al., 2000). The difference in structure of flavonoids contained in tested extracts may therefore explain the differences in inhibitory activity obtained, which could also be a result of the presence of different concentrations and types of phenolic compounds.
Declaration of interest
The authors report no conflicts of interest.
References
- Ali H, Houghton PJ, Soumyanath A. (2006). α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J Ethnopharmacol, 107, 449–455.
- Bhuiyan MA, Mia MY, Rashid MA. (1996). Antibacterial principles of the seeds of Eugenia jambolana. Bangladesh J Biol, 25, 239–241.
- Chakraborty D, Mahapatra PK, Chaudhuri AK. (1986). A neuropsychopharmacological study of Syzygium cuminii. Planta Med, 2, 139–143.
- Chatterjee A. (1994). The Treatise on Indian Medicinal Plants. Vol. III. Publication and Information Directorate. Council of Scientific and Industrial Research. New Delhi.
- Chaudhuri N, Pal AK, Gomes S, Bhattacharya A. (1990). Anti-inflammatory and related action of Syzygium cumini seed extract. Phytother Res, 4, 5–10.
- Costa-Lotufo LV, Khan MT, Ather A, Wilke DV, Jimenez PC, Pessoa C, de Moraes ME, de Moraes MO. (2005). Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacol, 99, 21–30.
- Hara Y, Honda M. (1990). The inhibition of α-amylase by tea polypphenols. Agricul and Biol Chem, 54, 1939–1945.
- Jagetia GC, Venkatesh P, Baliga MS. (2004). Evaluation of the radioprotective effect of bael leaf (Aegle marmelos) extract in mice. Int J Radiat Biol, 80, 281–290.
- Kamalakkannan N, Prince PS. (2003). Hypoglycaemic effect of water extracts of Aegle marmelos fruits in streptozotocin diabetic rats. J Ethnopharmacol, 87, 207–210.
- Kamble SS, Varsha M, Jadhav V, Kadam J. (2009). Development and validation of HPTLC method to detect 3-hydroxy androstane [16,17-C](6 methyl, 2-1-hydroxy isopropene-1-YL) 4,5,6 H pyran in polyherbal formulation. Int J Pharm Res Dev, 7, 1–9.
- Kim JS, Kwon CS, Son KH. (2000). Inhibition of α-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem, 64, 2458–2461.
- Kusumoto IT, Nakabayashi T, Kida H, Miyashira H, Hattari H, Namba T, Shimotohno K. (1995). Screening of various plant extracts used in Ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-I) protease. Phytother Res, 12, 488–493.
- Matsui T, Tanaka T, Tamura S, Toshima A, Tamaya K, Miyata Y, Tanaka K, Matsumoto K. (2007). α-Glucosidase inhibitory profile of catechins and theaflavins. J Agric Food Chem, 55, 99–105.
- Mazumder S, Bhattacharya A, Majumder AK, Pattnaik P, Tiwari M, Chaudhary S. (2006). Anti bacterial evaluation of Aegle marmelos (Correa) Linn. root extract. Phytother Res, 20, 82–84.
- Miller EL. (1998). Botanical influences on cardiovascular disease. Alt Med Rev, 3, 422–431.
- Nickavar B, Abolhasani L, Izadpanah H. (2008). α-Amylase inhibitory activities of six Salvia species. Iran J Pharm Res, 7, 297–303.
- Porter JR, Barrett TG. (2005). Monogenic syndromes of abnormal glucose homeostasis: Clinical review and relevance to the understanding of the pathology of insulin resistance and β cell failure. J Med Genet, 42, 893–902.
- Rana BK, Jain AK. (1997). Evaluation of anti-fungal activity of essential oil isolated from leaves of the A. marmelos. J Ethnopharmacol, 57, 29–37.
- Ravi K, Rajasekaran S, Subramanian S. (2005). Antihyperlipidemic effect of Eugenia jambolana seed kernel on streptozotocin-induced diabetes in rats. Food Chem Toxicol, 43, 1433–1439.
- Sahare KN, Anandhraman V, Meshram VG, et al. (1981). Antimicrofilarial activity of methanol extract of Vitex negundo and Aegle marmelos and their phytochemical analysis. Indian J Exp Biol, 3, 245–249.
- Sagrawat H, Mann AS, Kharya MD. (2006). Pharmacological potential of Eugenia jambolana: A review. Pharmacog Magazine, 2, 96–105.
- Shoba FG, Thomas M. (2001). Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol, 76, 73–76.
- Veerappan A, Shigeru M, Rengnathan D. (2005). Studies on the anti-inflammatory, antipyretic and analgesic properties of the leaves of Aegle marmelos Corr. J Ethnopharmacol, 96, 159–163.