Abstract
Context: Halophila spp. is a strong medicine against malaria and skin diseases and is found to be very effective in early stages of leprosy. Seagrasses are nutraceutical in nature and therefore of importance as food supplements.
Objective: The antibacterial, antioxidant, and anti-inflammatory activities of Halophila ovalis R. Br. Hooke (Hydrocharitaceae) methanol extract were investigated and the chemical constituents of purified fractions were analyzed.
Materials and methods: Plant materials were collected from Pondicherry coastal line, and antimicrobial screening of crude extract, and purified fractions was carried out by the disc diffusion method and the minimum inhibitory concentration (MICs) of the purified fractions and reference antibiotics were determined by microdilution method. Antioxidant and anti-inflammatory activities were investigated in vitro. Chemical constituents of purified fractions V and VI were analyzed by gas chromatography–mass spectrometry (GC–MS), and the phytochemicals were quantitatively determined.
Results: Methanol extract inhibited the growth of Bacillus cereus at a minimum inhibitory concentration of 50 µg/mL and other Gram-negative pathogens at 75 µg/ml, except Vibrio vulnificus. Reducing power and total antioxidant level increased with increasing extract concentration. H. ovalis exhibited strong scavenging activity on 2,2-diphenyl-1-picrylhydrazyl (DPPH) and superoxide radicals at IC50 of 0.13 and 0.65 mg/mL, respectively. Methanol extract of H. ovalis showed noticeable anti-inflammatory activity at IC50 of 78.72 µg/mL. The GC–MS analysis of H. ovalis revealed the presence of triacylglycerols as major components in purified fractions. Quantitative analysis of phytochemicals revealed that phenols are rich in seagrass H. ovalis.
Discussion and conclusion: These findings demonstrated that the methanol extract of H. ovalis exhibited appreciable antibacterial, noticeable antioxidant, and anti-inflammatory activities, and thus could be use as a potential source for natural health products.
Introduction
Seagrasses are submerged marine angiosperms that grow successfully in tidal and subtidal marine environments except in polar regions. The possibility of collecting organisms directly from the ocean with the use of SCUBA opened a new gate to a largely untapped resource with a wide range of unique structures and novel compounds. Seagrasses are well documented for the presence of potent diverse secondary metabolites (CitationPuglisi et al., 2007).
Vibrio spp., especially luminous Vibrio harveyi Johnson & Shunk (Vibrionaceae), and Vibrio parahaemolyticus Fujino et al. (Vibrionaceae), have been implicated as the main bacterial pathogens of shrimp in hatcheries as well as farms (CitationSandip et al., 2009). Problems including solubility, palatability, toxicity, cost, delivery, and governmental restrictions have limited the available antibiotics, especially in food fish culture (CitationChoudhury et al., 2005). Decreased efficacy and increased resistance of pathogens to antibiotics has necessitated the development of new alternative drugs/compounds (CitationSmith et al., 1994).
Acinetobacter baumannii has emerged as an important and problematic human pathogen as it is the causative agent for several types of infections including pneumonia, meningitis, septicemia, and urinary tract infections. Clinical impact of A. baumannii infection has been a matter of continuing debate. Since pathogens gaining resistance to drugs is common due to haphazard use of antibiotics, much attention is needed to kill or control the pathogens using bioactive substances. herefore, seagrasses have received comparatively less bioassay attention than seaweeds. Hence analyses of secondary metabolites toward seagrasses possessing antimicrobial and antifungal properties are found to be imperative to emphasize.
Reactive oxygen species such as O2, hydroxyl radical (OH˙), peroxide radical (ROO˙), and nitric acid radical are involved in extensive oxidative damage to the cells that leads to age-related degenerative diseases. Because of the carcinogenicity of commercially available antioxidants, pharmacologists have turned their attention toward natural antioxidants. Application of bioactive substances derived from marine organisms with respect to functional foods, cosmetics, cosmeceuticals, and pharmaceuticals have been reviewed (CitationKim et al., 2008). Prima facie, our investigation represents a prelude approach to characterize the antibacterial activity of crude methanol extract of seagrass Halophila ovalis R. Br. Hooke (Hydrocharitaceae) against marine and human pathogens, antioxidant potential, and anti-inflammatory effect on peripheral blood mononuclear cells (PBMCs). Chemical constituents of purified fractions were analyzed by GC–MS and phytochemicals were quantitatively determined.
Materials and methods
Collection of seagrass
Fresh seagrass samples of H. ovalis were collected in low tide from Chunnambar estuary (Pondicherry), India, during September 2009. Plant specimen was identified by Prof. N. Parthasarathy, Salim Ali School of Ecology, Pondicherry University. Specimen was preserved in 5% formalin solution.
Preparation of extracts
The seagrass sample was washed with sea water three times and then successively with tap water and distilled water to remove the epiphytes and other wastes. Finally, the sample material was air-dried under the shade for 2 weeks. The dried plant materials (10 g dry weight) were ground to fine powder and extracted in 100 mL methanol for 24 h using a Soxhlet extraction apparatus and the extract was filtered through a Buchner funnel with Whatman No. 1 filter paper. This was repeated three times for the complete extraction of compounds, and all the three methanol extracts were pooled. The solvent was evaporated from the crude extract by rotatory evaporation. The dried extract (1 g) was dissolved in 2 mL of methanol and stored at 4°C until use.
Test organisms
Bacteria, Vibrio parahaemolyticus MTCC 451, V. fischeri MTCC 1738, V. vulnificus MTCC 1145, Bacillus cereus MTCC 430, and Escherichia coli MTCC 1687 were procured from Microbial type culture collection, IMTECH, Chandigarh, India. V. anguillarum was procured from Central Institute of Brackish-water Aquaculture (CIBA), Chennai. The multidrug resistant bacteria Acinetobacter baumannii was obtained from Pondicherry Institute of Medical Sciences (PIMS), Pondicherry, India, and the strain was biochemically characterized (CitationPrashanth et al., 2008). The pathogenic bacteria were cultured individually on tryptic soy broth at 37°C for 18 h, before inoculation for assay. Broth culture (100 µL), which contained 107–108 bacteria per milliliter was added to tryptic soy agar medium (Hi-media, Mumbai), poured into sterile Petri dishes and allowed to solidify.
Antibacterial assay
Growth inhibition of pathogens by seagrass extract was assessed using the paper disc diffusion method. Briefly, sterile filter paper discs impregnated with crude extract (200 µg/mL), positive control (ampicillin 50 µg/mL), and negative control (methanol) were allowed to air dry and subsequently placed equidistantly onto the surface of the pathogen-seeded tryptic soy agar plates. The plates were kept in an inverted position and incubated at 37°C for 18 h. The growth inhibition was assessed as the diameter (in mm) of the zone of inhibited microbial growth. The experiment was carried out in triplicate. Experimental data represent mean ± SD of each sample, unless otherwise stated.
Minimum inhibitory concentration assay
A broth microdilution method was used to determine the minimum inhibitory concentration (MIC) (CitationMazzanti et al., 2000; CitationDevienne & Raddi, 2002; CitationNCCLS, 2008). All tests were performed in Mueller–Hinton agar medium (Hi-media).
Antioxidant assays
Reducing power
Total reducing power of seagrass extract was determined as described by CitationOyaizu (1986). The crude methanol extract at different concentrations (100–500 µg/mL) was dissolved in minimum quantity of methanol, and volume was made up to 1 mL with phosphate buffer, and 1% potassium ferricyanide was mixed with phosphate buffer (0.2 M, pH 6.6), and the mixture was incubated at 50°C for 20 min. About 2.5 mL of 10% trichloroacetic acid was added to the reaction mixture which was centrifuged at 1000 × g for 10 min. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL), FeCl3 (1.5 mL, 0.1%), and the absorbance was measured at 700 nm. Ascorbic acid at aforementioned concentration was used as standard. The higher the absorbance of the reaction mixture, the greater is the reducing power.
Total antioxidant activity
Total antioxidant activity of seagrass extract was determined according to the method of CitationPrieto et al. (1999). Briefly, 0.2 mL of sample at different concentrations (0.5–2.5 mg/mL) was mixed with 0.1 mL distilled water and 3.0 mL reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate, and 4.0 mM ammonium molybdate). The reaction mixture was incubated at 95°C for 90 min under water bath. After incubation, absorbance of all the sample mixtures was measured at 695 nm. Ascorbic acid was used as standard. Increase in absorbance of the reaction mixture indicates greater total antioxidant activity.
DPPH radical scavenging activity
The free-radical scavenging activity of seagrass extract was measured by DPPH according to CitationBlois method (1958). The DPPH solution (150 µL) was added to 3 mL methanol, and the absorbance was taken immediately at 516 nm for control reading. Different concentration of test samples (0.2–1.0 mg/mL) was mixed with 3 mL of methanol, and 150 µL of DPPH (0.1 mM) solution was added to each test tube. Absorbance was measured at 516 nm in a UV-Visible spectrophotometer (U-2000 model, Hitachi, Japan). A low absorbance of reaction mixture indicated a high free-radical scavenging activity. Butylated hydroxyanisole (BHA) was used as reference compound. The percentage DPPH scavenging effect was calculated as follows:
where, Acont was the absorbance of the control reaction, and Atest was the absorbance in the presence of the seagrass sample.
Superoxide anion scavenging activity
Measurement of superoxide anion scavenging activity was based on the method described by CitationNishikimi et al. (1972). Superoxide radicals were generated in the PMS-NADH system containing 3 mL Tris-HCl buffer (16 mM, pH 8.0), 468 µM NADH, 300 µM NBT, 60 µM PMS, and varying concentrations of sample (0.2–1.0 mg/mL); the mixture prepared earlier was incubated at room temperature for 5 min, and the absorbance read at 560 nm against the blank. In the control, the sample was substituted with Tris-HCl buffer. Decreased absorbance of the reaction mixture indicated increased superoxide anion scavenging activity. Gallic acid was used as reference compound. The capability of scavenging superoxide radical was calculated using the following equation.
where, Acont was the absorbance of the control reaction, and Atest was the absorbance in the presence of the seagrass sample.
Anti-inflammatory assay
Preparation of PBMCs
Mononuclear cells were isolated from human peripheral blood by Histopaque-1077 (Sigma) density gradient using standard procedures (CitationBignold & Ferrante, 1987). The PBMCs from the buffy layer were finally suspended in RPMI–1640 medium (Sigma) containing 10% fetal calf serum (FCS), and the cells were counted and assessed for cell viability using trypan blue exclusion test.
Mitogen-induced lymphocyte proliferation and its inhibition by anti-inflammatory agents
The PBMCs (2 × 105 cells/well) in a volume of 200 µL in RPMI-1640 medium containing 10% FCS and 1 µg/mL of phytohemagglutinin (PHA) were seeded in a 96-well U bottom plate followed by the addition of anti-inflammatory agent at various concentrations. The culture was then incubated at 37°C for 24 h in CO2 incubator containing 5% CO2 and 90% humidity. Assays were conducted in triplicate for each concentration of seagrass extract. Experimental data represent mean ± SD of each compound, unless otherwise stated.
Cytotoxic studies by MTT assay
To confirm the suppressive effect of crude methanol extract on lymphocyte proliferation, 2 µL of the compound was added at various concentrations (1, 10, 50, 100 µg/mL). Triton X was used as negative control and DMSO was used as solvent control. Following the removal of medium from the wells, 10 µL of MTT (5 mg/mL resuspended in PBS) to each well was added. After incubation at 37°C for 4 h, the floating cells were carefully removed, and 50 µL of DMSO was added to each well to lyse the cells, and the absorbance was measured at 570 nm using a microplate reader. Finally, the percentage of cell viability was calculated as follows:
Purification of fractions by thin layer chromatography
To investigate the chemical constituents, concentrated crude methanol extract of seagrass was purified by thin layer chromatography using ethyl acetate-hexane as solvent systems. The crude extract was separated in a pre-coated aluminum TLC sheet with silica gel G 60 as stationary phase, and ethyl acetate-hexane mixture in the ratio of 9.5:0.5 as mobile phase. The eluted spots, representing various fractions were visualized under UV transilluminator at 254 nm, and also in the iodine chamber. The TLC resolved spots of methanol extract at various Rf values were scrapped from the TLC plate, and the scrapped spots were dissolved in methanol, mixed well, and centrifuged at 12,000 × g for 5 min. A total of six different fractions were collected and the supernatant (40 µL) of each fraction was used to check the antibacterial activity against pathogens using the disc diffusion method in triplicate. Ampicillin was used as positive control. Experimental data represent mean ± SD of each sample, unless otherwise stated.
Gas chromatography – mass spectrometry analysis
After the initial thin layer chromatography, the purified fractions were analyzed using an Agilent 6890 series high-temperature gas chromatography–mass spectrometer (GC–MS), fitted with an auto injector. For GC–MS analysis, a high-temperature column (DB-5 ht, 30 m × 0.25 mm id × 0.25 μm film thickness) was purchased from Agilent Technologies (Agilent, USA). By employing a high-temperature column, we eliminated the need for derivatization of each sample. The injector and detector temperatures were set at 350°C, while the initial column temperature was set at 80°C. A 2 μL sample volume was injected into the column and ran using split less mode. After 2 min, the oven temperature was raised to 150°C at a ramp rate of 10°C/min. The oven temperature was then raised to 250°C at a ramp rate of 5°C/min, and finally the oven temperature was raised to 280°C at a ramp rate of 20°C/min and maintained at this temperature for 40 min. The helium carrier gas was programmed to maintain a constant flow rate of 1 mL/min, and the mass spectra were acquired and processed using both Agilent ChemStation (Agilent, USA) and AMDIS32 software. The major compounds were identified by comparison of their mass with authentic standards.
Quantitative analysis of phytochemicals
The dried, powdered sample of seagrass H. ovalis was analyzed for quantification of alkaloids (CitationHarborne, 1973), carbohydrates (CitationSheifter et al., 1950), flavanoids (CitationBohm & Kocipai-Abyazan, 1974), phenols (CitationSadasivam & Manickam, 1992), proteins (CitationLowry et al., 1951), saponins (CitationObadoni & Ochuko, 2001), and tannins (CitationVan-burden & Robinson, 1981).
Statistical analysis
All results were expressed as mean ± standard deviation. Statistical differences between the experimental groups were determined by one-way analysis of variance (ANOVA), and differences were considered to be statistically significant, if p < 0.05. Tukey’s post hoc test was performed for multiple group comparison between concentrations. All computations were done by employing statistical software (SPSS Version 7.0, SPSS Inc., Chicago, IL, USA).
Results
Antibacterial assay
The results of the antibacterial activity of the crude methanol extract of seagrass H. ovalis against Gram-positive and Gram-negative pathogens were summarized in . In this study, the methanol extract of H. ovalis (200 µg/mL) displayed a good antibacterial activity of 17 mm against Bacillus cereus followed by 14 mm against Acinetobacter baumannii.
Table 1. Antibacterial screening of crude extract and minimum inhibitory concentration of seagrass H. ovalis against pathogens (inhibition zone was measured to nearest millimeter).
Minimum inhibitory concentration assay
Minimum inhibitory concentration of the methanol extract of H. ovalis which showed maximum activity against the pathogens was depicted (). The growth of Bacillus cereus was inhibited at a minimum inhibitory concentration of 50 µg/mL followed by Gram-negative pathogens at 75 µg/mL, whereas, Vibrio vulnificus growth was inhibited at a minimum inhibitory concentration of 100 µg/mL.
Antioxidant assays
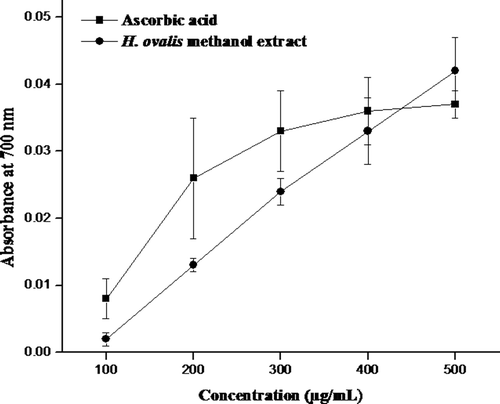
The reducing power of H. ovalis methanol extract is portrayed in . As shown in , the reducing power was found to be increasing in concentration dependent. The absorption was increased from 0.002 ± 0.001 to 0.042 ± 0.005 with the concentration increased from 100–500 µg/mL. Similarly, the reducing power increased in positive control (ascorbic acid) from 0.008 ± 0.003 to 0.037 ± 0.002 with increasing concentration. Also, it was observed that at given concentration (500 µg), the methanol extract of H. ovalis had higher reducing power than ascorbic acid. The reducing capacity of H. ovalis the methanol extract was statistically not significant (p > 0.05) as compared to ascorbic acid. However, both the sample and ascorbic acid showed a significant difference between the concentrations (p < 0.05). This property is associated with the presence of reductones that are reported to be terminators of free radical chain reaction.
Figure 1. Reducing power of H. ovalis methanol extract. Results are representative of three separated experiments.
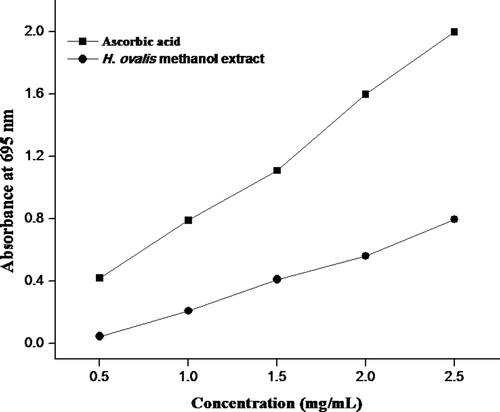
The total antioxidant activity of methanol extract of H. ovalis by phosphomolybdenum method is depicted in . In this method, Mo (VI) is reduced to form a green phosphate/Mo (V) complex. The total antioxidant activity increased with increase in absorption from 0.045 ± 0.002 to 0.795 ± 0.003 at increasing concentration (0.5–2.5 mg/mL). The sample was statistically not significant (p > 0.05) as compared to standard but showed a significant difference between the concentrations (p < 0.05).
Figure 2. Total antioxidant activity of H. ovalis methanol extract. Results are representative of three separated experiments.
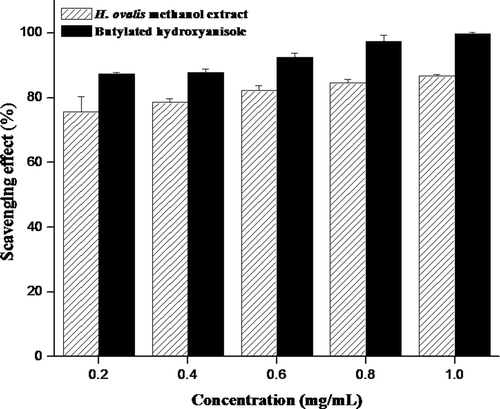
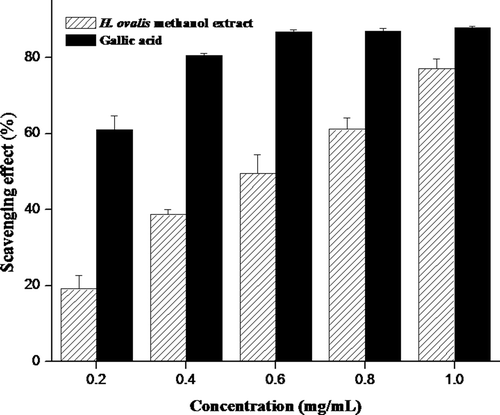
The DPPH and superoxide have been used extensively as free radicals to evaluate reducing substances and are useful reagents for investigating the free radical scavenging activities of compounds. The antiradical power of an antioxidant activity by measurement of the decrease in the absorbance of DPPH radical at 517 nm was determined. The methanol extract of H. ovalis showed significant DPPH radical scavenging activity (p < 0.05) between concentrations and IC50 was observed at 0.13 mg/mL (). Similarly, superoxide radicals scavenged by methanol extract of H. ovalis were found to be statistically significant (p < 0.05) and scavenging activity at 50% was observed at the concentration of 0.65 mg/mL (). The scavenging activities were found to be increased with increasing concentration of extract in DPPH as well as superoxide assays. In fact, the inhibitory ability of the methanol extract was inferior to those of the commercial counterparts such as butylated hydroxyanisole and gallic acid.
Mitogen-induced lymphocyte proliferation
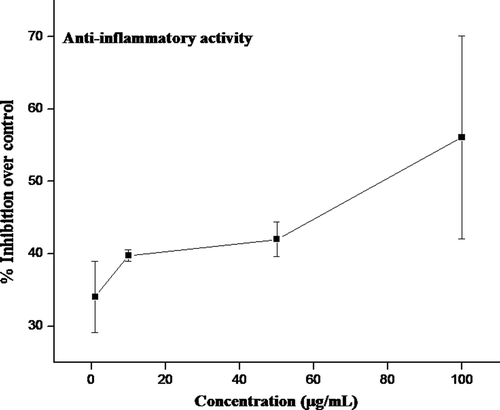
To find out whether the crude methanol extract obtained from seagrass H. ovalis possess anti-inflammatory activity, their effect at various concentrations on the inhibition of proliferation of mitogen-induced PBMCs was investigated and depicted in . There was a significant increase in proliferation of PBMCs on induction with PHA but this response was considerably inhibited by crude methanol extract from H. ovalis. A 50% inhibition (IC50) of proliferation of PBMCs was observed at a concentration of 78.72 µg/mL of crude methanol extract.
Figure 5. Inhibitory effect of crude extract from H. ovalis on mitogen-induced proliferation. Mitogen PHA (1 µg/mL)-induced PBMC (2 × 105/well) was treated with different concentrations of crude extracts and the percentage inhibition of lymphocyte proliferation was determined over control. Results were representative of three separated experiments.
Purification of antibacterial fractions
Purified fractions V and VI of seagrass H. ovalis showed an inhibition zone of more than 10 mm against Vibrio parahaemolyticus (12 mm), Acinetobacter baumannii (12 mm), V. anguillarum (11 mm), and V. fischeri (11 mm), whereas a diameter of less than 10 mm was observed in other fractions against the pathogens tested ().
Table 2. Antibacterial screening of purified fractions of seagrass H. ovalis against pathogens (inhibition zone was measured to nearest millimeter).
GC–MS analysis
The GC–MS analysis revealed the presence of saturated fatty acids, and aromatic carboxylic acid, in the purified fractions of H. ovalis ( and ). The major component was 9-octadecenoic acid (27.01%) followed by hexadecanoic acid (21.63%), and octadecanoic acid (10.42%) in fraction V, whereas, benzoic acid (11.11%) was found to be a major chemical constituent followed by tetradecanoic acid (6.12%), and hexadecane (3.47%) in fraction VI.
Table 3. The GC – MS analysis of fraction V of H. ovalis.
Table 4. The GC–MS analysis of fraction VI of H. ovalis.
Quantitative analysis of phytochemicals
The phytochemicals of seagrass H. ovalis were quantitatively determined and depicted in . Phytochemical analysis revealed that phenols were found to be rich in H. ovalis followed by saponins, flavanoids, proteins, carbohydrates, and alkaloids, whereas, tannins were found to be less than other phytochemicals.
Table 5. Biochemical composition of seagrass H. ovalis (n = 3; means ± SD).
Discussion
Natural products are considered as an important source of new antibacterial agents. Many chemically unique compounds of marine origin with different biological activities have been isolated and a number of them are under investigation and/or are being developed as new pharmaceuticals (CitationFaulkner, 2000a,Citationb; CitationKim et al., 2008). Several species of seagrass produce antimicrobial compounds that may act to reduce or control microbial growth. In the present study, the methanol extract of seagrass H. ovalis collected from Chunnambar estuary, Pondicherry coastal line, exhibited antibacterial activity against Gram-positive Bacillus cereus and Gram-negative pathogens such as Vibrio parahaemolyticus, V. fischeri, V. anguillarum, V. vulnificus, and Acinetobacter baumannii. The results of the present study consisted with some preceding studies of seagrass antibacterial activity (CitationRengasamy et al., 2008). Similarly extracts from Cymodocea rotundata Ehrenberg and Hemprich ex Ascherson (Cymodoceaceae) was effective against Bacillus species (CitationBernard & Pesando, 1989). It was reported that ethanol and methanol extracts of seagrasses showed better zones of inhibition against bacterial pathogens (CitationThirumaran et al., 2009). The methanol and diethyl methyl formamide extracts of seagrass sp. was found to be active against Gram-positive pathogens (CitationShelat, 1979). We also observed that crude methanol extract of seagrass H. ovalis exhibited good antibacterial activity against Gram-positive as well as to Gram-negative pathogens. The presence of antibacterial substances could be varied from algal species to species (CitationLustigman & Brown, 1991). In the present study, the methanol extract of H. ovalis controlled the growth of B. cereus at a minimum concentration of 50 µg/mL. Similarly, crude methanol extract of H. ovalis was effective in controlling the growth of Micrococcus luteus at the minimum inhibitory concentration of 50 µg/mL (CitationRengasamy et al., 2008).
Recently, extensive studies have been conducted on the utility of seagrasses as a source of natural antioxidants. Hence, in the present study, we screened the antioxidant activity for methanol extract of seagrass H. ovalis using different methods. Concentration dependency of antioxidant activity was investigated as a function of reducing power. The reducing power increased with increasing concentration in methanol extract of H. ovalis. The same trend has also been reported in methanol extracts of higher plants (CitationKumaran & Karunakaran, 2007). Also, the total antioxidant activity of H. ovalis methanol extract was found to be increased with increasing concentration. The DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. Therefore, DPPH is often used as a substrate to evaluate antioxidant activity of an antioxidant. Our study showed that methanol extract of H. ovalis exhibited 50% of scavenging activity on DPPH radicals at 0.13 mg/mL and superoxide radicals at 0.65 mg/mL, respectively, whereas seagrass Posidonia oceanica (L.) Delile (Posidoniaceae) showed relatively a less scavenging activity 13.9% at 2 mg/mL (CitationKartal et al., 2009). However, in vivo studies revealed that P. oceanica extract restored antioxidant enzymes and decreased lipid peroxidation in diabetic animals (CitationGokce & Haznedaroglu, 2008). This present finding corroborates well with earlier reports in other higher plants including brown and red seaweeds (CitationKuda et al., 2005; CitationKumaran & Karunakaran, 2007).
In the present work, an attempt was made to study the anti-inflammatory activity, if any, in methanol extract of seagrass H. ovalis by evaluating the suppression of mitogen-induced lymphocyte proliferation, respectively. The ability of the methanol extract from H. ovalis on proliferation of mononuclear cells, which are crucial for generation of effective immune responses, was analyzed in the presence of the mitogen PHA. Interestingly, the methanol extract of H. ovalis inhibited the PBMC proliferation. Organic extracts from different plants were reported to inhibit proliferation of PBMC (CitationManjula et al., 2006; CitationGayathri et al., 2007; CitationWu et al., 2007). In our study, inhibition of proliferation of mitogen-induced PBMC in the presence of added methanol extract was observed. For the first time, this finding suggests that the crude methanol extract of seagrass H. ovalis do contain compounds, capable of suppressing PBMC proliferation. Further study is needed to see that the suppression of proliferation of PBMC is by means of cytotoxic effects of this methanol extract. Our preliminary results encourage us to further investigate the immunomodulatory properties of the crude extract.
In this study, the purified fractions of seagrass H. ovalis displayed good antibacterial activity against Gram-positive as well as Gram-negative pathogens. Fractions V and VI of H. ovalis extract effectively inhibited V. parahaemolyticus, V. vulnificus, and A. baumannii. Similarly, purified fractions of selected South African seaweeds showed broad spectrum activity against Gram-positive as well as Gram-negative pathogens than crude extracts of the same seaweeds (CitationVlachos et al., 1997). The antibacterial activity of marine algae and mangrove plants against fish pathogens was observed, and the fractions of methanol extract of red seaweed Gracilaria corticata J. Agardh (Gracilariaceae) showed good activity against fish pathogens Pseudomonas aeruginosa and V. alginolyticus (CitationSree et al., 2005).
There are numerous reports of compounds derived from macro algae with a broad range of biological activities such as antibiotics (CitationScheuer, 1990), and triacylglycerols in model diatoms (CitationEizadora et al., 2009). The GC–MS analysis of active fractions of H. ovalis in this study revealed the presence of triacylglycerols such as hexadecanoic acid, tetradecanoic acid, 9-octadecenoic acid, octadecanoic acid, and hydrocarbon, hexadecane. Similar group of triacylglycerols were reported in Zosteraceae species Zostera japonica Aschers. & Graeben (CitationHua et al., 2006), and Zostera marina L. (CitationSanina et al., 2004), respectively. The presence of hydrocarbon, hexadecane in the H. ovalis extract corroborated well with earlier reports in brown algae Cystoseira barbata (Good et Woodw.) J. Agardh (Cystoseiraceae), and Dictyotaceae species Dictyopteris membranaceae (Stackhouse) batters (CitationOzdemir et al., 2006).
In this study, a wide range of phytochemicals were analyzed quantitatively. The protein content in the present investigation was found to be less than the level of protein reported in an earlier study on seagrass H. ovalis (CitationAthiperumalsami et al., 2008). The amount of carbohydrate was found to be more in H. ovalis. The amount of phenol in the seagrass H. ovalis investigated in the present study appears to be more than reported in pulses (CitationVadivel & Janarthanan, 2000). Soluble phenolic acids have been shown to be abundant in a variety of seagrasses (CitationZapata & McMillan, 1979; CitationBuchsbaum et al., 1991). Phenol rich seagrass indicated that extracts may have antioxidant activity and antimicrobial activity which may help in preventing a number of diseases through free-radical scavenging activity (CitationHarrison & Chan, 1980; CitationAnggadiredja et al., 1997; CitationRuberto et al., 2001; CitationVasanthi et al., 2006). The amount of tannin in the seagrass was found in the average level (2.940–9.800 mg/g) corroborated with the earlier study on seagrasses (CitationAthiperumalsami et al., 2008). In addition, we quantitatively determined the saponins, flavanoids, and alkaloids content of H. ovalis, which so far has not been reported by any authors. In the present study, phytochemical analysis showed that phenols and saponins were found to be in high concentration in H. ovalis than other phytochemicals.
In conclusion, our study showed that the crude and purified fractions of H. ovalis showed appreciable antibacterial activity against Gram-positive as well as Gram-negative human and fish pathogens. The methanol extract of H. ovalis was found to be a promising candidate as antioxidant and anti-inflammatory agent. The GC–MS analysis of purified fractions revealed the presence of oleic acid, palmitic acid, and benzoic acid as major constituents. Further study is underway to elucidate the structure of compound, and the mechanism of inhibition of pathogens. However, the abundant availability of seagrasses along the Indian coastline opens up a new avenue for the entry of pharmaceutical industries in developing new drugs for aquaculture remedies.
Acknowledgments
The authors thank Prof. N. Parthasarathy, Salim Ali School of Ecology, Pondicherry University, Pondicherry for the identification of seagrasses. We also thank Dr. Babu Rajendar, Dept. of Environmental Biotechnology, Bharathidasan University, Tiruchirappalli for providing the GC–MS facility.
Declaration of interest
This work was supported by a grant from Department of Biotechnology (DBT), New Delhi, India. The authors declare that there are no other conflicts of interest.
References
- Anggadiredja J, Andyani R, Murawanah H. (1997). Antioxidant activity of Sargassum polycystum (Phaeophyta) and Laurencia obtusa (Rhodophyta) from Seribu Islands. J Appl Phycol, 9, 477–479.
- Athiperumalsami T, Kumar V, Louis Jesudass L. (2008). Survey and phytochemical analysis of seagrasses in the Gulf of Mannar, southeast coast of India. Bot Mar, 51, 269–277.
- Bernard P, Pesando D. (1989). Antibacterial and antifungal activity of extracts from the rhizomes of the Mediterranean seagrasses Posidonia oceanica (L.) Delile. Bot Mar, 32, 85–88.
- Bignold LP, Ferrante A. (1987). Mechanism of separation of polymorphonuclear leukocytes from whole blood by the one-step Hypaque-Ficoll method. J Immunol Methods, 96, 29–33.
- Blois MS. (1958). Antioxidant determination by the use of a stable free radical. Nature, 181, 1199–1200.
- Boham BA, Kocipai-Abyazan R. (1974). Flavonoids and condensed tannins from leaves of Hawaiian vaccinium vaticulatum and V. calycinium. Pacific Sci, 48, 458–463.
- Buchsbaum R, Valiela I, Swain T, Dzierzeski M, Allen S. (1991). Available and refractory nitrogen in detritus of coastal vascular plants and macroalgae. Mar Ecol Prog Ser, 72, 131–143.
- Choudhury S, Sree A, Mukherjee SC, Pattaik P, Bapuji M. (2005). In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fish Sci, 18, 285–294.
- Devienne KF, Raddi MSG. (2002). Screening for antimicrobial activity of natural products using a microplate photometer. Braz J Microbiol, 33, 166–168.
- Eizadora TYU, Zendejas FJ, Lane PD, Gaucher S, Simmons BA, Lane TW. (2009). Triacylglycerol accumulation and profiling in the model diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum (Baccilariophyceae) during starvation. J Appl Phycol, 21, 669–681.
- Faulkner DJ. (2000a). Marine natural products. Nat Prod Rep, 17, 7–55.
- Faulkner DJ. (2000b). Marine pharmacology. Antonie Van Leeuwenhoek, 77, 135–145.
- Gayathri B, Manjula N, Vinaykumar KS, Lakshmi BS, Balakrishnan A. (2007). Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFalpha, IL-1beta, NO and MAP kinases. Int Immunopharmacol, 7, 473–482.
- Gokce G, Haznedaroglu MZ. (2008). Evaluation of antidiabetic, antioxidant and vasoprotective effects of Posidonia oceanica extract. J Ethnopharmacol, 115, 122–130.
- Harborne JB. (1973). Phytochemical methods. Chapman and Hall, New York. pp. 288.
- Harrison PG, Chan AT. (1980). Inhibition of the growth of microalgae and bacteria by extracts of eelgrass (Zostera marina) leaves. Mar Biol, 61, 21–26.
- Hua KF, Hsu HY, Su YC, Lin IF, Yang SS, Chen YM, Chao LK. (2006). Study on the antiinflammatory activity of methanol extract from seagrass Zostera japonica. J Agric Food Chem, 54, 306–311.
- Kartal M, Orhan I, Abu-asaker M, Senol FS, Atici T, Sener B. (2009). Antioxidant and anticholinesterase assets and liquid chromatography-mass spectrometry preface of various fresh-water and marine macroalgae. Pharmacognosy Magazine, 20, 291–297.
- Kim SK, Ravichandran YD, Khan SB, Kim YT. (2008). Prospective of the cosmeceuticals derived from marine organisms. Biotechnol Bioprocess Eng, 13, 511–523.
- Kuda T, Tsunekawa M, Goto H, Araki Y. (2005). Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J Food Comp Ana, 18, 625–633.
- Kumaran A, Karunakaran RJ. (2007). In vitro antioxidant properties of methanol extracts of five Phillanthus species from India. LWT, 40, 344–352.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Lustigman B, Brown C. (1991). Antibiotic production by marine algae isolated from the New York/New Jersey coast. Bull Environ Contam Toxicol, 46, 329–335.
- Manjula N, Gayathri B, Vinaykumar KS, Shankernarayanan NP, Vishwakarma RA, Balakrishnan A. (2006). Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down regulation of TNF-alpha, IL-1beta and IL-2. Int Immunopharmacol, 6, 122–132.
- Mazzanti G, Mascellino MT, Battinelli L, Coluccia D, Manganaro M, Saso L. (2000). Antimicrobial investigation of semipurified fractions of Ginkgo biloba leaves. J Ethnopharmacol, 71, 83–88.
- NCCLS. (2008). Performance Standards for Antimicrobial Susceptibility testing; Ninth Informational Supplement. NCCLS document M100-S9. National Committee for Clinical Laboratory Standards, Wayne, PA, pp. 120–126.
- Nishikimi M, Appaji N, Yagi K. (1972). The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun, 46, 849–854.
- Obadoni BO, Ochuko PO. (2001). Phytochemical studies and comparative efficacy of the crude extracts of some homostatic plants in Edo and Delta States of Nigeria. Global J Pure Appl Sci, 8, 203–208.
- Oyaizu M. (1986). Studies on product of browning reaction prepared from glucose amine. Jap J Nut, 44, 307–315.
- Ozdemir G, Horzum Z, Sukatar A, karabay-yavasoglu NU. (2006). Antimicrobial activities of volatile components and various extracts of Dictyopteris membranaceae and Cystoseira barbata from the Coast of Izmir, Turkey. Pharm Biol, 44, 183–188.
- Prashanth K, Srinivasa rao R, Uma karthika R, Singh SP, Shashikala P, Kanungo R, Jayachandran S. (2008). Correlation between biofilm production and multiple drug resistance in imipenem resistant clinical isolates of Acinetobacter baumannii. Ind J Med Micro, 26, 333–337.
- Prieto P, Pineda M, Aguilar M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem, 269, 337–341.
- Puglisi MP, Engel S, Jensen PR, Fenical W. (2007). Antimicrobial activities of extracts from Indo-Pacific marine plants against marine pathogens and saprophytes. Mar Biol, 150, 531–540.
- Rengasamy R, Kumar CS, Sarada DVL, Gideon TP. (2008). Antibacterial activity of three South Indian seagrasses, Cymodocea serrulata, Halophila ovalis and Zostera capensis. World J Microbiol Biotechnol, 24, 1989–1992.
- Ruberto G, Baratta MT, Biondi DM, Amico V. (2001). Antioxidant activity of extracts of the marine algal genus Cystoseira in a micellar model system. J App Phycol, 13, 403–407.
- Sadasivam S, Manickam A. (1992). Biochemical methods for agricultural sciences. Wiley Eastern Ltd., Madras. pp. 240.
- Sandip MS, Singh C, Arul V. (2009). Inhibitory activity of Streptococcus phocae PI80 and Enterococcus faecium MC13 against vibriosisin shrimp Penaeus monodon. World J Microbiol Biotech, 25, 697–703.
- Sanina NM, Goncharova SN, Kostetsky EY. (2004). Fatty acid composition of individual polar lipid classes from marine macrophytes. Phytochemistry, 65, 721–730.
- Scheuer PJ. (1990). Some marine ecological phenomena: chemical basis and biomedical potential. Science, 248, 173–177.
- Sheifter S, Bayton S, Novic B, Muntwyler E. (1950). The estimation of glycogen with the anthrone reagent. Arch Biochem Biophy, 25, 190–200.
- Shelat YA. (1979). Bioactive substances from Indian marine algae. Ph.D Thesis Saurashtra University, Rajkot, India.
- Smith P, Hiney MP, Samuelsen OB. (1994). Bacterial resistance to antimicrobial agents used in fish farming. Ann Rev fish Dis, 4, 273–313.
- Sree A, Choudhury S, Mukherjee SC, Pattnaik P, Bapuji M. (2005). In vitro antibacterial activity of extracts of selected marine algae and mangroves against fish pathogens. Asian Fis Sci, 18, 285–294.
- Thirumaran G, Umamaheshwari R, Anantharaman P. (2009). Potential antibacterial activities of seagrasses from Vellar estuary; Southeast Coast of India. Adv Biol Res, 3, 140–143.
- Vadivel V, Janardhanan K. (2000). Chemical composition of the underutilized legume Cassia hirsuta L. Plant Foods Hum Nutr, 55, 369–381.
- Van-burden TP, Robinson WC. (1981). Formation of complexes between protein and tannin acid. J Agric Food Chem, 1, 77–82.
- Vasanthi HR, Charles dorni AI, Vidyalakshmi KS, Rajamanickam GC. (2006). Free radical scavenging and antioxidant activity of a red algae Acanthophora spicifera. Relation to its chemical composition. Seaweed Res Utiln, 28, 119–125.
- Vlachos V, Critchley AT, Von holy A. (1997). Antimicrobial activity of extracts from selected Southern African marine macroalgae. S Afr J Sci, 93, 328–332.
- Wu MH, Tsai WJ, Don MJ, Chen YC, Chen IS, Kuo YC. (2007). Tanshinlactone A from Salvia miltiorrhiza modulates interleukin-2 and interferon-gamma gene expression. J Ethnopharmacol, 113, 210–217.
- Zapata O, McMillan C. (1979). Phenolic acids in seagrasses. Aquat Bot, 7, 307–317.