Abstract
Context: The problem of hypertension has gained enormous proportions in the past decade. Multifactorial etiology and complex pathophysiology of the disease has rendered the treatment of the disease a hard task. Sympathetic nervous system and the renin–angiotensin–aldosterone system are primary contributors of blood pressure homeostasis.
Objective: Structural similarities were identified among AT1 and α1-antagonists, initiating a speculation that α1-antagonists could possibly block the AT1 receptor and vice-versa.
Methods: To corroborate this speculation, we screened prototypical α1-antagonists such as prazosin, doxazosin, and terazosin for antagonism of angiotensin II on rat aortic strips. We also examined the AT1 antagonists losartan, valsartan, and olmesartan for their possible antagonistic effect, on contractions of rat aortic strips induced by phenylephrine.
Results: To our astonishment, we found that prazosin and its analogs which have been reported to have α1-antagonistic activity only, were able to shift concentration response curves of angiotensin II.
Conclusion: Our findings suggest that the potent antihypertensive effect of prazosin-type α1-antagonists is not purely due to α1-receptor blocking activity of these compounds but also due to blockade of AT1 receptors. This finding may lead to the development of more potent dual inhibitors which would prove to be of immense value in the control of the scourge of hypertension.
Keywords::
Introduction
Hypertension is recognized as one of the leading risk factors for human morbidity and mortality. On a worldwide basis, hypertension has been ranked first as a cause of disability adjusted life years (CitationGlobal Health Risks, 2009). Hypertension is known as a silent killer because the symptoms do not appear until it is severely high. The blood pressure is derived from the hemodynamic properties of the systemic circulation.
Hypertension is influenced by both function and structure of blood vessels. The purpose of the “blood pressure system” is to maintain blood flow to all of the tissues of the body at rest or during movements. There are two important contributors to the control of blood pressure, sympathetic nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS). Over the years, a number of experimental and clinical investigations have shed light on the key role exerted by RAAS and SNS in the homeostatic control of blood volume and blood pressure (CitationSealey & Laragh, 1995; CitationShepherd & Mancia, 1986). Straightforward evidence has also been provided that these two systems do not operate independently but interact mutually with each other in accomplishing their cardiovascular regulatory functions. (CitationGrassi, 2001; CitationRang et al., 2007).
Stimulation of SNS results into vasoconstriction and increased inotropic and chronotropic effect of heart, while stimulation of RAAS results in increased production of active hormone angiotensin II (Ang II), which raises blood pressure in two ways. First, Ang II is a potent vasoconstrictor that raises systemic vascular resistance and second, it indirectly influences blood pressure through release of aldosterone and noradrenaline.
Both SNS and RAAS also appear to modulate fluid volume through the kidney. The kidney is a vital organ involved in long-term control of blood pressure. The renal-body fluid feedback mechanism couples the long-term regulation of arterial pressure to extracellular volume (sodium and water) homeostasis via pressure natriuresis whereby the kidneys respond to changes in arterial pressure by altering urinary sodium and water excretion (CitationCowley, 1992). Both SNS and RAAS systems are primary modulators of renal effects on circulating blood volume. The α-adrenergic receptors are involved only when associated renal hemodynamic changes occur with decrease in renal blood flow (RBF), glomerular filtration rate (GFR) and urinary sodium excretion. Renal α1-adrenergic receptors mediate renal (including preglomerular) vasoconstriction and tubular gluconeogenesis. These effects are coupled to tubular Na+ reabsorption. The direct effects of renal nerve stimulation through renal tubular α1-adrenergic receptors were observed in vivo in the dog (CitationSummers, 1984) and rabbit (CitationWolff et al., 1985) and in vitro in isolated buffer-perfused kidney preparation of the rat (CitationDrew & Whiting, 1979; CitationHorn et al., 1982; CitationWolff et al., 1984). On the other hand, Ang II causes vasoconstriction and diminishes blood flow through the kidneys, thereby increasing the reabsorption of salt and water retention (CitationHall, 2004).
Information on the renin–angiotensin–sympathetic interactions has also been extended to the possible sites of these interactions:
Stimulation of the sympathetic nervous system leads to renin secretion and Ang II formation (CitationDiBona, 1989).
It has been shown that released norepinephrine negatively regulates Ang II receptors in cultured brain neurons (CitationMancia et al., 1995) and in vascular tissue through its interactions with α1-adrenoceptor (α1-ARs) (CitationDu et al., 1997). In neonatal rat, cardiac myocytes Ang II selectively down-regulates α1-AR subtype mRNA and its corresponding receptors (CitationLi et al., 1997).
Evidence has also been provided that Ang II:
Triggers a sympathetically mediated blood pressure rise associated with systemic vasoconstriction when dosed intracerebrally. It suggested a central facilitatory effect of Ang II on sympathetic outflow (CitationWolff et al., 1984; CitationHall, 2004; CitationZimmerman, 1984).
Plays a facilitatory role on the neuroadrenergic transmission across sympathetic ganglia (CitationZimmerman, 1984; CitationReid, 1992; CitationReit, 1972)
Potentiates norepinephrine release from sympathetic nerve terminals via stimulation of presynaptic angiotensinergic receptors (CitationZimmerman, 1984; CitationReid, 1992; CitationStarke, 1977).
Amplifies the α-receptor mediated vasoconstrictor responses to exogenously administered or endogenously produced norepinephrine. Furthermore, Ang II has been shown to exert inhibitory effects on baroreceptor reflex control of heart rate and sympathetic nerve traffic (CitationZimmerman, 1984; CitationReid, 1992).
The renin–angiotensin–sympathetic interactions have physiological, as well as pathophysiological relevance; a reciprocal reinforcement of the favorable as well as unfavorable cardiovascular, renal, metabolic and reflex effects of the two systems have been reported in a variety of cardiovascular conditions like hypertension (CitationZimmerman, 1984; CitationReid, 1992; CitationReit, 1972). Multiple signaling pathways and redundant feedback mechanisms, both positive and negative, contribute to the hypertensive disease process.
Due to its multifactorial etiology, it has become difficult to control the hypertension with one particular class of therapy. Each class of antihypertensive agents has its own limitations. Chronic administration of particular class of antagonist causes one or the other adverse effects. Therefore, combinations of two or more types of antihypertensive agents are preferred in medical practice for effective control of blood pressure (CitationElliott, 2002; CitationPaulis & Unger, 2010).
Parallel modulation of multiple biological targets can be beneficial for the treatment of disease with complex etiologies like hypertension. Both SNS and RAAS become important targets in order to control the blood pressure as these systems work in coordination. Simultaneous blockade of both systems would be beneficial. A number of clinically used drugs have been found to have activity at more than one target, which in some cases is associated with increased efficacy (CitationMorphy & Rankovic, 2009).
This laboratory is actively engaged in developing newer antihypertensive agents. While studying the structure–activity relationship (SAR) of prazosin class of α1-antagonists and AT1 antagonist, it was realized that molecules with dual activities (α1- and AT1-receptors blocking) could be designed and synthesized. Working on the hypothesis of developing dual inhibitors, a large number of molecules were synthesized and evaluated biologically for α1 and Ang II receptor blocking activities (unpublished results). Some of the synthesized compounds showed dual inhibition of both the receptors, comparable in potency to prazosin and losartan. Since these compounds were chemically related to the structure of prazosin and losartan and possessed dual activity, it struck to us whether prazosin and losartan could also possess dual activities and their potencies as antihypertensive agents could be due to dual inhibition of both the types of receptors and not due to singular effects. To support this assumption it was decided to cross-screen the clinically used drug molecules (α1- and AT1-receptor blockers) of one class on the other class of receptors. The α1 receptor antagonist like prazosin, doxazosin, and terazosin were thus screened for AT1 receptor antagonistic and AT1 antagonist like losartan, valsartan, olmesartan were screened for α1 receptor antagonistic activities.
Materials and methods
In vitro functional antagonism and potency assay
Male Wistar rats (200–250 g) were used for the study. They were sacrificed by cervical dislocation; descending thoracic aortas were removed immediately and placed in ice-cold Kreb’s bicarbonate solution of the following composition (mM): NaCl 112, NaHCO3 12, glucose 11.1, KCl 5.0, MgSO4 1.2, KH2PO4 1.0, and CaCl2 2.5. The tissue was aerated with 95% O2 and 5% CO2. Periadventitial tissue was removed, taking care not to stretch the tissue. A spinal needle was inserted in the tissue and rotated gently to denude the endothelium. Following this, the tissue was cut spirally into a helical strip (20 mm × 3 mm) using a surgical blade. The strip was tied at both ends using a cotton thread and suspended in a 25 mL organ tube under an initial resting tension of 2 g. The pH of the Kreb’s solution was 7.4 and maintained at 37°C using a thermostat. The Kreb’s solution in the organ tube was changed every 10 min during an equilibration period of about 90 min. Denudation of the endothelium was confirmed by observing the “absence of relaxation” on strips precontracted with phenylephrine. Isometric contractions were recorded using a force transducer (UGO BASILE, Italy) coupled to a Gemini 7070 recorder (UGO BASILE, Italy). All the experimental protocols were approved by the Institutional Animal Ethics Committee of Pharmacy Department, the M. S. University of Baroda (Protocol No. PHR/GHPC/CPCSEA/183/06). The experiments were carried out in accordance to the guidelines of CPCSEA, India.
Contractions were induced in rat aortic strips with graded, cumulative concentrations of phenylephrine or Ang II. Losartan, valsartan, olmesartan, or solvent were added to organ tubes 30 min prior to the addition of phenylephrine. Similar procedure was followed to record graded cumulative response curves of Ang II against prazosin, doxazosin, and terazosin. Control strips were incubated with solvent [DMSO (0.5%) or normal saline] for 30 min before recording the concentration response curves. The pA2 values were calculated by the method described by CitationArunlakshana and Schild (1959).
Results and discussion
Calculations based on Schild plots revealed that the α1-antagonists prazosin, doxazosin, and terazosin cause a dose-dependent shift in the concentration response curves to Ang II. Any effect mediated by the endothelium was not expected since the endothelial layer was removed while preparing the aortic strip. The pA2 values obtained after regression analysis were found to be 8.26 ± 0.7, 6.61 ± 0.4, and 6.39 ± 0.4 for prazosin, doxazosin, and terazosin, respectively. The slopes in these cases were not very different from unity. As opposed to these findings, the AT1 blockers like losartan, valsartan, and olmesartan did not cause any major shifts in the concentration response curves to phenylephrine. All the tissues were incubated with antagonists for 30 min but incubation up to 45 min did not affect the results. Inhibition induced by the antagonists was reversible after the antagonists were removed following repeated washings. The pA2 value is an index of antagonist potency and a higher pA2 reflects strong competitive antagonism. As is evident from , prazosin causes major shift in the Ang II concentration response curves suggesting that this might be the subsidiary supporting mechanism by which prazosin acts, ensuing a higher potency as compared to its counterparts, doxazosin ().
Figure 1. Inhibition observed with prazosin and doxazosin in Ang II-induced contractions of rat thoracic aorta. Results are expressed as percentage of maximum contractions obtained on aortic strips incubated with solvent. Values are represented as mean ± SEM of 4–5 experiments.
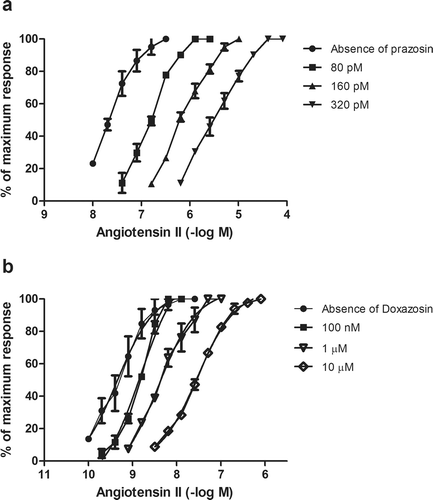
The pA2 value of prazosin (8.26 ± 0.7) is almost equal to losartan (8.08 ± 0.3) on Ang II receptor. This is a totally new and surprising finding unreported in the literature. It may be because of prazosin possessing some stereochemical features required to bind the AT1 receptor or may possess some three-dimensional features required to fill in AT1 receptor pockets. Further work on the structurally related analogs of prazosin is in progress.
Postural hypotension is one of the common side effects of α1-receptor blockers frequently reported by patients. Prazosin, being the most prominent in this regard, causes orthostatic hypotension in majority of the patients. Based on our findings, we take the liberty to suggest that prazosin mediates fall in blood pressure through blockade of not only α1-receptors but also AT1 receptor as well. An absence of baroreceptor-mediated reflex in patients receiving prazosin therapy is responsible for orthostatic hypotension. The baroreceptor reflex is regulated through the sympathetic as well as the parasympathetic nervous system. An activation of the SNS results in a consequential release of norepinephrine. The primary action of norepinephrine released in this manner is to increase the resistance of blood vessels, ultimately maintaining the blood pressure. Second, the released norepinephrine also stimulates the secretion of renin and, ultimately, formation of Ang II. This de novo formation of Ang II can cause direct vasoconstriction mediated through AT1 receptors. But as per our findings, prazosin blocks this arm through competitive blockade of AT1 receptors. As a novel finding, we can therefore assume that potency of α1-blockers is additionally a function of AT1 receptor antagonism also. These results are amazing and throw new light on the antihypertensive effect of prazosin type of compounds. Such a novel finding will be able to delineate new facets of mechanism of α1-antagonists. The high potency of this class of antihypertensive agents receives a good stand based on our findings. This finding will be of use in designing potent dual-acting antihypertensive agents.
Declaration of interest
This work was financially supported by University Research Fellowship Contingency (No. ADM/3/468) to H. P. Gandhi.
References
- Arunlakshana O, Schild HO. (1959). Some quantitative uses of drug antagonists. Br J Pharmacol Chemother, 14, 48–58.
- Cowley AW Jr. (1992). Long-term control of arterial blood pressure. Physiol Rev, 72, 231–300.
- DiBona GF. (1989). Sympathetic nervous system influences on the kidney. Role in hypertension. Am J Hypertens, 2, 119S–124S.
- Drew GM, Whiting SB. (1979). Evidence for two distinct types of postsynaptic α-adrenoceptor in vascular smooth muscle in vivo. Br J Pharmacol, 67, 207–215.
- Du Y, Qiu J, Nelson SH, Wang DH. (1997). Regulation of type 1 ANG II receptor in vascular tissue: Role of α1-adrenoreceptor. Am J Physiol, 273, R1224–R1229.
- Elliott WJ. (2002). Is fixed combination therapy appropriate for initial hypertension treatment? Curr Hypertens Rep, 4, 278–285.
- Global Health Risks. (2009). Mortality and burden of disease attributable to selected major risks, WHO report, I–VI.
- Grassi G. (2001). Renin-angiotensin-sympathetic crosstalks in hypertension: Reappraising the relevance of peripheral interactions. J Hypertens, 19, 1713–1716.
- Hall RA. (2004). beta-adrenergic receptors and their interacting proteins. Semin Cell Dev Biol, 15, 281–288.
- Horn PT, Kohli JD, Listinsky JJ, Goldberg LI. (1982). Regional variation in the α-adrenergic receptors in the canine resistance vessels. Naunyn Schmiedebergs Arch Pharmacol, 318, 166–172.
- Li HT, Long CS, Gray MO, Rokosh DG, Honbo NY, Karliner JS. (1997). Cross talk between angiotensin AT1 and α1-adrenergic receptors: Angiotensin II downregulates α1a-adrenergic receptor subtype mRNA and density in neonatal rat cardiac myocytes. Circ Res, 81, 396–403.
- Mancia G, Saino A, Grassi G. (1995). Interactions between the sympathetic nervous system and the renin-angiotensin system. In: Laragh JG, Brenner BM, eds. Hypertension: Pathophysiology, Diagnosis and Management. New York: Raven Press, 399.
- Morphy R, Rankovic Z. (2009). Designing multiple ligands – medicinal chemistry strategies and challenges. Curr Pharm Des, 15, 587–600.
- Paulis L, Unger T. (2010). Novel therapeutic targets for hypertension. Nat Rev Cardiol, 7, 431–441.
- Rang HP, Dale MM, Ritter JM, Flower RJ. (2007). Pharmacology. Philadelphia, USA: Churchill Livingstone.
- Reid IA. (1992). Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol, 262, E763–E778.
- Reit E. (1972). Actions of angiotensin on the adrenal medulla and autonomic ganglia. Fed Proc, 31, 1338–1343.
- Sealey JE, Laragh JH. (1995). The renin-angiotensin-aldosterone system for the normal regulation of blood pressure and sodium and potassium homeostasis. In: Laragh JG, Brenner BM, eds. Hypertension: Pathophysiology, Diagnosis and Management. New York: Raven Press, 1763–1797.
- Shepherd JT, Mancia G. (1986). Reflex control of the human cardiovascular system. Rev Physiol Biochem Pharmacol, 105, 1–99.
- Starke K. (1977). Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol, 77, 1–124.
- Summers RJ. (1984). Renal α adrenoceptors. Fed Proc, 43, 2917–2922.
- Wolff DW, Buckalew VM Jr, Strandhoy JW. (1984). Renal α1- and α2-adrenoceptor mediated vasoconstriction in dogs: Comparison of phenylephrine, clonidine, and guanabenz. J Cardiovasc Pharmacol, 6 Suppl 5, S793–S798.
- Wolff PW, Gesek FA, Strandhoy JW. (1985). In vivo assessment of rat renal vascular α adrenoceptors [Abstract]. Fed Proc, 44, 76–92.
- Zimmerman BG, Sybertz EJ, Wong PC. (1984). Interaction between sympathetic and renin-angiotensin system. J Hypertens, 2, 581–587.