Abstract
Context: Studies have shown that pomegranate, Punica granatum Linn. (Lythraceae), has remarkable biological and medicinal properties.
Objective: This work aimed to explore and compare the analgesic and anti-inflammatory activities of the methanol extract (MoE) obtained from fruit peels of two varieties of pomegranate: Amrouz (MoEA) and Sefri (MoES).
Materials and methods: Antinociceptive activity of MoEA and MoES was examined using four models of pain. The extracts were administered by the intraperitoneal route (i.p.) in writhing (50, 100 and 150 mg/kg) and formalin tests (25, 50 and 100 mg/kg) and by intra-cerebroventricular injection (i.c.v.) in hotplate and tail-immersion tests (10, 25 and 50 µg/3 µl/rat). anti-inflammatory activity was studied using the hind paw egg albumin test (50, 100 and 150 mg/kg, i.p.).
Results: In the writhing test, the index of pain inhibition (IPI) was 52% for MoEA (150 mg/kg, i.p.) and 29% for MoES (150 mg/kg, i.p.). In the formalin test, the IPI of early and late phase were, respectively, 75% and 82% for MoEA (100 mg/kg, i.p.) and 8% and 63% for MoES (100 mg/kg, i.p.). In the hotplate and tail-immersion test, MoEA and MoES increased in a dosedependent manner the reaction latency to the thermal stimuli. MoEA seems to be more potent than MoES. Only the analgesic effect of MoEA was partially inhibited by pretreatment with naloxone. Both extracts exerted a significant anti-inflammatory effect.
Discussion and conclusions: The results demonstrated that P. granatum contains active constituents, which possess antinociceptive and anti-inflammatory activity, justifying its popular uses.
Introduction
Punica granatum Linn. (Lythraceae), the pomegranate plant, inherently develops numerous trunks. In orchards, plants are normally trained to a single trunk, forming a large shrub or small tree, and reaching a height of 12 to 20 ft at maturity. P. granatum has a deep association with the cultures of the Mediterranean region and Near East, where it is savored as a delicacy and is an important dietary component (CitationPolunin & Huxley, 1987). Additionally, almost all parts of this plant are used in traditional medicine for the treatment of various ailments (CitationLansky & Newman, 2007). In Moroccan medicine, pomegranate fruit peels, flowers and leaves have been used to treat mild pyrexia, visceral pain, gastritis and diarrhea (CitationBellakhdar, 1997; CitationJafri et al., 2000). The methanol extract of P. granatum seed and dried peels have antidiarrheal activity (CitationDas et al., 1999) and the rind extract has been shown to have gastroprotective activity through an antioxidant mechanism (CitationAjaikumar et al., 2005).
Despite the relatively wide use of this plant in popular medicine in Morocco, there is a lack of reports about the Moroccan varieties of pomegranate. CitationOukabli (2004) have reported that Moroccan clones of P. granatum carry different names, allocated according to fruit shape (Ounk Hmam), the region (Bzou, Gjebali), or the color of berry skin (Sefri, yellow or red pomegranate). Their characteristics remain relatively close to other foreign varieties (Roja in Spain, Hicaznar in Turkey and Jolore seedless in India) from the point of view of the color of the skin and seeds. The differences, concern some pomological traits, in particular the gustative quality and the texture of the berries, which determine the quality of fruits.
In recent years, several investigators have attempted to unravel the underlying mechanisms of beneficial effects of pomegranate. These investigations have focused mainly on the antioxidant, anti-inflammatory, and antibacterial potentials of pomegranate (CitationAviram et al., 2000, Citation2002, Citation2004; CitationCerda et al., 2003a,Citationb; Citationde Nigris et al., 2007). Pomegranate juice and fruit extracts normalized to punicalagins and a total pomegranate tannin fraction (TPT) from the fruit have been shown to inhibit the proliferation of human cancer cells and modulate inflammatory subcellular signaling pathways (CitationAfaq et al., 2005; CitationSeeram et al., 2005; CitationAdams et al., 2006). The effects of pomegranate juice and extracts on molecular and cellular mechanisms has been researched on a systemic level where benefits for cardiovascular, prostate, dental, and metabolic health have been shown in the clinical literature (CitationAviram et al., 2000, Citation2002, Citation2004; CitationEsmaillzadeh et al., 2006; CitationMenezes et al., 2006; CitationPantuck et al., 2006).
Several types of phytochemicals have been identified in the different parts of P. granatum; amongst them are the polyphenols, which are the predominating class of bioactive compounds. Pomegranate polyphenols include flavonoids (flavonols, flavanols and anthocyanins) (CitationSantagati et al., 1984; CitationHernandez et al., 1999; Citationvan Elswijk, 2004), tannins (proanthocyanidins) and hydrolysable tannins (ellagitannins and gallotannins) (CitationTanaka et al., 1986; CitationSatomi et al., 1993; CitationHussein et al., 1997; CitationMavlyanov et al., 1997). The flowers also contain triterpenoids (CitationBatta & Rangaswami, 1973; CitationHuang et al., 2005), and alkaloids were identified from the bark of tree and root (CitationNeuhofer et al., 1993).
Two varieties of P. granatum will be discussed in this study: variety A and variety S. Variety S, known as “Sefri”, is widespread in the region of Marrakech, Morocco. Its fruit has a standard size and a sweet taste (CitationOukabli, 2004). Variety A is the most commonly used by herbalists and is known as “Amrouz”. It is well recognized for its remedial properties. Compared to variety S, variety A produces small size, highly acidic fruits with a thick rind. No previous studies have been done on the variety A and its biological properties.
The ethno-medicinal use of pomegranate fruit peel suggests that it has analgesic and anti-inflammatory properties. To verify these assumptions, several experimental models were conducted on the fruit peel methanol extract of the variety A (MoEA) and the variety S (MoES).
Materials and methods
Plant material
Fresh pomegranates (of both varieties) were collected from an orchard in the region of Sidi Rahal near Marrakech, in the fall of 2008. Voucher samples representing Amrouz (No.6291) and Sefri (No.6290) were identified by Professor A.Ouhammou, a taxonomist from the Faculty of Sciences Semlalia and were deposited in the Faculty of Sciences Semlalia Herbarium, Marrakech, Morocco.
Extraction and phytochemical analysis
Dried pomegranate peels (200 g) were powdered and extracted with analytical grade MeOH in a Soxhlet apparatus for 12 h; the extract was concentrated to dryness under reduced pressure at 40 ± 5°C until it became a brownish solid residue. The extraction yield (w/w) was 38% for MoEA and 30% for MoES. Extracts were stored as dried powder at 4°C.
Preliminary phytochemical analysis of MoEA and MoES implicated qualitative determinations of the following classes of phytoconstituents: alkaloids, flavonoids, tannins, anthocyanes, sterols and/or terpenes, quinons and saponins (CitationFarouk et al., 2008).
Drugs and treatment
For the intraperitoneal injection (i.p.), MoEA and MoES (25, 50, 100 and 150 mg/kg, 10 ml/kg b.w.) were dissolved in the vehicle (saline) and administered to the animals, 30 min before the procedure. For the intracerebroventricular injection (i.c.v.), filtered and stabilized stock solutions (through a filter membrane of 0.22 µm) were used to prepare clear aliquots of MoEA and MoES. The extracts were given to rats, 5 min before the experiment, at the doses of 10, 25 and 50 µg/rat in the volume of 3 µl of saline. The vehicle at the same volume was injected into the control group. Morphine sulfate (5 mg/kg; Actiskenan®) and dl-lysine acetylsalicylates (100 mg/kg; Aspegic®) were used as the reference agents. In order to investigate possible involvement of opioid system in the antinociceptive effect of MoEA and MoES, rats were pre-treated with the non-selective opioid receptor antagonist, naloxone chlorhydrate (1 mg/kg; Narcan®) 15 min before the administration of the extracts.
Animals
Adult Wistar rats (190–260 g) and albino mice (25–30 g) of both sexes were used. The animals were supplied by the Animal Care Facility of the Faculty of Sciences Semlalia, Cadi Ayyad University, Marrakech, Morocco. They were group-housed in stainless steel cages (males separated from females) and kept under standard environmental conditions (25 ± 2°C; 12/12 h light/dark cycle); food and water were given ad libitum. All animals were allowed to acclimatize to the laboratory environment for 1 week before being subjected to experiments.
The experiments were performed following the guidelines set for the International Association for the Study of Pain (CitationZimmerman, 1983) and the National Institute of Health “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 85–23, 1985) regarding the care and treatment of experimental animals.
Surgical preparation and technique of intracerebroventricular injection
Before they were scheduled to undergo the surgical cannula implantation, the animals were handled for 2 weeks to reduce stress and discomfort levels.
Just before the surgery, rats were anesthetized with ketamine (1.7 mg/kg), than they were stereotaxically implanted with a unilateral guide stainless steel cannula (26 G). Cannulae were positioned at 0.65 mm above the lateral ventricle (coordinates: 1.3 mm posterior to bregma, lateral 1.6 mm from midline, deep 3.2 mm from dura) and were fixed to the skull using jeweler’s screws and dental acrylic. In addition, dummy cannulae were inserted into the guide cannulae to prevent them from clogging. The animals were individually housed and allowed to recover for at least 10 days between the surgery and the testing. During this time, the dummy cannulae were changed daily to keep the guide cannulae clear and to accustom the rats to the handling.
On the day of the experiment, an injection cannula was attached to a 30 cm polyethylene tube, fitted to a 10 µl Hamilton microsyringe. Then, the rat was restrained by hand, and the injection cannula (0.15 mm inner diameter) was introduced into the guide cannula. The volume of the solutions injected into lateral ventricle was 3 µl and the injection was made over a period of 30 s. One specific group of rats was assigned to one specific drug treatment condition and each group comprised six rats. No mortality was observed in i.c.v. treated animals, and all of them displayed normal feeding and drinking behaviors postoperatively. Rats showing neurological deficits were not studied.
After experiments, all rats were sacrificed with 1 ml of sodium pentobarbital (70 mg/ml, i.p.) and then intracardially perfused with 0.9% saline followed by a 10% formaldehyde solution. The brains were removed and placed in formaldehyde (10%) solution for 2 days, and cut into 80 µm slices. Localization of the cannulae tips was determined according to the atlas of CitationPaxinos and Watson (1986). Data from rats with an incorrect placement of the cannula were excluded from analysis.
Acute toxicity
To identify the limit dose to be used for an acute treatment, intraperitoneal LD50 of the extracts were calculated for rats and mice. The test consists of a single dose injection (i.p.) of the extract dissolved in saline. The animals were than observed twice daily during the first 12 h for mortality and for any abnormal physical or behavioral changes.
The doses used for the rats were: 0, 100, 200, 300 and 400 mg/kg; for mice, the doses were 0, 100, 150, 200, 250, 300 and 350 mg/kg. Dosing volume was 10 ml/kg. Observation continued for 14 days to verify that the number of animals per dose that remained alive did not change. Animals were always allowed food and water ad libitum. Values for LD50 were calculated by means of the method of Leitchfield and Wilcoxon (1949).
Acetic acid-induced writhing test
The method described by CitationCollier et al. (1968) was used on albino mice of either sex. The writhing was elicited by an intraperitoneal injection of 0.7% acetic acid at the dose of 0.1 ml/kg body weight).
Eight groups of mice (n = 6) were used. Saline (control), MoEA or MoES (50, 100 and 150 mg/kg, i.p.) and the acetylsalicylic acid (100 mg/kg, i.p.) were given to animals (single dose injection, 10 ml/kg, i.p.) 30 min before acetic acid and the number of writhes was recorded during a 20 min period. The index of pain inhibition (IPI) was calculated as follows:
Xo is the number of writhes observed in control group. Xi the number of writhes in tested groups (MoEA, MoES, or acetylsalicylic acid).
Formalin test
The method used in the present study was similar to that described previously by Citationde Miranda et al. (2001) with slight changes. Before testing, the mice were placed individually for 5 min in a transparent enclosure (testing box) and were free to explore and habituate to this environment (habituation phase). Next, formaldehyde (20 µl of 2%) was injected subcutaneously under the plantar surface of the right posterior paw and immediately replaced into the testing box.
The time spent by animals licking or biting the injected paw was timed with a chronometer and was considered indicative of pain. Two mice (control and treated) were simultaneously observed from 0 up to 30 min after formalin injection. The initial nociceptive scores normally should peak at 5 min (first phase, representing the neurogenic pain) and at 15–30 min (second phase, representing inflammatory pain) after formalin injection (CitationHunskaar & Hole, 1987).
Twelve groups of mice (n = 6) were used, and 30 min before the formalin injection, animals were treated (10 ml/kg, i.p.) with a single dose of: saline (control), MoEA and MoES (50, 100 and 150 mg/kg, i.p.). Reference groups were treated with morphine (5 mg/kg, i.p.) or with acetylsalicylic acid (100 mg/kg, i.p.).
To investigate the possible involvement of opioidergic system, naloxone (1 mg/kg, s.c.) was administrated 15 min before injection of the extracts (150 mg/kg, i.p.) and morphine (5 mg/kg, i.p.). The index of pain inhibition was calculated as follows:
Xo is the pain score for control group. Xi is the pain score of tested groups (MoEA, MoES or reference drugs).
Tail-immersion test
The posterior half of the rat’s tail was dropped into a container filled with hot water maintained at a constant temperature of 55°C. The latency to tail withdrawal was recorded (using a chronometer) before the injection (baseline) and repeated at 10, 20, 30, 40 and 60 min after the injection. The cut-off time for the response was set at 20 s to avoid tissue damage to the tail of rats (CitationPalanichamy & Nagarajan, 1990; CitationChakraborty et al., 2004).
Eleven groups of stereotaxically implanted rats (n = 6) were used. Five min before testing, animals received a single i.c.v. injection of: MoEA (10, 25, and 50 µg/3 µl/rat), MoES (10, 25, 50 µg/3 µl/rat) and saline (control). The reference group was treated with a single injection of morphine (5 mg/kg, s.c.).
To investigate the possible involvement of opioidergic system, naloxone (1 mg/kg, s.c.) was administrated 15 min before administration of morphine (2 mg/kg, s.c.) or the extracts (50 µg/3 µl/rat, i.c.v.).
Hotplate test
The heated surface of a hotplate analgesia meter (Ugo Basil, Italy; Socrel DS-37) was maintained at 55 ± 0.2°C. Each animal was placed into a glass cylinder (20 cm diameter) on the heated surface of the plate. Each rat was only time tested twice, before and at 5, 10, 15 or 20 min after i.c.v. injection. The pain threshold is considered to be reached when the animals lift and lick their paws or attempt to jump out of the breaker. To minimize damage to the animal paws, the cut-off time was 20 s (CitationShalheen et al., 2000).
Eight groups of stereotaxically implanted rats (n = 6) were used. They received, 5 min before testing, MoEA or MoES (10, 25 or 50 µg/3 µl/rat), saline (control) by a single i.c.v. injection. The reference group received a single injection of morphine (5 mg/kg, s.c.).
Fresh egg albumin induced-edema
The rats hind paw edema was induced by intra-plantar injection of fresh egg albumin (0.5 ml/kg) as previously described by CitationEkpendu et al. (1994). Acute inflammation of the hind paw was induced by injecting 0.5 ml/kg of fresh egg albumin into the sub-plantar surface of the right hind paw. The increases in the linear diameter of the right hind paw were taken as an indicator of pedal inflammation. Measurement of paw size was carried out by wrapping a piece of cotton thread around the paw, the circumference was determined by measuring the length of the thread using a meter rule (CitationHess & Miloning, 1972; CitationOlajide et al., 2000).
The measurements were done immediately before and after inflammation induction at different intervals. Eight groups of animals were used (n = 6). Saline (control), MoEA or MoES (50, 100 and 150 mg/kg, i.p.) and acetylsalicylic acid (100 mg/kg, i.p.) were given to animals 30 min before the albumin injection. The index of edema inhibition (IEI) was calculated from the formula:
C0 is the average inflammation (circumference of hind paws edema) of the control group. Ct is the average inflammation of a tested group.
Statistical analysis
To appreciate the global effect of drug treatments over the total testing period in the hotplate, tail-immersion and albumin induced-edema tests; the areas under the curve (AUC) were calculated (using the trapezoidal rule). Data were represented as mean ± standard error of mean (SEM). The results were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test for post hoc comparisons between the groups. The statistical analyses were carried out using SPSS-15.0 software (SPSS Inc., Chicago, IL). Values with p < 0.05 were considered significant.
Results
Phytochemical analysis ()
Preliminary phytochemical analysis of MoEA and MoES revealed the presence of anthocyanes, flavonoids, tannins and saponosides. Dragendorff’s assay, which detects the presence of alkaloids, shows a positive result for MoEA and a negative result for MoES.
Table 1. Lists of chemical compound found in MoEA and MoES by the phytochemical analysis.
Acute toxicity
The acute toxicity studies were performed following intraperitoneal injection of pomegranate extracts. The LD50 of the extract in Wistar rats was determined as 320.5 mg/kg for MoEA and 355.8 mg/kg for MoES. In albino mice, the intraperitoneal LD50 was determined as 300 mg/kg and 348.2 mg/kg for MoEA and MoES, respectively.
Acetic acid-induced writhing test
The results in showed that the MoEA and MoES (50, 100 and 150 mg/kg, i.p.) reduced in a dose dependent manner the number of writes induced by acetic acid [F7, 40 = 30.84; p < 0.001]. IPI were 52% (p < 0.001) for MoEA (150 mg/kg, i.p.) and 29% (p < 0.01) for MoES (150 mg/kg, i.p.). Acetylsalicylic acid (100 mg/kg, i.p.) exhibited 68% (p < 0.001) of pain inhibition. The comparison by Tukey’s test showed that at equal dose, MoEA was more active than MoES (p < 0.01).
Table 2. The antinociceptive effect (i.p.) of P.granatum extracts and acetylsalicylic acid on acid acetic-induced visceral pain in mice.
Formalin test ()
Intraplantar injection of 2% formalin produced a typical reaction of biphasic licking. The treatment with MoEA and MoES (25, 50 and 100 mg/kg, i.p.) reduced significantly the formalin nociceptive response [F11, 60 (early phase) = 29.7, (late phase) = 60.2; p < 0.001]. MoEA (50 and 100 mg/kg, i.p.) reduced the duration of licking (p < 0.001) in the early (61 and 75% of pain inhibition respectively) and late phase (74 and 82% of pain inhibition respectively). MoES (50 and 100 mg/kg, i.p.), showed significant pain inhibition (44 and 63%, respectively, p < 0.01) in the late phase, but not in the early phase (5.2 and 8.9%, respectively, p > 0.05). Tukey’s multiple range test revealed that, at equal dose, MoEA scored higher than MoES in the early phase (p < 0.001) and the late phase (p < 0.05). Morphine (5 mg/kg, s.c.) caused marked pain inhibition (p < 0.001) in the early phase (80.2%) and the late phase (90.3%). Naloxone reversed, very significantly, the analgesic effect of MoEA (100 mg/kg, i.p.) only in the early phase (p < 0.001). The analgesic effect of MoES (100 mg/kg, i.p.) was not affected by this pretreatment (p > 0.05). The effect of morphine (5 mg/kg, i.p.) was reversed in both phases (p < 0.001).
Table 3. The antinociceptive effect of P.granatum extracts, morphine, acetylsalicylic acid and reversal effect of naloxone on formalin-induced pain in mice.
Tail-immersion test
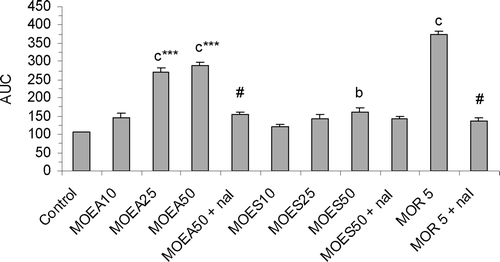
The different pretreatments decreased significantly the response to the heat stimulus [F10, 55 = 84.54; p < 0.001] (), and both extracts have increased, in a dose- dependent manner, the reaction latency. Compared to control, the effect of MoEA (25 and 50 µg/3 µl/rat, i.c.v.) over the total period of 60 min was highly significant (p < 0.001), and the effect of MoES (50 µg/3 µl/rat, i.c.v.) was significant (p < 0.01). MoEA (25 and 50 µg/3 µl/rat, i.c.v.) was more potent than MoES, when administrated at the same doses (p < 0.001). Naloxone, significantly (p < 0.001) reversed the effect of MoEA (50 µg/3 µl/rat, i.c.v.) and morphine (5 mg/kg, s.c.), while the analgesic activity of MoES (50 µg/3 µl/rat, i.c.v.) was not affected by this pretreatment (p > 0.05) ().
Figure 1. Effect of P.granatum extracts (10, 25 and 50 µg/3 µl/rat, i.c.v.), morphine (5 mg/kg, s.c.) and reversal effect of naloxone (1 mg/kg, s.c.) on tail-immersion test, as revealed by change of the estimated areas under the curves (AUC). Values are represented as the mean ± SEM (n = 6). Differences between groups were statistically analyzed by ANOVA followed by Tukey’s multiple range test. bp < 0.01, cp < 0.001 vs. control (saline). ***p < 0.001 vs. the group receiving the same dose of MoES. #p < 0.001 vs. the group receiving the appropriated drug at the same dose without naloxone. MoEA: fruit peel methanol extract of the variety A; MoES: fruit peel methanol extract of the variety S; nal: naloxone; MOR 5: morphine (5 mg/kg, s.c.).
Hotplate test
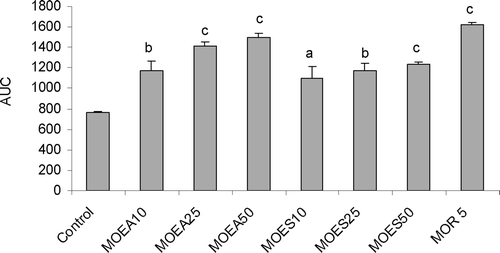
One way ANOVA showed highly significant difference in AUC between control and treated groups [F7, 40 = 17.06; p < 0.001]. Compared to control, MoEA and MoES (25 and 50 µg/3 µl/rat, i.c.v.) have increased, in a dose dependent manner, the reaction latency (). No significant difference was observed between MoEA and MoES groups (p > 0.05). Morphine (5 mg/kg, s.c.), the reference drug, exhibited a highly significant analgesic effect (p < 0.001).
Figure 2. Effect of P.granatum extracts (10, 25 and 50 µg/3 µl/rat, i.c.v.), morphine (5 mg/kg, s.c.) on pain threshold in hotplate test in rats, as revealed by change of the estimated areas under the curves (AUC). Values are represented as the mean ± SEM (n = 6). Differences between groups were statistically analyzed by ANOVA followed by Tukey’s multiple range test. ap < 0.05, bp < 0.01, cp < 0.001 vs. control (saline). MoEA: fruit peel methanol extract of the variety A; MoES: fruit peel methanol extract of the variety S; MOR 5: morphine (5 mg/kg, s.c.).
Fresh egg albumin induced-edema
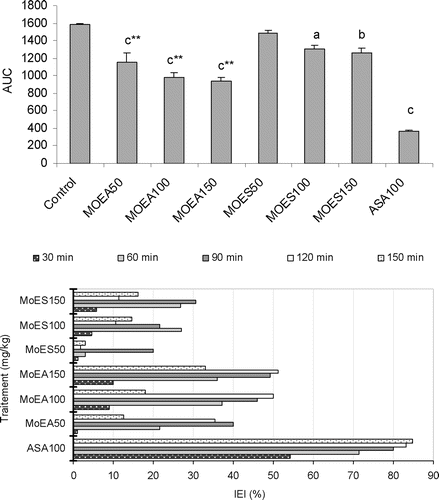
Sub-plantar injections of fresh egg albumin provoked distinct time related and progressive increase in the hind paw circumference of the control group. Pedal edema was always evident within 8 min following the administration of the phlogistic agent. The maximal circumference was observed approximately at 90 min after albumin injection. , summarizes the results obtained in this experiment. The analysis of AUC values revealed that there was a highly significant difference among the groups [F7, 40 = 55.10; p < 0.001]. MoEA (50, 100 and 150 mg/kg, i.p.) showed a highly significant anti-inflammatory activity (p < 0.001), the maximum IEI obtained was 51% with the dose of 150 mg/kg at 120 min after treatment. The effect of MoEA was significant (p < 0.01) at the doses of 150 mg/kg (i.p.), and the highest IEI (30%) was obtained at 90 min after extract administration. MoEA (50, 100 and 150 mg/kg, i.p.) was always more potent than MoES (50, 100 and 150 mg/kg, i.p.) (p < 0.001). Acetylsalicylic acid (100 mg/kg, i.p.) exhibited a highly significant anti-inflammatory activity during all the experiments (p < 0.001), and its effect was evident 30 min after injection (IEI 55%).
Figure 3. Upper panel: The effect of P.granatum extracts (50, 100 and 150 mg/kg, i.p.) and acetylsalicylic acid (100 mg/kg, i.p.) on induced edema in rats, as revealed by change of the estimated areas under the curves (AUC). Values are represented as the mean ± SEM (n = 6). Differences between groups were statistically analyzed by ANOVA followed Tukey’s multiple range test. ap < 0.05, bp < 0.01, cp < 0.001 vs. control (saline). ***p < 0.001 vs. the group receiving the same dose of MoES. Lower panel: the index of edema inhibition (IEI%) at different time intervals.
Discussion
The aim of this study was to evaluate the pharmacological effects of P. granatum fruit peels, which are commonly used in Moroccan folk medicine for its anti-inflammatory and analgesic properties (CitationBellakhdar, 1997). We also focused on making a preliminary comparison between the two varieties, Amrouz is particularly used by herbalists and Sefri is a common variety. To investigate the analgesic and the anti-inflammatory potential of the methanol extract (MoEA and MoES) of P. granatum, five different experiments were conducted. The peripheral analgesic effect of the extracts was tested by using the writhing test. The involvement of central mechanisms was studied by using the hot-plate and tail-immersion tests, known to activate supraspinal nociceptive and spinal nociceptive pathways, respectively (CitationPaulino et al., 2003; CitationBektas & Arslan, 2010). The formalin test was used to investigate both peripheral and central mechanisms (CitationTjolsen et al., 1992). The results clearly show that MoEA and MoES possess evident analgesic potential which suggests the existence of both centrally and peripherally mediated mechanisms.
The extracts MoEA and MoES produced a significant dose-related analgesic effect in writhing test. This test is widely accepted as a model visceral pain (CitationBektas & Arslan, 2010), the abdominal constriction response is induced by the local peritoneal receptors activation by mediators of pain (CitationBentley et al., 1983). Therefore, the analgesic and anti-inflammatory actions of MoEA and MoES seem to be mediated by inhibition of the release of these endogenous nociceptive mediators (CitationReibero et al., 2000).
The formalin test was selected to continue this investigation since it is more precise and allows identifying two distinct phases of nociception. Results showed that the time spent in licking the injured paw was significantly reduced by intraperitoneal administration of MoEA in both phases and only in the late phase for MoES. The early phase is thought to be caused by direct activation of nociceptive neurons by formalin, whereas the late phase reflects pain generated in acutely injured tissue (CitationHunskaar & Hole, 1987). Centrally acting drugs, such as opioids, inhibit both phases of pain by equally involving the effects produced by prostaglandins and endogenous opioids (CitationShibata et al., 1989; CitationJaffe & Martin, 1990). Peripheral acting drugs such as acetylsalicylic acid reduce nociception only in the late phase by inhibiting the inflammatory process (CitationHunskaar & Hole, 1987; CitationRosland et al., 1990). These data suggest that MoEA and MoES exert their analgesic action at both central and peripheral levels. It also implies that the extracts not only possess an antinociceptive activity, but also an anti-inflammatory activity.
The hotplate and tail-immersion tests, respectively, represent spinal and supra-spinal assays. Although both of them are considered as central model that has selectivity for opioid-derived analgesics (CitationJanssen et al., 1963; CitationAbbott & Melzack, 1982), the results show that, morphine (5 mg/kg, s.c.) demonstrated significant antinociceptive effect in the tail-immersion and hotplate tests. Injections (i.c.v.) of MoEA and MoES exert a potent analgesic in these models confirming their central activity. MoES has produced a statistically significant effect, but it was less potent than MoEA. The use of naloxone has partially reversed the antinociceptive effect of MoEA in the first phase, but not in the second phase of the formalin assay. The effect of MoES was not modified by naloxone.
The acute inflammatory assay (albumin paw inflammation) results showed that all the extracts at the tested doses exhibited inhibition of inflammation after 30 min of albumin injection, while acetylsalicylic acid showed significant inhibition of inflammation at all the time intervals studied. Acetylsalicylic acid like the other NSAIDs prevents inflammation by inhibition of cyclooxygenase conversion of arachidonic acid into prostaglandins (CitationToshihiro et al., 2001). Based on data obtained during this study, it was concluded that the antinociceptive effect of MoEA may partially involve the opioid system at the spinal and supraspinal level like related opioid analgesic drugs (CitationReisine & Pasternak, 1996), whereas MoES seems to act mainly like NSAIDs in its analgesic effect. The observed anti inflammatory activity of both extracts may be related to peripheral acting components, that inhibit the release of endogenous inflammatory mediators.
Phytochemical analysis revealed that phenolic compounds such as flavonoids and tannins are present in large amounts in both varieties, whereas the alkaloids are only present in MoEA. Previous reports suggested that plant materials, which contain tannins, alkaloids, flavonoids, and phenolic acids possess analgesic and anti-infammatory effects on experimental animals and these pharmacological effects are resulted from these contents (CitationMills & Bone, 2000; CitationMorteza-Semnani et al., 2006). Recent studies about pomegranate fruit peels have reported that it contains these active compounds (CitationLansky & Newman, 2007). Ellagic acid in particular, was found to possess in vitro anti-inflammatory properties (CitationPanichayupakaranant et al., 2010). On the other hand, there are few reports on the role of tannins in anti-nociceptive and anti-infammatory activities (CitationStarec et al., 1988), whereas flavonoids have demonstrated antinociceptive effect through opioid mechanisms (CitationAnjaneyulu & Chopra, 2003; CitationKatavic et al., 2007).
In all these experiments, the extract of the MoEA was more potent than MoES in all the experiments. Both extracts have virtually the same chemical composition, but phytochemical screening is a qualitative method, and therefore cannot solely be relied on to explain the difference between the two studied varieties. In Morocco, herbalists mainly use Amrouz and most of them consider it to be a wild variety, which contains more active compounds than the cultivated counterparts. The literature survey revealed that there are no scientific studies carried out regarding the North-African varieties of P. granatum, especially those used in traditional medicine. According to CitationSchmidt et al. (2008), the phytochemical composition of many plants has changed over time with domestication. Conventional plant breeding has often reduced the content of bioactive compounds in domesticated plants (CitationNho & Jeffery, 2001; CitationFriedman, 2006). Besides, it is reported that flavonoids and tannins are more abundant in the peels of wild-crafted pomegranates compared to cultivated fruits (CitationOzcal & Dinc, 1993). This assumption may explain the observed difference between the studied varieties.
Conclusion
Based on the results of this study, it can be concluded that MoEA and MoES have both analgesic and anti-inflammatory properties. These findings seem to justify the use of this plant in traditional medicine to manage pain and inflammation. Further investigations are needed to better understand the extracts actions and to find the reasons behind the dissimilarities observed between Amrouz and Sefri varieties.
Acknowledgments
We are very thankful to Pr. A. Ouhamou for helping with the plant specimen’s identification and to Mr. A. Regragui for his technical assistance.
Declaration of interest
The authors report no declarations of interest.
References
- Abbott FV, Melzack R. (1982). Brainstem lesions dissociate neural mechanisms of morphine analgesia in different kinds of pain. Brain Res, 251, 149–155.
- Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. (2006). Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem, 54, 980–985.
- Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. (2005). Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer, 113, 423–433.
- Ajaikumar KB, Asheef M, Babu BH, Padikkala J. (2005). The inhibition of gastric mucosal injury by Punicagranatum L. (pomegranate) methanolic extract. J Ethnopharmacol, 96, 171–176.
- Anjaneyulu M, Chopra K. (2003). Quercetin, a bioflavonoid, attenuates thermal hyperalgesia in a mouse model of diabetic neuropathic pain. Prog Neuropsychopharmacol Biol Psychiatry, 27, 1001–1005.
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B. (2000). Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr, 71, 1062–1076.
- Aviram M, Dornfeld L, Kaplan M, Coleman R, Gaitini D, Nitecki S, Hofman A, Rosenblat M, Volkova N, Presser D, Attias J, Hayek T, Fuhrman B. (2002). Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: studies in atherosclerotic mice and in humans. Drugs Exp Clin Res, 28, 49–62.
- Aviram M, Rosenblatt M, Gaitani D, Nitecki S, Hoffman A, Dornfield L. (2004). Pomegranate juice consumption for 3 years by patients with carotid artery stenosis (CAS) reduces common carotid intima-media thickness (IMT), blood pressure and LDL oxidation. Clin Nutr, 23, 423–433.
- Batta AK, Rangaswami S. (1973). Crystalline chemical components of some vegetable drugs. Phytochemistry, 12, 214–261.
- Bektas N, Arslan R. (2010).Antinociceptive effect of methanol extract of Capparis ovata in mice. Pharm Biol, 48, 1185–1190.
- Bellakhdar J (1997). La pharmacopée marocaine traditionnelle médecine arabe ancienne et savoirs populaires. Paris, Ibis Press Eds, pp. 320–321.
- Bentley GA, Newton SH, Starr J. (1983). Studies on the antinociceptive action of α-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol, 79, 125–134.
- Cerdá B, Cerón JJ, Tomás-Barberán FA, Espín JC. (2003). Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J Agric Food Chem, 51, 3493–3501.
- Cerdá B, Llorach R, Cerón JJ, Espín JC, Tomás-Barberán FA. (2003). Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur J Nutr, 42, 18–28.
- Chakraborty A, Devi RKB, Rita S, Sharatchandra Kh, Singh TI. (2004). Preliminary studies on anti-inflammatory and analgesic activities of Spilanthes acmella in experimental animal model. Indian J Pharmacol, 36, 148–150.
- Collier HO, Dinneen LC, Johnson CA, Schneider C. (1968). The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother, 32, 295–310.
- Das AK, Mandal SC, Banerjee SK, Sinha S, Das J, Saha BP, Pal M. (1999). Studies on antidiarrhoeal activity of Punica granatum seed extract in rats. J Ethnopharmacol, 68, 205–208.
- de Miranda FG, Vilar JC, Alves IA, Cavalcanti SC, Antoniolli AR. (2001). Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol, 1, 6.
- de Nigris F, Balestrieri ML, Williams-Ignarro S, D’Armiento FP, Fiorito C, Ignarro LJ, Napoli C. (2007). The influence of pomegranate fruit extract in comparison to regular pomegranate juice and seed oil on nitric oxide and arterial function in obese Zucker rats. Nitric Oxide, 17, 50–54.
- Ekpendu TO, Akah PA, Adesomuju AA, Okogun JC. (1994). Anti-inflammatory and antimicrobial activities of Mitracarpus scaber extracts. Int J Pharmacog, 32, 191–196.
- Esmaillzadeh A, Tahbaz F, Gaieni I, Alavi-Majd H, Azadbakht L. (2006). Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int J Vitam Nutr Res, 76, 147–151.
- Farouk L, Laroubi A, Aboufatima R, Benharref A, Chait A. (2008). Evaluation of the analgesic effect of alkaloid extract of Peganum harmala L.: possible mechanisms involved. J Ethnopharmacol, 115, 449–454.
- Friedman M. (2006). Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem, 54, 8655–8681.
- Hernandez F, Melgarejo P, Tomas-Barberan FA, Artes F. (1999). Evolution of juice anthocyanins during ripening of new selected pomegranate (Punica granatum) clones. Eur Food Res Technol, 210, 39–42.
- Hess SM, Miloning RC. (1972). Assay for anti-inflammatory drugs. In: Lepow IH, Ward PA eds. Inflammation, Mechanisms and Control. New York: Academic Press.
- Huang TH, Yang Q, Harada M, Li GQ, Yamahara J, Roufogalis BD, Li Y. (2005). Pomegranate flower extract diminishes cardiac fibrosis in Zucker diabetic fatty rats: modulation of cardiac endothelin-1 and nuclear factor-kappaB pathways. J Cardiovasc Pharmacol, 46, 856–862.
- Hunskaar S, Hole K. (1987). The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain, 30, 103–114.
- Hussein SAM, Barakat HH, Merfort I, Nawwar MAM. (1997). Tannins from the leaves of Punica granatum. Phytochemistry, 45, 819–823.
- Jaffe JH, Martin WR. (1990). Opioid analgesics and antagonists. In: Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 9th edn. New York: Macmillan Press, 485–519.
- Jafri MA, Aslam M, Javed K, Singh S. (2000). Effect of Punica granatum Linn. (flowers) on blood glucose level in normal and alloxan-induced diabetic rats. J Ethnopharmacol, 70, 309–314.
- Janssen PAJ, Niemegeers CJE, Dony JGH. (1963). The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawal reflex in rats. Drug Res, 6, 502–507.
- Katavic PL, Lamb K, Navarro H, Prisinzano TE. (2007). Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships. J Nat Prod, 70, 1278–1282.
- Lansky EP, Newman RA. (2007). Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol, 109, 177–206.
- Litchfield JT Jr, Wilcoxon F. (1949). A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther, 96, 99–113.
- Mavlyanov SM, Islambekov SH YU, Karimdzhanov AK, Ismailov AI. (1997). Polyphenols of the fruits of some varieties of pomegranate growing in Uzbekistan. Chem Nat Comp, 33, 98–99.
- Menezes SM, Cordeiro LN, Viana GS. (2006). Punica granatum (pomegranate) extract is active against dental plaque. J Herb Pharmacother, 6, 79–92.
- Mills S, Bone K. (2000). Principles and Practice of Phytotherapy. Edinburgh: Churchill Livingstone, 23–24, 31–34, 229–231.
- Morteza-Semnani K, Mahmoudi M, Heidar MR. (2006). Analgesic activity of the methanolic extract and total alkaloids of Glaucium paucilobum. Methods Find Exp Clin Pharmacol, 28, 151–155.
- Neuhofer H, Witte L, Gorunovic M, Czygan FC. (1993). Alkaloids in the bark of Punica granatum L. (pomegranate) from Yugoslavia. Pharmazie, 48, 389–391.
- Nho CW, Jeffery E. (2001). The synergistic upregulation of phase II detoxification enzymes by glucosinolate breakdown products in cruciferous vegetables. Toxicol Appl Pharmacol, 174, 146–152.
- Olajide OA, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, Okpako DT. (2000). Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J Ethnopharmacol, 71, 179–186.
- Oukabli, A. (2004). Transfert de technologie en agriculture: le Grenadier, des variétés performantes pour la culture. Bulletin mensuel d’information et de liaison du PNTTA 123.
- Ozcal N, Dinc S. (1993). Evaluation of the pomegranate (Punica granatum L.) peels from the standpoint of pharmacy. Eczacılık Fakültesi Dergisi, 22, 21–29.
- Palanichamy S, Nagarajan S. (1990). Analgesic activity of Cassia alata leaf extract and kaempferol 3-o-sophoroside. J Ethnopharmacol, 29, 73–78.
- Panichayupakaranant P, Tewtrakul S, Yuenyongsawad S. (2010). Antibacterial, anti inflammatory and anti-allergic activities of standardised pomegranate rind extract. Food Chem, 123, 400–403.
- Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, Seeram N, Liker H, Wang H, Elashoff R, Heber D, Aviram M, Ignarro L, Belldegrun A. (2006). Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res, 12, 4018–4026.
- Paulino N, Dantas AP, Bankova V, Longhi DT, Scremin A, de Castro SL, Calixto JB. (2003). Bulgarian propolis induces analgesic and anti-inflammatory effects in mice and inhibits in vitro contraction of airway smooth muscle. J Pharmacol Sci, 93, 307–313.
- Paxinos G, Watson C. (1986). The Rat Brain in Stereotaxic Coordinates, 2nd edn. San Diego (CA): Academic Press.
- Polunin O, Huxley A. (1987). Pomegranate. In: Flowers of the Mediterranean. London, Hogarth Press, 54–57.
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. (2000). Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol, 387, 111–118.
- Reisine T, Pasternak G. (1996). Opioid analgesics and antagonist. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds. Goodman Gilman’s The Pharmacological Basis of Therapeutics, 9th edn. New York, NY: McGraw-Hill Companies; 521–555.
- Rosland JH, Tjølsen A, Maehle B, Hole K. (1990). The formalin test in mice: effect of formalin concentration. Pain, 42, 235–242.
- Santagati NA, Duro R, Duro F. (1984). Study on pigments present in pomegranate seeds. J Commodity Sci, 23, 247–254.
- Satomi H, Umemura K, Ueno A, Hatano T, Okuda T, Noro T. (1993). Carbonic anhydrase inhibitors from the pericarps of Punica granatum L. Biol Pharm Bull, 16, 787–790.
- Schmidt B, Ribnicky DM, Poulev A, Logendra S, Cefalu WT, Raskin I. (2008). A natural history of botanical therapeutics. Metab Clin Exp, 57, S3–S9.
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. (2005). In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem, 16, 360–367.
- Shalheen HM, Badreldin HA, Alquarawi AA, Bashir AK. (2000). Effect of Psidium guajava leaves on some aspects of the central nerveus system in mice. Phytother Res, 14, 107–111.
- Shibata M, Ohkubo T, Takahashi H, Inoki R. (1989). Modified formalin test: characteristic biphasic pain response. Pain, 38, 347–352.
- Starec M, Waitzová D, Elis J. (1988). [Evaluation of the analgesic effect of RG-tannin using the “hot plate” and “tail flick” method in mice]. Cesk Farm, 37, 319–321.
- Tanaka T, Nonaka G, Nishioka I. (1986). Tannins and related compounds. XLI. Isolation and characterization of novel ellagitannins, punicacorteins A, B, C and D, and punigluconin from the bark of Punica granatum L. Chem Pharm Bull, 34, 656–663.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. (1992). The formalin test: an evaluation of the method. Pain, 51, 5–17.
- Toshihiro K, Uchida AIO, Koichiro N, Kenji H, Terunobu S (2001). Selective prostaglandin biosynthesis inhibition of zaltoprofen at the inflammatory site. Inflamm Regen, 21, 235–241.
- van Elswijk DA, Schobel UP, Lansky EP, Irth H, van der Greef J. (2004). Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry, 65, 233–241.
- Zimmerman M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16(2), 109–10.