Abstract
Context: Ursolic acid is a pentacyclic triterpenoid which has hepatoprotective and antihepatotoxic activities.
Objective: This study investigated whether ursolic acid is able to stimulate liver regeneration in partially hepatectomized mice.
Materials and methods: Ursolic acid or the vehicle solution was orally administered to the experimental, sham-operated and vehicle-treated group mice for 7 days, positive control animal (mice) was treated with recombinant human hepatocyte growth factor (rhHGF), and then the 70% liver partial hepatectomy was performed. The liver mass recovery rate was estimated by measuring the ratios of mice liver weight to body weight. The liver cells undergoing DNA synthesis were identified by immunohistochemistry analysis using monoclonal anti-BrdU antibodies. The expression levels of cyclin D1, cyclin E and C/EBP proteins (C/EBPα and C/EBPβ) were detected by the Western blotting technique.
Results: Our results showed administration of ursolic acid significantly increased the ratio of the liver to body weight and BrdU labeling index at 36 and 48 h after partial hepatectomy, and the potency of UA is similar to rhHGF treated positive control mice. In addition, ursolic acid treatment significantly increased cyclin D1, cyclin E and C/EBPβ protein expression levels at 36 h after liver PHx compared with the vehicle-treated control mice.
Discussion and conclusion: All these results suggest that ursolic acid stimulates liver proliferation after partial hepatectomy, and this effect may be associated with the stimulation of C/EBPβ expression.
Keywords::
Introduction
Liver has the unique ability to regenerate itself after major tissue loss. The remaining liver can proliferate and fully restores its original size (CitationMichalopoulos & DeFrances, 1997; CitationCourt et al., 2002; CitationMarkiewski et al., 2006). Hence, if the liver fails completely, a surgical resection (partial hepatectomy) or a liver transplant offers a chance to cure serious liver diseases. However, liver resection involves the risk of liver failure, and the survival of patients depends on the ability of the remaining hepatocytes to regenerate (CitationClavien et al., 2007; CitationClavien, 2008). Thus, developing an agent that can stimulate hepatic regeneration has tremendous therapeutic relevance. Experimental liver partial hepatectomy, which is applied by excising about 70% of the liver in mice or rats, is widely used as a model of liver regeneration and surgical stress (CitationMichalopoulos & DeFrances, 1997; CitationAndiran et al., 2000; CitationFuruta et al., 2000).
Ursolic acid (UA), a naturally occurring pentacyclic triterpenoid compound, is the major component of some traditional medicinal herbs (CitationPrice et al., 1987). It has broad bioactive use including antifungal, antiinflammatory, antiulceric, antihyperlipidemic, antihyperglycemic, and antitumorgenic activity (CitationYan et al., 2010; CitationLiu, 1995; CitationGayathri et al., 2009). Moreover, multiple lines of evidence indicate that UA has hepatoprotective and antihepatotoxic activities (CitationMa et al., 1986; CitationLin et al., 1988; CitationLiu et al., 1994; CitationSaraswat et al., 2000; CitationOvesna et al., 2004). However, the potential role of UA in liver regeneration has not been studied. Hepatocyte growth factor (HGF) is the most potent mitogen for mature hepatocytes and seems to act as a hepatotropic factor (CitationMatsumoto & Nakamura, 1992). CitationIshiki et al. (1992, Citation1995) and others have shown that injection of recombinant human hepatocyte growth factor (rhHGF) after 70% hepatectomy produced dose-dependent increases in hepatic DNA synthesis and proliferation (CitationKaibori et al., 1997). In the present study, we evaluated the effect of the exogenous administration of UA on the stimulation of liver regeneration in vivo in 70% partially hepatectomized mice by measuring the liver mass recovery, Brdu uptake, cell cycle proteins and C/EBP proteins (C/EBPα and C/EBPβ) expression.
Materials and methods
Chemicals
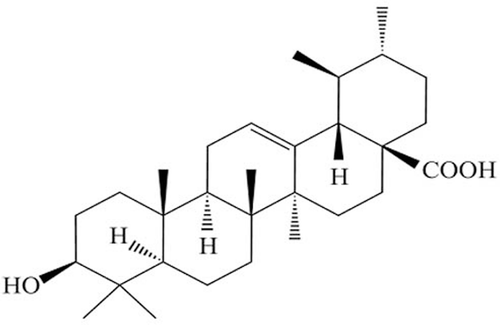
Ursolic acid (UA, 3β-hydroxy-12-ursen-28-ic acid, formula: C30H48O3, molecular weight: 456.70) () was obtained from Sigma Aldrich (St. Louis, MO, USA). BrdU was from Invitrogen (Carlsbad, CA, USA). rhHGF was from R&D Systems (Minneapolis, MN, USA). All other chemicals used were of analytical grade and purchased from commercial suppliers.
Animals and drug administration
C57BL/6 male mice, 10–12 weeks of age, were provided by the Experimental Animal Center of Yanbian University Medical College (Yanji, Jilin, China). The mice were kept at controlled temperature 22 ± 2°C and humidity under a 12 h light-dark cycle and fed standard laboratory chow and water. All experiments were performed in accordance with the National Research Council’s guidelines. The experimental procedures were approved by the Ethical Committee for the Experimental Use of Animals at Yanbian University. The mice were randomly divided into four groups. UA (Sigma Chemical Co., St Louis, MO, USA, 80 mg/kg/day) was orally administered to the experimental group animals daily for 7 days before surgery and continually until sacrifice. The dose of the UA was selected on the basis of the literature data (CitationAparecida Resende et al., 2006). The sham-operated group animals were submitted to UA treatment same as experimental group animals without liver PHx. The vehicle-treated group animals were submitted to the treatment of 0.5% sodium carboxymethyl cellulose (CMC-Na) solution without or instead of UA, and subjected to PHx. The positive control mice were administered with rhHGF (1.0 μg/kg/day, i.p.) once daily before surgery and continually until sacrifice.
Liver partial hepatectomy
Experimental liver partial hepatectomy was used as a model of liver regeneration and surgical stress (CitationMichalopoulos & DeFrances, 1997; CitationAndiran et al., 2000; CitationFuruta et al., 2000). Under anesthesia, median and left lateral lobes of the liver (about 70%, two-thirds) were surgically removed and regeneration was allowed to proceed for 24, 36 and 48 h. The liver mass recovery rate was calculated by measuring the ratios of mice liver weight to body weight at different times. Liver samples were also collected, frozen in liquid nitrogen for further analyses.
BrdU incorporation index
Mice were injected with 50 mg/kg BrdU intraperitoneally. After 2 h, mice were anesthetized and the livers were removed. Liver specimens were made into paraffin-embedded tissue sections (5 μm thick), and the liver cells undergoing DNA synthesis were immunohistochemically identified using monoclonal anti-BrdU antibodies on formalin-fixed and paraffin-embedded liver tissues according to manufacturer’s instructions and counting the number of labeled nuclei per 1000 nuclei in randomly selected fields under a light microscope.
Protein isolation and Western blotting
Nuclear extracts were isolated from livers as described previously (CitationWang et al., 2001; CitationJin et al., 2009). Nuclear extracts (50 to 100 µg) were loaded on the gradient (4–20%, Bio-rad) polyacrylamide gels, and transferred onto membranes, and probed with antibodies. To verify protein loading, each filter was re-probed with TATA binding protein (TBP) and/or stained with Coomassie blue. Antibodies to cyclin D1, cyclin E, C/EBPα and C/EBPβ were purchased from Santa Cruz Biotechnology. Monoclonal anti-TBP antibody was from Abacam.
Statistical analysis
All data are expressed as means ± SD. Unpaired Student’s t-test was used in all the experiments. P values <0.05 were considered statistically significant.
Results
UA enhances liver mass recovery rate after PHx
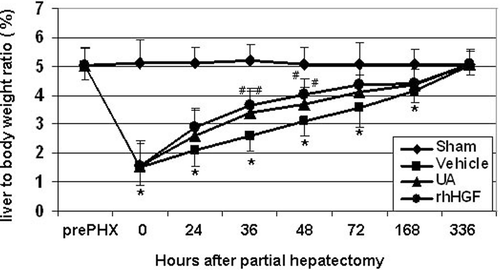
After UA treatment, the ratios of the liver to body weight were significantly increased at 36 and 48 h after PHx compared with those of the vehicle-treated control group, the potency is similar to the rhHGF treated group, while the application of UA for 7 days did not change the liver to body weight in the sham-operated mice (). In addition, UA did not induce liver overgrowth after 14 days of PHx. Moreover, after careful examination of liver histopathology, we have not observed any liver damage on control normal mice liver after 14 days of treatment by UA (data not shown). These results suggest that UA accelerates liver regeneration to its original size, and this stimulatory effect does not induce overgrown liver or liver damage.
Figure 2. Stimulatory effects of UA on liver mass recovery after 70% PHx. liver to body weight ratio was examined in the livers at pre PHx, and at 0, 24, 36, 48, 72, 168 and 336 h after PHx. Data are expressed as means ± SD. n = 5 in each group. *P < 0.05 versus sham group; #P < 0.05 versus vehicle-treated control group. UA, ursolic acid.
UA stimulates hepatocyte DNA synthesis after PHx
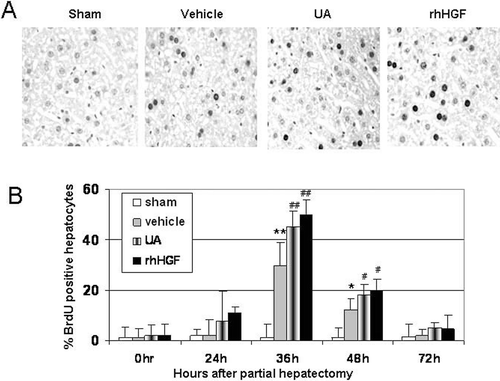
To observe if liver regeneration by UA is due to the stimulation of the hepatocyte DNA synthesis, we measured the mitotic index of nuclear thymidine analog BrdU incorporation into hepatocyte DNA, which is a useful indicator of cell proliferation and cell kinetics (CitationIatropoulos & Williams, 1996; CitationNolte et al., 2005). As shown in , after UA treatment, the BrdU labeling indexes increased significantly compared with the vehicle-treated control animals, the potency is similar to the rhHGF treated positive control animals at 36 and 48 h after PHx, while no BrdU positive cell were observed in the sham-operated control animals. Overall, these results indicate that UA markedly stimulates DNA synthesis in hepatocytes and thereafter accelerates liver regeneration after PHx in vivo.
Figure 3. Hepatocyte proliferation enhancement by UA following 70% PHx (A) Representative sections of BrdU labeling of hepatocytes nuclei in the sham, control, UA and HGF treated mice at 36 h following PHx. (Magnification, × 200). (B) Summary of BrdU labeling index. Values are means ± SD (n = 5). *P < 0.05; **P < 0.01 versus sham group; #P < 0.05, # #P < 0.01 versus vehicle-treated control group.
UA activates cell cycle regulatory proteins and CCAAT/enhancer binding protein beta expression after PHx
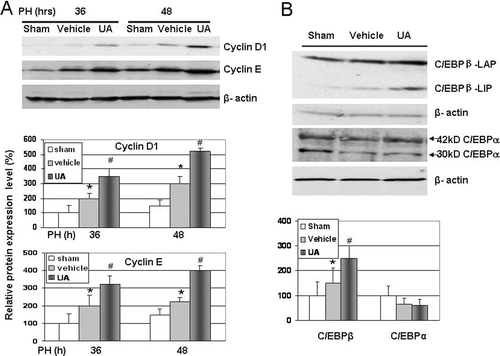
To investigate the possible mechanism of UA-induced liver regeneration, we first examined the expression of cell cycle proteins in liver. We found that the protein levels of cyclin D1 and cyclin E were significantly increased in livers of UA-treated mice at 36 and 48 h after PHx, compared with the sham-operated and vehicle-treated control mice (). Since the activation of C/EBPβ has been reported to contribute to an increase in cyclin E expression level (CitationCho et al., 2009), and both C/EBPα and C/EBPβ and plays a critical role in the regulation of liver biology and liver regeneration (CitationCressman et al., 1996; CitationGreenbaum et al., 1998; CitationAnkoma-Sey, 1999; CitationWang & Timchenko, 2005), we measured the hepatic expression of C/EBPα and C/EBPβ. Our results showed that after the UA treatment, both C/EBPα and C/EBPβ expressions did not change before PHx (data not shown). However, after PHx, UA significantly increased the expression of both LAP and LIP, which are the isoforms of C/EBPβ, compared with the vehicle-treated animals (). No significant differences were observed in the expression level of C/EBPα in experiment group animal compared with the vehicle-treated control mice after PHx. Therefore, these results suggest that the UA-induced enhancement in the liver proliferation after PHx may be associated with the stimulation of C/EBPβ expression and the consequent activation of the cyclin E expression ().
Figure 4. Western blotting for cell cycle proteins and C/EBPβ expression. (A) Expression of cyclin D1 and cyclin E proteins were increased at 36 and 48 h after PHx in UA treated group. (B) Expression of C/EBPβ proteins was increased at 36 h after PHx in UA treated group animals. Changes in the protein levels relative to control were assessed by scanning densitometry of the immunoblots. Data represent mean ± SD (n = 3). *P < 0.05 versus sham group; #P < 0.05 versus vehicle-treated control group.
Discussion
Normal liver is well known for its capacity to regenerate itself after partial hepatectomy (CitationFrancavilla et al., 1990; CitationCourt et al., 2002). Liver resection is often the best option in primary and secondary hepatic malignancies patients (CitationTsim et al., 2010). Partial hepatectomy induces a rapid regenerative process in the remnant liver tissue, and the liver returns to its original size and the liver regeneration is terminated within 2 weeks (CitationMichalopoulos & DeFrances, 1997; CitationAndiran et al., 2000; CitationFuruta et al., 2000). However, the major liver resection, both in patients with liver tumors and for organ donation, involves the risk of liver failure (CitationClavien et al., 2007; CitationClavien, 2008). Therefore, it is very important to investigate and find an effective therapeutic agent to improve the liver regeneration after partial hepatectomy without side-effects. In the present study, we discovered that UA can improve liver regeneration after PHx through increasing the liver mass recovery and accelerating liver proliferation.
Previous studies demonstrated that UA has pro-apoptotic effect on hepatoma cell line (CitationLauthier et al., 2000; CitationAchiwa et al., 2005; CitationHarmand et al., 2005). However, on normal livers, we have not observed any pro-apoptotic or toxic effect after UA treatment. Moreover, the application of UA produced a more rapid increase in the BrdU labeling indexes at 36 and 48 h after PHx, suggesting that UA can more rapidly stimulate DNA synthesis and thereafter accelerate liver regeneration after PHx.
Liver proliferation is controlled by a complex cooperation of several signal transductions pathways (CitationChamuleau & Bosman, 1988; CitationMichalopoulos & DeFrances, 1997). Two members of C/EBP family, C/EBPα and C/EBPβ, are expressed in the liver and play a critical role in the regulation of liver biology. It has been also shown that the C/EBPα is involved in the inhibition of cell proliferation (CitationWang & Timchenko, 2005); while C/EBPβ, is a vital component of the hepatic regenerative response (CitationCressman et al., 1996; CitationGreenbaum et al., 1998; CitationAnkoma-Sey, 1999). Liver regeneration normally begins with a priming phase, which is followed by a spurt of regeneration during which most of the hepatocytes enter the cell cycle (CitationMichalopoulos, 2007). Cyclin D1 and cyclin E are important cell cycle proteins which are induced after PHx, and are involved in the promotion of liver proliferation (CitationSchwabe et al., 2003; CitationKato et al., 2005). Activation of C/EBPβ enhances cyclin E expression (CitationNakano et al., 2001). We found that UA increase the C/EBPβ and cell cycle proteins cyclin D1 and cyclin E expression after PHx, suggesting that the activation of C/EBPβ expression by UA after PHx may induce the cyclin E expression and contribute to the stimulation of mice liver regeneration. We do not know yet mechanisms by which UA regulates C/EBPβ in livers after PHx. However, from previous observations (Zhang et al., 2010), it is possibly through regulation of the ERK pathway, which can also be activated by HGF (CitationLi et al., 2011). Further studies are required to elucidate how UA regulates C/EBPβ expression.
Taken together, our findings indicate that UA may play a role in accelerating liver proliferation, recovering liver function, and protecting the integrity of hepatocytes against liver damage. And we postulate that this function may be through an activation of C/EBPβ expression. Hence it merits further development for exploiting it as a therapeutic agent.
Conclusion
In conclusion, ursolic acid accelerates liver mass recovery, stimulates hepatocyte proliferation after partial hepatectomy, and this effect may be associated with the stimulation of C/EBPβ expression.
Acknowledgement
This study was supported by the Yanbian University Foundation of Jilin Province, China, No. 200009.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper
References
- Achiwa Y, Hasegawa K, Komiya T, Udagawa Y. (2005). Ursolic acid induces Bax-dependent apoptosis through the caspase-3 pathway in endometrial cancer SNG-II cells. Oncol Rep, 13, 51–57.
- Andiran F, Ayhan A, Tanyel FC, Abbasoglu O, Sayek I. (2000). Regenerative capacities of normal and cirrhotic livers following 70% hepatectomy in rats and the effect of alpha-tocopherol on cirrhotic regeneration. J Surg Res, 89, 184–188.
- Ankoma-Sey V. (1999). Hepatic regeneration-revisiting the myth of Prometheus. News Physiol Sci, 14, 149–155.
- Aparecida Resende F, de Andrade Barcala CA, da Silva Faria MC, Kato FH, Cunha WR, Tavares DC. (2006). Antimutagenicity of ursolic acid and oleanolic acid against doxorubicin-induced clastogenesis in Balb/c mice. Life Sci, 79, 1268–1273.
- Chamuleau RA, Bosman DK. (1988). Liver regeneration. Hepatogastroenterology, 35, 309–312.
- Cho IJ, Sung DK, Kang KW, Kim SG. (2009). Oltipraz promotion of liver regeneration after partial hepatectomy: The role of PI3-kinase-dependent C/EBPbeta and cyclin E regulation. Arch Pharm Res, 32, 625–635.
- Clavien PA. (2008). Liver regeneration: a spotlight on the novel role of platelets and serotonin. Swiss Med Wkly, 138, 361–370.
- Clavien PA, Petrowsky H, DeOliveira ML, Graf R. (2007). Strategies for safer liver surgery and partial liver transplantation. N Engl J Med, 356, 1545–1559.
- Court FG, Wemyss-Holden SA, Dennison AR, Maddern GJ. (2002). The mystery of liver regeneration. Br J Surg, 89, 1089–1095.
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. (1996). Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science, 274, 1379–1383.
- Francavilla A, Panella C, Polimeno L, Giangaspero A, Mazzaferro V, Pan CE, Van Thiel DH, Starzl TE. (1990). Hormonal and enzymatic parameters of hepatic regeneration in patients undergoing major liver resections. Hepatology, 12, 1134–1138.
- Furuta K, Kakita A, Takahashi T, Tomiya T, Fujiwara K. (2000). Experimental study on liver regeneration after simultaneous partial hepatectomy and pancreatectomy. Hepatol Res, 17, 223–236.
- Gayathri R, Priya DK, Gunassekaran GR, Sakthisekaran D. (2009). Ursolic acid attenuates oxidative stress-mediated hepatocellular carcinoma induction by diethylnitrosamine in male Wistar rats. Asian Pac J Cancer Prev, 10, 933–938.
- Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R. (1998). CCAAT enhancer- binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest, 102, 996–1007.
- Harmand PO, Duval R, Delage C, Simon A. (2005). Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int J Cancer, 114, 1–11.
- Iatropoulos MJ, Williams GM. (1996). Proliferation markers. Exp Toxicol Pathol, 48, 175–181.
- Ishii T, Sato M, Sudo K, Suzuki M, Nakai H, Hishida T, Niwa T, Umezu K, Yuasa S. (1995). Hepatocyte growth factor stimulates liver regeneration and elevates blood protein level in normal and partially hepatectomized rats. J Biochem, 117, 1105–1112.
- Ishiki Y, Ohnishi H, Muto Y, Matsumoto K, Nakamura T. (1992). Direct evidence that hepatocyte growth factor is a hepatotrophic factor for liver regeneration and has a potent antihepatitis effect in vivo. Hepatology, 16, 1227–1235.
- Jin J, Wang GL, Shi X, Darlington GJ, Timchenko NA. (2009). The age-associated decline of glycogen synthase kinase 3beta plays a critical role in the inhibition of liver regeneration. Mol Cell Biol, 29, 3867–3880.
- Kaibori M, Kwon AH, Nakagawa M, Wei T, Uetsuji S, Kamiyama Y, Okumura T, Kitamura N. (1997). Stimulation of liver regeneration and function after partial hepatectomy in cirrhotic rats by continuous infusion of recombinant human hepatocyte growth factor. J Hepatol, 27, 381–390.
- Kato A, Bamba H, Shinohara M, Yamauchi A, Ota S, Kawamoto C, Yoshida Y. (2005). Relationship between expression of cyclin D1 and impaired liver regeneration observed in fibrotic or cirrhotic rats. J Gastroenterol Hepatol, 20, 1198–1205.
- Lauthier F, Taillet L, Trouillas P, Delage C, Simon A. (2000). Ursolic acid triggers calcium-dependent apoptosis in human Daudi cells. Anticancer Drugs, 11, 737–745.
- Li F, Jiang Y, Zheng Q, Yang X, Wang S. (2011). TEC protein tyrosine kinase is involved in the Erk signaling pathway induced by HGF. Biochem Biophys Res Commun, 404, 79–85.
- Lin CN, Chung MI, Gan KH. (1988). Novel antihepatotoxic principles of Solanum incanum. Planta Med, 54, 222.
- Liu J. (1995). Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol, 49, 57–68.
- Liu J, Liu Y, Mao Q, Klaassen CD. (1994). The effects of 10 triterpenoid compounds on experimental liver injury in mice. Fundam Appl Toxicol, 22, 34–40.
- Ma XH, Zhao YC, Yin L, Xu RL, Han DW, Wang MS. (1986). [Studies on the preventive and therapeutic effects of ursolic acid (UA) on acute hepatic injury in rats]. Yao Xue Xue Bao, 21, 332–335.
- Markiewski MM, DeAngelis RA, Lambris JD. (2006). Liver inflammation and regeneration: two distinct biological phenomena or parallel pathophysiologic processes? Mol Immunol, 43, 45–56.
- Matsumoto K, Nakamura T. (1992). Hepatocyte growth factor: molecular structure, roles in liver regeneration, and other biological functions. Crit Rev Oncog, 3, 27–54.
- Michalopoulos GK. (2007). Liver regeneration. J Cell Physiol, 213, 286–300.
- Michalopoulos GK, DeFrances MC. (1997). Liver regeneration. Science, 276, 60–66.
- Nakano K, Chijiiwa K, Tanaka M. (2001). Lower activity of CCAAT/enhancer-binding protein and expression of cyclin E, but not cyclin D1, activating protein-1 and p21(WAF1), after partial hepatectomy in obstructive jaundice. Biochem Biophys Res Commun, 280, 640–645.
- Nolte T, Kaufmann W, Schorsch F, Soames T, Weber E. (2005). Standardized assessment of cell proliferation: the approach of the RITA-CEPA working group. Exp Toxicol Pathol, 57, 91–103.
- Ovesná Z, Vachálková A, Horváthová K, Tóthová D. (2004). Pentacyclic triterpenoic acids: new chemoprotective compounds. Minireview. Neoplasma, 51, 327–333.
- Price KR, Johnson IT, Fenwick GR. (1987). The chemistry and biological significance of saponins in foods and feedingstuffs. Crit Rev Food Sci Nutr, 26, 27–135.
- Saraswat B, Visen PK, Agarwal DP. (2000). Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother Res, 14, 163–166.
- Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. (2003). c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology, 37, 824–832.
- Tsim NC, Frampton AE, Habib NA, Jiao LR. (2010). Surgical treatment for liver cancer. World J Gastroenterol, 16, 927–933.
- Wang GL, Timchenko NA. (2005). Dephosphorylated C/EBPalpha accelerates cell proliferation through sequestering retinoblastoma protein. Mol Cell Biol, 25, 1325–1338.
- Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler WJ, Timchenko NA. (2001). C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell, 8, 817–828.
- Yan SL, Huang CY, Wu ST, Yin MC. (2010). Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol in Vitro, 24, 842–848.
- Zhang T, He YM, Wang JS, Shen J, Xing YY, Xi T. (2011). Ursolic acid induces HL60 monocytic differentiation and upregulates C/EBPß expression by ERK pathway activation. Anticancer Drugs, 22, 158–165.