Abstract
Context: Ficus racemosa Linn. (Moraceae) bark is a rich source of phenolic compounds having diverse biological properties including antioxidant activity. The present study evaluated the cardioprotective activity of sequential acetone extract of Ficus racemosa bark against doxorubicin-induced cardiotoxicity in rats.
Materials and methods: The extract was standardized by high-performance liquid chromatography (HPLC) and subjected to acute toxicological evaluation in mice. Cardiotoxicity was induced by administration of doxorubicin (10 mg kg−1 i.v.) to the extract pretreated rats (250 and 500 mg kg−1) and compared with that of Arjuna, a standard cardiotonic. Biochemical parameters included CK-MB, LDH, AST, ALT, troponin I, thiobarbituric acid reactive substances (TBARS), and glutathione.
Results: The HPLC fingerprinting of the extract indicated the presence of bergenin (0.89%) and bergapten (0.07%). In an acute toxicity study, the extract at a dose of 2 g kg−1 did not cause any adverse changes and no mortality was observed. Administration of doxorubicin significantly increased (p ≤ 0.05) serum levels of creatine kinase, lactate dehydrogenase, aspartate aminotransferase, and alanine aminotransferase, which were decreased to an extent of 68, 63, 41, and 65%, respectively, in extract pretreated group (500 mg kg−1). Troponin I was undetected in control group, while it was found in serum of all the experimental groups. The extract pretreatment significantly decreased (p ≤ 0.05) TBARS and increased glutathione levels in serum and cardiac tissue. These observations were further substantiated by the histopathological studies.
Conclusion: The acetone extract of F. racemosa bark possesses potential cardioprotective activity against doxorubicin-induced cardiotoxicity in rats by scavenging free radicals generated by the administration of the drug.
Keywords::
Introduction
Doxorubicin (Dox) is one of the most potent broad spectrum antitumor anthracycline antibiotic, widely used to treat a variety of cancers, including severe leukemias, lymphomas, and solid tumors (CitationPriestman, 2008). The clinical use of Dox is restricted because of its serious toxicity on various organs viz., heart, liver, lung, kidney, and testis (CitationLui et al., 1986; CitationNg et al., 2006; CitationInjac & Strukelj, 2008). Doxorubicin administration can induce a wide variety of acute cardiotoxic effects including transient cardiac arrhythmia, nonspecific electrocardiographic abnormalities, pericarditis, and acute heart failure (CitationBillingham et al., 1978). Although, the precise mechanism is unclear, production of free radicals as a byproduct of its metabolism is considered to be the primary mechanism of Dox toxicity, consequently warranting some new approaches, such as the potential use of natural antioxidants, most commonly used and investigated compounds being vitamins (A, C, and E), coenzyme Q, flavonoids, polyphenols, herbal antioxidants, selenium, and virgin olive oil (CitationQuiles et al., 2002).
Ficus racemosa Linn. (syn. Ficus glomerata Roxb.) (Moraceae), commonly known as Gular, is widely distributed all over India, northern Australia, and other parts of Asia. In the Indian traditional system of medicine, all parts of this plant are considered important and have been extensively used in biliary disorders, dysentery, diabetes, diarrhea, and inflammatory conditions (CitationKirtikar & Basu, 1975; CitationNadkarni et al., 1976). F. racemosa bark being rich source of polyphenolic compounds has shown to possess excellent antioxidant properties in terms of radical scavenging activity, reducing power and antilipidperoxidative activity in rat liver homogenate (CitationAhmed & Urooj, 2009a). Further, it has also exhibited significant TBARS lowering and glutathione restoration effect in streptozotocin-induced diabetic rats (CitationAhmed & Urooj, 2009b). We have also reported F. racemosa stem bark to exhibit potential hepatoprotective effect against CCl4-induced hepatotoxicity in rats (CitationAhmed & Urooj, 2010).
In view of the above, since there are no reports on the cardioprotective activity of F. racemosa, the present study evaluated the cardioprotective activity of standardized extract of F. racemosa stem bark against doxorubicin-induced cardiotoxicity in rats.
Materials and methods
Chemicals and reagents
Doxorubicin and 5,5-dithio(bis)nitro benzoic acid (DTNB) were purchased from Sigma Aldrich, Bangalore, India. Creatine kinase (CK-MB), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), troponin I (Agappe Diagnostics, Ernakulam, India) assay kits, and Arjuna (Himalaya Healthcare, Bangalore, India), a standard cardio tonic were used. All the other chemicals and reagents used in the study were of extra pure analytical grade.
Preparation of the extract and high-performance liquid chromatography fingerprinting
Ficus racemosa stem bark was collected from Mukkadahally, Chamarajanagar district of Karnataka, India during September 2007, and was subsequently identified by Dr. Shivprasad Hudeda, JSS Ayurvedic Medical College, Mysore; a voucher specimen (BOT-001/2008) was deposited at the herbarium of the Department of Studies in Botany, University of Mysore, Mysore, India. The bark was cut into small pieces, dried (50°C) and powdered, passed through a 60 mesh sieve (BS), and the powder was extracted sequentially with solvents of increasing polarity (petroleum ether – chloroform – acetone – methanol – water) in a Soxhlet apparatus for 8 h each. Acetone extract (FRSACE) containing highest amount of phenolic compounds (CitationAhmed & Urooj, 2009a) was selected and analyzed by high-performance liquid chromatography (HPLC) (Shimadzu Corporation, Japan) using 1000 ppm solutions on a reverse phase packed column (RP C-18 column; Supleco, USA; 250 × 4.6 mm2; particle size 5 µm) using gradient elution with water and acetonitrile at a flow rate of 1.0 mL min−1 and run time of 30 min, monitored by a PDA detector at 270 nm. Gradient composition (min, % acetonitrile): 0, 20%; 5, 40%; 8, 75%; 12, 90%; 15, 95%; 25, 95%; 27, 20%; 30, 20% was used. The compounds were identified using external standards, wherein the standard solutions (1000 ppm) were repeatedly injected (n = 4) to confirm reproducibility of peak area and retention time. The relative standard deviation (RSD) values were less than 1.1% for peak areas and less than 0.5% for retention times.
Acute toxicity studies
Acute toxicity tests were carried out in Swiss albino mice of both the sexes weighing 20 ± 2 g according to OECD 423 guidelines (CitationOECD, 2001). The animals were divided into following two groups consisting of six animals each (three male and three female).
Group I (Control): normal mice received olive oil (1 mL kg−1 p.o.)
Group II (FRSACE): received sequential acetone extract (2 g kg−1 p.o.)
The mice were housed in polyacrylic cages and maintained at 23 ± 2°C, 45–60% RH and 12 h photo period. The extracts were given in the form of suspension after homogenization with olive oil by gavage using a stomach tube. They were provided with a standard pellet diet (Amrut feeds, Pune, India) and water ad libitum.
The animals were observed individually after dosing at least once during the first 30 min interval during the first 24 h, with special attention given during the first 4 h, and daily thereafter, for a total of 14 days. All observations were systematically recorded with individual records being maintained for each animal. The observations included changes in skin and fur, eyes and mucous membranes, and also respiratory, circulatory, autonomic, and central nervous systems, and somatomotor activity, behavior pattern, and mortality. Attention was given to observations of tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma.
Cardioprotective activity
Healthy male Wistar rats between 8 and 9 weeks of age and weighing 140–160 g were divided into following five groups (n = 6).
Group I: Control group; received distilled water (1 mL kg−1 BW, p.o.) for 9 days followed by sterile water for injection (1 mL kg−1 BW, i.v.) on 10th day.
Group II: Dox group; received distilled water (1 mL kg−1 BW, p.o.) for 9 days followed by a single dose of Dox injection (10 mg kg−1 BW, i.v.) on the 10th day.
Group III: FR250 group; received FRSACE (250 mg kg−1 BW, p.o.) for 9 days followed by a single dose of Dox injection (10 mg kg−1 BW, i.v.) on the 10th day.
Group IV: FR500 group; received FRSACE (500 mg kg−1 BW, p.o.) for 9 days followed by a single dose of Dox injection (10 mg kg−1 BW, i.v.) on the 10th day.
Group V: AR100 group; received Arjuna (100 mg kg−1 BW, p.o.) for 9 days followed by a single dose of Dox injection (10 mg kg−1 BW, i.v.) on the 10th day.
The rats were housed in polyacrylic cages and maintained at 27 ± 2°C, 45–60% RH and 12 h photo period. The rats were provided with a standard pellet diet (Amrut feeds, Pune, India) and water ad libitum. All animal procedures have been approved by the Animal Ethical Committee of University of Mysore in accordance with animal experimentation and care. After 48 h of the injection of either Dox or vehicle, the animals were starved overnight (to minimize metabolic variations), euthanized and blood was collected by direct cardiac puncture and used for serum separation. The activities of CK-MB, LDH, ALT, AST, and troponin I were determined as markers of cardiotoxicity in serum. Heart was immediately excised and a portion was homogenized (1:5 w/v) in phosphate-buffered saline (pH 7.4) for estimation of TBARS and glutathione (GSH) as markers of oxidative stress according to the methods reported earlier (CitationAhmed & Urooj, 2009a), while the other portion was fixed in 10% formalin, dehydrated in graduated ethanol (50%–100%), cleared in xylene and embedded in paraffin. The sections (4–5 μm) were then examined with a photomicroscope (Leica DM LS2, Switzerland) after staining with hematoxylin and eosin (H–E) dye. The morphological changes included cell necrosis, mononuclear infiltration, vacuolation, and degenerative changes.
Statistical analysis
Data are presented as mean ± SD and analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test for significant differences using SPSS version 14.0 software (SPSS Inc., Chicago, IL, USA). The values were considered significant at p ≤ 0.05.
Results and discussion
Acute toxicity study
In the acute oral toxicity test, the extract (2 g kg−1) did not show any signs of toxicity such as behavioral, ANS/CNS changes, dermatitis, scaling, and no mortality was observed during the 14-day observation. Based on these findings, according to the OECD 423 guidelines, an LD50 value of > 2000 mg kg−1 was deduced for the extract.
HPLC fingerprinting
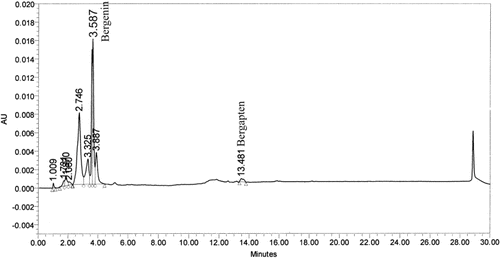
The HPLC chromatogram of FRSACE was found to contain constituents eluting between 1.009 min and 13.481 min with major peaks at 2.746 and 3.587 min (). The peak found at 3.587 min was identified as bergenin and a minor peak found at 13.481 min was identified as bergapten (5-methoxy psoralen). The concentrations of bergenin and bergapten were 0.89% and 0.07%, respectively. CitationVeerapur et al. (2007) have earlier reported the presence of bergenin in the ethanol extract of F. racemosa bark.
Figure 1. HPLC chromatogram of sequential acetone extract. The HPLC chromatogram was separated on a RP-C18 column (250 × 4.6 mm2; 5 µm) using gradient elution-water and acetonitrile at a total flow rate of 1.0 mL min−1; gradient composition (min,% acetonitrile): 0, 20%; 5, 40%; 8, 75%; 12, 90%;15, 95%; 25, 95%; 27, 20%; 30, 20%. The chromatogram at 270 nm was analyzed and compared.
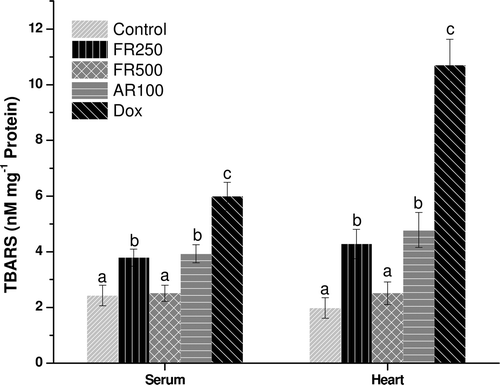
Cardioprotective effect
Cardiotoxic compounds such as doxorubicin are known to cause elevation in CK-MB, LDH, and serum transaminases. The activities of LDH, CK-MB, AST, ALT, and the presence of troponin I in serum were determined as markers of cardiotoxicity (). As expected, doxorubicin administration induced severe cardiac damage as the activities of LDH and CK-MB were found to be significantly (p ≤ 0.001) elevated compared to control groups. Pretreatment with FR250, FR500, and AR100 significantly attenuated (p ≤ 0.01) the increase in their activities. The percentage reductions in LDH activity by FR250, FR500 and AR100 were 38, 68, and 36% respectively, while, the activity of CK-MB was decreased to an extent of 43, 63, and 38% by FR250, FR500, and AR100, respectively. Troponin I was undetected only in the serum of control, while it was found in all the experimental groups.
Table 1. Effect of FRSACE on key cardiac and hepatic enzymes in serum.
These findings are in good agreement with the observations of CitationShanmugarajan et al. (2008) regarding Ficus hispida; another very important plant of genus Ficus exhibited significant cardioprotective effect against cyclophosphamide provoked oxidative injury in rats. The significant reductions in the activities of CK-MB and LDH brought about by FRSACE-pretreatment could be due to the presence of racemosic acid (CitationLi et al., 2004), quercetin (CitationKhan & Sultana, 2005), gallic acid and ellagic acid (CitationRao et al., 2008), which are known to exhibit potent antioxidant activity. The cardioprotective activity of Arjuna is very well established, wherein its protective effect is ascribed to the presence of various phenolics, tannins, glycosides, saponins, alkaloids, and flavonoids having potent antioxidant activity (CitationSingh et al., 2008).
A significant increase (p ≤ 0.001) in the activities AST and ALT observed in the Dox group were effectively counteracted and restored toward normalization by FR250, FR500, and AR100 pretreatment. Although, FR250, FR500, and AR100 pretreatment did not decrease serum transaminase activities to control levels, they brought about 21%, 41%, and 23% reduction in the activity of AST and 44%, 65%, and 46% reduction in the activity of ALT, respectively. The results indicated that 500 mg kg−1 dose of FRSACE offers optimal hepatoprotection. The hepatoprotective effect of FRSACE could conclusively be attributed to the presence of bergenin; an isocoumarin, since it is reported to exhibit significant hepatoprotective activity against CCl4-induced hepatotoxicity in rats (CitationLim et al., 2000).
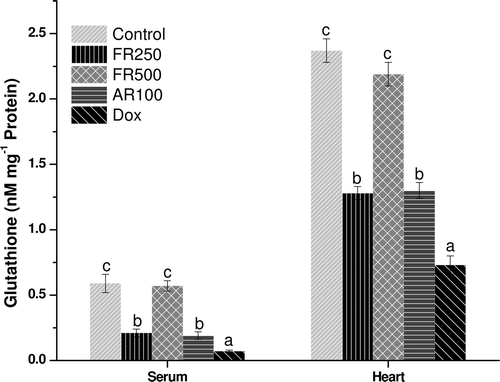
Effect on oxidative stress
In states of oxidative stress, GSH is converted to glutathione disulfide (GSSG) and depleted, leading to lipid peroxidation resulting in higher TBARS levels. Therefore, the role of GSH as a reasonable marker for the evaluation of oxidative stress is important (CitationRecknagel et al., 1989). The FR250, FR500, and AR100 pretreatment significantly decreased (p ≤ 0.05) lipid peroxidation induced by doxorubicin as reflected by lower TBARS and higher GSH values in heart and serum. The AR100 also decreased the oxidative stress significantly (p ≤ 0.05) and it was comparable with that of FR250 ( and ). However, except FR500, none of the extracts resulted in complete reversal of the oxidative stress to normal levels. The complete reversal of the oxidative stress to normal levels by FR500 could be attributed to various phenolic compounds and flavonoids such as quercetin, gallic acid, ellagic acid, and trepenoids; lupeol, lupeol acetate, and α-amyrin (CitationJoy et al., 2001; CitationKhan & Sultana, 2005; CitationRao et al., 2008) that are reported to act as strong antioxidant and anti-inflammatory agents (CitationKhan & Sultana, 2005).
Effect on histopathology of the heart
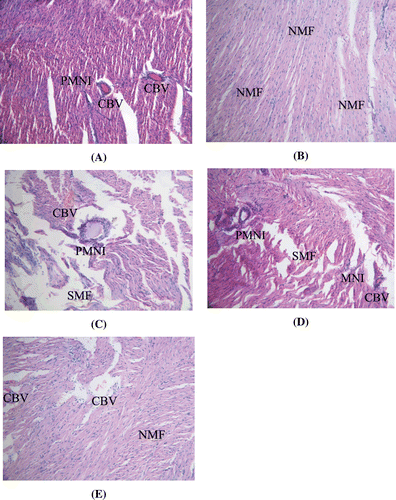
Dox administration exerted serious adverse effects on the cardiac muscle fibers. Perivascular mononuclear infiltrate extending into the muscle fibers was seen in Dox-treated group, suggesting inflammatory changes (). Edema was also observed with separation of the cardiac muscle fibers compared to control group wherein normal muscle fibers with vesicular nuclei were found (). In all the other groups (FR250, FR500, and AR100) the cardiac architecture was significantly restored toward normalization; however congested blood vessels associated with hemorrhages were seen in the sections of FR250 and AR100 group ( and ). In FR500, although no congested blood vessels and perivascular neutrophil infiltrate was seen, separation of cardiac muscle fibers was evident ().
Figure 4. (A) Section of the heart of Dox-treated rats showing inflammatory changes. (B) Section of the heart of control rats showing normal cardiac muscle fibers with vesicular nuclei. (C) Section of the heart of FRSACE (250 mg kg−1) + Dox-treated rats showing congested blood vessels and inflammatory changes. (D) Section of the heart of Arjuna (100 mg kg−1) + Dox-treated rats showing inflammatory changes. (E) Section of the heart of FRSACE (500 mg kg−1) + Dox-treated rats showing normal cardiac muscle fibers and some congested blood vessels; NMF: normal cardiac muscle fibers, PNMI: perivascular mononuclear infiltrate, CBV: congested blood vessel.
Conclusion
In conclusion, doxorubicin exposure results in pronounced oxidative stress and administration of F. racemosa stem bark extract protects the heart by scavenging free radicals. Although, the compounds responsible were traced from the HPLC fingerprinting and reported literature, there is need to isolate specific compound(s) for its optimal utilization as a therapeutic agent to derive maximum benefits of doxorubicin as an anticancer drug by reducing its toxic effects.
Declaration of interest
The authors acknowledge University Grants Commission, New Delhi, India, for the financial assistance (F.31-278/2005).
References
- Ahmed F, Urooj A. (2009a). Antioxidant activity of various extracts of Ficus racemosa stem bark. Nat J Life Sci, 6, 69–74.
- Ahmed F, Urooj A. (2009b). Glucose lowering, hepatoprotective and hypolipidemic activity of stem bark of Ficus racemosa in streptozotocin-induced diabetic rats. J Young Pharm, 1, 160–164.
- Ahmed F, Urooj A. (2010). Hepatoprotective effects of Ficus racemosa stem bark against carbon tetrachloride-induced hepatic damage in albino rats. Pharm Biol, 48, 210–216.
- Billingham ME, Mason JW, Bristow MR, Daniels JR. (1978). Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep, 62, 865–872.
- Injac R, Strukelj B. (2008). Recent advances in protection against doxorubicin-induced toxicity. Technol Cancer Res Treat, 7, 497–516.
- Joy PP, Thomas J, Mathew S, Skaria BP. (2001). Medicinal Plants. Tropical Horticulture Vol. 2, Naya Prokash, Calcutta, pp. 449–632.
- Khan N, Sultana S. (2005). Chemomodulatory effect of Ficus racemosa extract against chemically induced renal carcinogenesis and oxidative damage response in Wistar rats. Life Sci, 77, 1194–1210.
- Kirtikar KR, Basu BD. (1975). Indian Medicinal Plants. 2nd Edn, Dehra Dun, India, pp. 2327–2328.
- Li RW, Leach DN, Myers SP, Lin GD, Leach GJ, Waterman PG. (2004). A new anti-inflammatory glucoside from Ficus racemosa L. Planta Med, 70, 421–426.
- Lim HK, Kim HS, Choi HS, Oh S, Choi J. (2000). Hepatoprotective effects of bergenin, a major constituent of Mallotus japonicus, on carbon tetrachloride-intoxicated rats. J Ethnopharmacol, 72, 469–474.
- Lui RC, Laregina MC, Herbold DR, Johnson FE. (1986). Testicular cytotoxicity of intravenous doxorubicin in rats. J Urol, 136, 940–943.
- Nadkarni KM, Nadkarni AK, Chopra RN. (1976). Indian Materia Medica. Popular Prakashan, Bombay, pp. 548–550.
- Ng R, Better N, Green MD. (2006). Anticancer agents and cardiotoxicity. Semin Oncol, 33, 2–14.
- OECD 423. (2001). Acute oral toxicity–Acute toxic class method. OECD guidelines for testing of chemicals, 423, 1–14.
- Priestman T. (2008). Cancer Chemotherapy in Clinical Practice. Springer-Verlag, London, UK.
- Quiles JL, Huertas JR, Battino M, Mataix J, Ramírez-Tortosa MC. (2002). Antioxidant nutrients and adriamycin toxicity. Toxicology, 180, 79–95.
- Rao ChV, Verma AR, Vijayakumar M, Rastogi S. (2008). Gastroprotective effect of standardized extract of Ficus glomerata fruit on experimental gastric ulcers in rats. J Ethnopharmacol, 115, 323–326.
- Recknagel RO, Glende EA Jr, Dolak JA, Waller RL. (1989). Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther, 43, 139–154.
- Shanmugarajan TS, Arunsundar M, Somasundaram I, Krishnakumar E, Sivaraman D, Ravichandiran V. (2008). Cardioprotective effect of Ficus hispida Linn, on cyclophosphamide provoked oxidative myocardial injury in a rat model. Int J Pharmacol, 4, 78–87.
- Singh G, Singh AT, Abraham A, Bhat B, Mukherjee A, Verma R, Agarwal SK, Jha S, Mukherjee R, Burman AC. (2008). Protective effects of Terminalia arjuna against doxorubicin-induced cardiotoxicity. J Ethnopharmacol, 117, 123–129.
- Veerapur VP, Prabhakar KR, Parihar VK, Kandadi MR, Ramakrishana S, Mishra B, Satish Rao BS, Srinivasan KK, Priyadarsini KI, Unnikrishnan MK. (2007). Ficus racemosa stem bark extract: A potent antioxidant and a probable natural radioprotector. Evid Based Complement Alternat Med, 119, 1–8.