Abstract
Context: Oxidative damage to cellular components such as lipids and cell membranes by free radicals and reactive oxygen species (ROS) is thought to be associated with the development of degenerative diseases. Fluoride intoxication is associated with oxidative stress and altered anti-oxidant defense mechanism. Lycopene is a lipid-soluble powerful anti-oxidant that scavenges free radicals and ROS.
Objective: This study was extended to investigate lycopene anti-oxidant efficacy in different organs of fluoride-intoxicated rats.
Methods: Twenty-four adult rats were randomly divided into four groups of six animals each. Rats in group I received daily doses of vehicle. Group II rats were given lycopene (10 mg/kg body weight/day), by tubes, dissolved in 0.5 ml of corn oil for 5 weeks. Group III rats were given sodium fluoride (NaF) (10.3 mg/kg body weight/day), by tubes, for 5 weeks. In group IV rats, lycopene was administered 1 h later and NaF was administered for 5 weeks.
Results: NaF administration induced oxidative stress as evidenced by elevated levels of lipid peroxidation (51.3, 65.9 and 67.6%) measured as malondialdehyde and total nitrate/nitrite (61.0, 59.7 and 68.9%) in red blood cells, heart and brain tissues. Moreover, significantly decreased reduced glutathione level, total anti-oxidant capacity and superoxide dismutase activity were observed in the examined tissues. The induced oxidative stress and the alterations in anti-oxidant system were normalized by the oral administration of lycopene treatment.
Conclusion: Lycopene administration could minimize the toxic effects of fluoride indicating its free-radical scavenging and powerful anti-oxidant activities.
Keywords::
Introduction
Fluoride is a well-known water contaminant that raises serious health issues all over the world (CitationTiwari & Rao, 2010). It could induce oxidative stress and DNA damage in rat cells (CitationMendoza-Schulz et al., 2009). In contrast, CitationJung et al. (2006) demonstrated the fluoride-induced apoptotic morphological changes in human cells with no production of reactive oxygen species (ROS). Increased ROS and lipid peroxidation (LP) productions have been implicated in the pathogenesis of toxic action of a wide range of compounds (CitationBencini et al., 2010). Epidemiological investigations indicated that long-term high-level fluoride intake can lead to severe damage in the metabolism of many systems and organs in animals and humans (CitationHassan & Yousef, 2009). Recent studies suggest that increased generation of ROS and enhanced LP have been shown to play an important role in fluorosis (CitationNarayanaswamy & Piler, 2010).
Consumers all over the world are becoming more aware of the nutrition value, health benefits and safety of their food and its ingredients present naturally in diets that are believed to be less subject to hazards than their artificial counterparts (CitationAlamed et al., 2009). One of the most important natural diets with anti-oxidant properties is lycopene. It has an acyclic isomer of β-carotene that gives red color to tomatoes. Humans and animals depend on dietary sources and do not synthesize lycopene. The dietary sources of lycopene include tomatoes, apricots, pink guava and watermelon (CitationKhan et al., 2008). It has anti-oxidant, anti-cancer and anti-inflammatory effects with molecular mechanisms not fully identified (CitationFeng et al., 2010). Several pharmacokinetic studies have evaluated the factors that affect bioavailability of lycopene (CitationRiso et al., 2010). Thus, in this study, lycopene was selected as the most potential natural compound to prevail over fluoride-induced toxicity in rats.
Materials and methods
Chemicals
Sodium fluoride (NaF) was obtained from Sigma-Aldrich Chemical Co., USA. Lycopene was purchased from Mankind Pharma Ltd., Mumbai, India. All other chemicals and solvents used were of the highest purity grade available.
Experimental animals
Twenty-four adult male albino rats weighing 125 ± 5 g were obtained from Egyptian Organization for Biological Products and Vaccines, Helwan, Cairo, Egypt and received humane care. This study complies with National Institutes of Health guidelines. The animals are maintained under standard rat housing conditions (artificial illumination; light:dark, 13 h:11 h; and thermally controlled) and had access to food and water ad libitum.
Experimental design
After a week of acclimation, rats were divided into four groups of six rats each. In the control group, rats received daily doses of vehicle. In the lycopene group, rats were given lycopene orally (10 mg/kg body weight), which is dissolved in 0.5 ml of corn oil as it is reported to be the most effective dose (CitationSandhir et al., 2010). In fluoride group, rats were given NaF orally (10.3 mg/kg body weight), which is chosen according to the study by CitationZabulyte et al. (2007). In the lycopene + fluoride group, rats were first treated with lycopene (10 mg/kg weight/day) and then with NaF after an hour (10.3 mg/kg body weight/day). All rats received their respective doses daily by tubes for 5 weeks.
Sampling of blood and tissue homogenates
At the end of the experimental durations, animals were sacrificed by decapitation. Blood samples were collected immediately in centrifuge tubes containing an acid–citrate–dextrose solution (1 ml/4 ml of blood). Plasma and buffy-coat were removed by centrifugation at 860g for 20 min. Red blood cells were washed three times with buffered saline (0.9% saline in 0.01 M phosphate buffer, pH 7.4). The packed cells were then suspended in an equal volume of the buffered saline and stored at −20°C for anti-oxidants analysis.
Hearts and brains were quickly excised, washed with saline, blotted with a piece of filter paper and homogenized (20% w/v) in ice-cold sodium, potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl using a Branson sonifier (250, VWR Scientific, Danbury, CT, USA). The homogenates were used for the determination of malondialdehyde (MDA) level, glutathione (GSH) content, total anti-oxidant capacity (TAC) and NO(x). The homogenates were centrifuged at 800g for 5 min at 4°C to separate the debris. The supernatant so obtained was centrifuged at 10,500g for 20 min at 4°C to get the postmitochondrial supernatant, which was used to assay superoxide dismutase (SOD) activity.
Biochemical analysis
MDA level was determined spectrophotometrically using the method of CitationBuege and Aust (1978). Total nitrate/nitrite [NO(x)] level was determined according to the method of CitationIgnarro et al. (1987). The assay is based on the diazotization of sulphanilic acid with nitric oxide at acidic pH and subsequent coupling with N-(10-naphthyl) ethylenediamine to yield an intensely pink colored product that is measured spectrophotometrically at 540 nm. Reduced GSH was determined according to the methods of CitationEllman (1959), which is based on the reduction of Ellman’s reagent [5,5-dithio-bis-(2-nitrobenzoic acid)] by SH groups to form 1 mole of 2-nitro-5-mercaptobenzoic acid per mole of SH. The nitro-mercaptobenzoic acid has an intense yellow color and can be determined spectrophotometrically. SOD activity was determined according to the method of CitationMinami and Yoshikawa (1979). This method is based on the generation of superoxide anions by pyrogallol auto-oxidation, detection of generated superoxide anions by nitro blue tetrazolium formazan color development, and measurement the amount of generated superoxide anions scavenged by SOD (the inhibitory level of formazan color development). TAC was determined using commercial kits obtained from Biodiagnostic Co. for diagnostic reagents, Giza, Egypt, based on the reaction of anti-oxidants in the sample with a definite amount of exogenously provide hydrogen peroxide (H2O2). The residual H2O2 was determined colorimetrically by an enzymatic reaction that evolves the conversion of 3,5-dichloro-2-hydroxy benzene sulphate to a colored product, measured at 505 nm according to the methods of CitationKoracevic et al. (2001).
Statistical analysis
Differences between obtained values (mean ± SD, n = 10) were obtained by one-way analysis of variance followed by the Tukey–Kramer multiple comparison test. A p value of ≤0.05 was taken as a criterion for a statistically significant difference (CitationSokal & Rahif, 1981).
Results
Clinical laboratory studies
There were no significant clinical signs, and mortalities or abnormal behavior were observed in all rat groups throughout the study period except rats of the fluoride group that displayed low behavioral activity from the second week of the study when compared with the control rats.
Biochemical measurements
The data did not show any significant changes in all tested parameters in red blood cells, heart and brain of rats that received lycopene compared with control group (–).
Figure 1. Effect of sodium fluoride (NaF), lycopene and their combination on the level of malondialdehyde (MDA) in blood, heart and brain tissues.
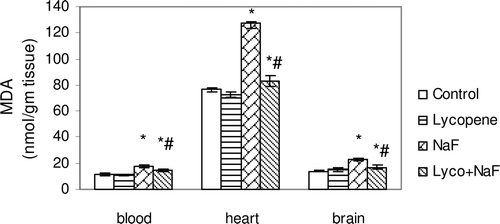
Figure 2. Effect of sodium fluoride (NaF), lycopene and their combination on the level of total nitrate/nitrite [NO(x)] in blood, heart and brain tissues.
![Figure 2. Effect of sodium fluoride (NaF), lycopene and their combination on the level of total nitrate/nitrite [NO(x)] in blood, heart and brain tissues.](/cms/asset/9008db00-983d-4722-beb6-d5aa42d3062e/iphb_a_618994_f0002_b.gif)
Figure 3. Effect of sodium fluoride (NaF), lycopene and their combination on superoxide dismutase activity (SOD) in blood, heart and brain tissues.
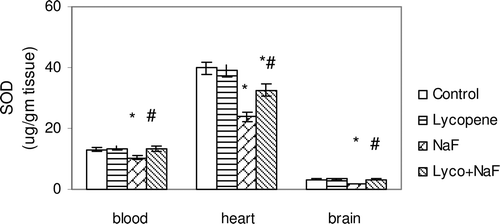
Figure 4. Effect of sodium fluoride (NaF), lycopene and their combination on reduced glutathione content (GSH) in blood, heart and brain tissues.
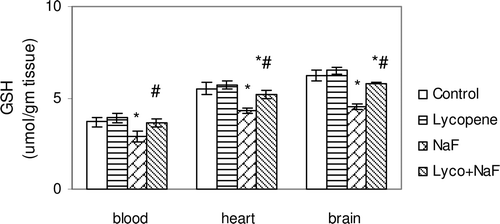
Figure 5. Effect of sodium fluoride (NaF), lycopene and their combination on total anti-oxidant capacity (TCA) in blood, heart and brain tissues.
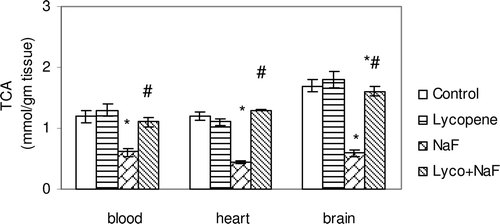
In fluoride-intoxicated rats, there were significant increase in MDA (51.3, 65.9 and 67.6%) and NO(x) (61.0, 59.7 and 68.9%) levels in the red blood cells, heart and brain, respectively, when compared with control rats each p < 0.05 ( and ). Furthermore, administration of NaF induced significant decrease in GSH content (26.6, 21.8 and 27.4%), SOD activity (21.4, 40.0 and 46.3%), and TAC activity (48.3, 63.3 and 64.4%) in the red blood cells, heart and brain tissues, respectively, when compared with those of controls (–).
Administration of lycopene to fluoride-intoxicated rats ameliorated the fluoride adverse effects as expressed by the significant reduction in the elevated MDA and NO(x) levels ( and ) and marked an elevation of endogenous anti-oxidant system testimony to GSH, SOD and TAC when compared with fluoride-intoxicated rats (p < 0.05) ().
Discussion
Recently, the relationship between fluoride toxicity and elevated oxidative stress has been reported in rats, suggesting that oxidative/nitrosative damage is the major mode of action of fluoride (CitationHassan & Yousef, 2009). Fluoride at millimolar concentrations inhibits several enzymes both in vivo and in vitro (CitationMendoza-Schulz et al., 2009). In this study, fluoride treatment increased LP and NO production, whereas the estimated anti-oxidant parameters were diminished in red blood cells and in examined heart and brain tissues. These results suggested that the balance between the oxidative system and anti-oxidant system in the rats was out of order during fluoride exposure (CitationNarayanaswamy & Piler, 2010). The present findings are in harmony with recent observations by CitationInkielewicz-Stepniak and Czarnowski (2010), indicating that the excessive fluoride exposure can induce LP, specifically polyunsaturated fatty acids.
In previous studies, altered levels of GSH, SOD and TAC were estimated in animals treated with fluoride enhancing increased heavy accumulation of free radicals in red blood cells, brain (CitationGhosh et al., 2008; CitationHassan & Abdel-Aziz, 2010) and heart (CitationSinha et al., 2008). All these results indicate that free radicals may play an important role in the pathogenesis of fluorosis. Red blood cells are more commonly employed in the evaluation of oxidative stress, since they are prone to oxidative reactions because of relatively high oxygen tension and the presence of polyunsaturated lipid-rich plasma membranes (CitationSkoumalova et al., 2008).
Fluoride exposure increases the generation of O2−, its concentration and its downstream consequences such as H2O2, ONOO−, and OH radicals seem particularly important in mediating fluoride’s effects (Garcia-Montalvo et al., 2009). Also, fluoride induces NO(x); on the other hand, NO(x) inhibits mitochondrial respiration level, leading to the damage of cellular components (CitationHassan & Yousef, 2009) and increases the permeability of the blood-brain barrier (CitationSandhir et al., 2010). Moreover, fluoride reacts with metal centres in proteins to form nitrosyl adducts resulting in the accumulation of misfolded proteins in the endoplasmic reticulum causing ROS production (CitationBarbier et al., 2010). Furthermore, when fluoride accumulates in the brain of rats, it causes stress and inhibits auto-oxidation mechanism resulting in oxidative damage of tissues (CitationChouhan et al., 2010).
The use of anti-oxidant-rich food ingredients as antidotes for fluoride toxicity was recently suggested (CitationTiwari & Rao, 2010). Several endeavors have been made to correlate the free-radical-scavenging capacity of compounds with their anti-oxidant activity in foods even though anti-oxidant activity is dependent on both physical and chemical properties (CitationAlamed et al., 2009). Lycopene’s anti-oxidant activity is higher than that of β-carotene, α-carotene and α-tocopherol. It is a 40-carbon atom, open-chain hydrocarbon containing 11 conjugated and two non-conjugated double bonds arranged in a linear array (CitationTopal et al., 2006).
CitationSrinivasan et al. (2007) reported that lycopene is very efficient ROS scavenging and also has the potential to increase the activity of SOD. The ability of it to detoxify cells by up-regulating the GSH-mediated detoxification process was recently evidenced (CitationKoul et al., 2010). In addition, lycopene exerts potent anti-inflammatory effect through its action as an anti-oxidant and free-radical scavenger, which may reduce cellular damage (CitationSaedisomeolia et al., 2009). These findings were confirmed by the existing reduction of MDA and NO(x) production, in addition to the obvious restoring of the endogenous anti-oxidant status through the increased levels of GSH, SOD and TAC in red blood cells and examined organs of rats that received both lycopene and fluoride.
Lycopene administration plays an imperative role in the avoidance of fluoride-induced oxidative stress and augments the cellular anti-oxidant defense system. The present work indicated that lycopene is an exceptional natural anti-oxidant and free-radical scavenger.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alamed J, Chaiyasit W, McClements DJ, Decker EA. (2009). Relationships between free radical scavenging and antioxidant activity in foods. J Agric Food Chem, 57, 2969–2976.
- Barbier O, Arreola-Mendoza L, Del Razo LM. (2010). Molecular mechanisms of fluoride toxicity. Chem Biol Interact, 188, 319–333.
- Bencini A, Failli P, Valtancoli B, Bani D. (2010). Low molecular weight compounds with transition metals as free radical scavengers and novel therapeutic agents. Cardiovasc Hematol Agents Med Chem, 8, 128–146.
- Buege JA, Aust SD. (1978). Microsomal lipid peroxidation. Meth Enzymol, 52, 302–310.
- Chouhan S, Lomash V, Flora SJ. (2010). Fluoride-induced changes in haem biosynthesis pathway, neurological variables and tissue histopathology of rats. J Appl Toxicol, 30, 63–73.
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 17, 214–226.
- Feng D, Ling WH, Duan RD. (2010). Lycopene suppresses LPS-induced NO and IL-6 production by inhibiting the activation of ERK, p38MAPK, and NF-kappaB in macrophages. Inflamm Res, 59, 115–121.
- García-Montalvo EA, Reyes-Pérez H, Del Razo LM. (2009). Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress. Toxicology, 263, 75–83.
- Ghosh J, Das J, Manna P, Sil PC. (2008). Cytoprotective effect of arjunolic acid in response to sodium fluoride mediated oxidative stress and cell death via necrotic pathway. Toxicol In Vitro, 22, 1918–1926.
- Hassan HA, Abdel-Aziz AF. (2010). Evaluation of free radical-scavenging and anti-oxidant properties of black berry against fluoride toxicity in rats. Food Chem Toxicol, 48, 1999–2004.
- Hassan HA, Yousef MI. (2009). Mitigating effects of antioxidant properties of black berry juice on sodium fluoride induced hepatotoxicity and oxidative stress in rats. Food Chem Toxicol, 47, 2332–2337.
- Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. (1987). Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA, 84, 9265–9269.
- Inkielewicz-Stepniak I, Czarnowski W. (2010). Oxidative stress parameters in rats exposed to fluoride and caffeine. Food Chem Toxicol, 48, 1607–1611.
- Jung JY, Park JH, Jeong YJ, Yang KH, Choi NK, Kim SH, Kim WJ. (2006). Involvement of Bcl-2 family and caspases cascade in sodium. Fluoride-induced apoptosis of human gingival fibroblasts. Korean J Physiol Pharmacol 10, 289–295.
- Khan N, Afaq F, Mukhtar H. (2008). Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal, 10, 475–510.
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. (2001). Method for the measurement of antioxidant activity in human fluids. J Clin Pathol, 54, 356–361.
- Koul A, Arora N, Tanwar L. (2010). Lycopene mediated modulation of 7,12 dimethlybenz (a) anthracene induced hepatic clastogenicity in male Balb/c mice. Nutr Hosp, 25, 304–310.
- Mendoza-Schulz A, Solano-Agama C, Arreola-Mendoza L, Reyes-Márquez B, Barbier O, Del Razo LM, Mendoza-Garrido ME. (2009). The effects of fluoride on cell migration, cell proliferation, and cell metabolism in GH4C1 pituitary tumour cells. Toxicol Lett, 190, 179–186.
- Minami M, Yoshikawa H. (1979). A simplified assay method of superoxide dismutase activity for clinical use. Clin Chim Acta, 92, 337–342.
- Narayanaswamy M, Piler MB. (2010). Effect of maternal exposure of fluoride on biometals and oxidative stress parameters in developing CNS of rat. Biol Trace Elem Res, 133, 71–82.
- Riso P, Brusamolino A, Contino D, Martini D, Vendrame S, Del Bo’ C, Porrini M. (2010). Lycopene absorption in humans after the intake of two different single-dose lycopene formulations. Pharmacol Res, 62, 318–321.
- Saedisomeolia A, Wood LG, Garg ML, Gibson PG, Wark PA. (2009). Lycopene enrichment of cultured airway epithelial cells decreases the inflammation induced by rhinovirus infection and lipopolysaccharide. J Nutr Biochem, 20, 577–585.
- Sandhir R, Mehrotra A, Kamboj SS. (2010). Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem Int, 57, 579–587.
- Sinha M, Manna P, Sil PC. (2008). Terminalia arjuna protects mouse hearts against sodium fluoride-induced oxidative stress. J Med Food, 11, 733–740.
- Skoumalová A, Herget J, Wilhelm J. (2008). Hypercapnia protects erythrocytes against free radical damage induced by hypoxia in exposed rats. Cell Biochem Funct, 26, 801–807.
- Sokal RR, Rahif FJ. (1981). The Principles and Practice of Statistics in Biological Research, second ed., San Francisco, USA: Freeman, W.H. Company.
- Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. (2007). Lycopene as a natural protector against gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochim Biophys Acta, 1770, 659–665.
- Tiwari H, Rao MV. (2010). Curcumin supplementation protects from genotoxic effects of arsenic and fluoride. Food Chem Toxicol, 48, 1234–1238.
- Topal U, Sasaki M, Goto M, Hayakawa K. (2006). Extraction of lycopene from tomato skin with supercritical carbon dioxide: effect of operating conditions and solubility analysis. J Agric Food Chem, 54, 5604–5610.
- Zabulyte D, Uleckiene S, Kalibatas J, Paltanaviciene A, Jascaniniene N, Stosik M. (2007). Experimental studies on effect of sodium fluoride and nitrate on biochemical parameters in rats. Bull Vet Inst Pulawy 51, 79–82.
