Abstract
Context: Polygala paniculata Linnaeus (Polygalaceae) has shown neuroprotective effects, but there is no report about its antidepressant potential.
Objective: The antidepressant-like effect of the hydroalcoholic extract from P. paniculata and some of the possible mechanisms involved in this effect were investigated in forced swimming test (FST).
Materials and methods: Mice received extract by oral route and were submitted to FST and open-field test. Animals were forced to swim and the total immobility time was registered (6-min period). A reduction in the immobility time is considered an antidepressant-like effect. In order to investigate the involvement of the monoaminergic systems, mice were treated with pharmacological antagonists before administration of the extract.
Results: The acute administration of the hydroalcoholic extract from P. paniculata produced an antidepressant-like effect, since it significantly reduced the immobility time in FST (0.01–30 mg/kg) as compared to control group, without changing locomotor activity. Pretreatment of mice with yohimbine (1 mg/kg, i.p., α2-adrenoceptor antagonist), propranolol (1 mg/kg, i.p., β-adrenoceptor antagonist), SCH23390 (0.05 mg/kg, s.c., dopamine D1 receptor antagonist) or sulpiride (50 mg/kg, i.p., dopamine D2 receptor antagonist) prevented the antidepressant-like effect of the extract in FST (30 mg/kg). Moreover, ketanserin (5 mg/kg, i.p., preferential 5-HT2A receptor antagonist) enhanced the effect of the extract in FST.
Discussion and conclusion: The results of the present study indicate that the extract from P. paniculata has an antidepressant-like action that is likely mediated by an interaction with the serotonergic (5-HT2A receptors), noradrenergic (α2 and β-receptor) and dopaminergic (D1 and D2 receptors) systems.
Introduction
Depression is one of the top ten causes of morbidity and mortality worldwide based on a survey by the CitationWorld Health Organization (2001). It is a chronic, recurring and potentially life-threatening disease that affects up to 20% of the population across the globe (CitationBerton & Nestler, 2006). The mechanisms underlying the pathogenesis of depression are not well understood and several theories were proposed since 1950s. Most of the antidepressants currently used today exert their primary biochemical effects by regulating synaptic concentrations of serotonin, noradrenaline and/or dopamine (CitationElhwuegi, 2004; CitationPáez-Pereda, 2005). However, not all depressed patients respond to available antidepressants (success rate about 60%), and the therapeutic response requires several weeks or months of treatment (CitationDuman et al., 2000; CitationWong & Licinio, 2001).
Natural products from plant origin are important sources of therapeutic agents and currently, about 25–30% of all drugs available as therapeutics are natural product derivatives. Brazil alone possesses about 20–22% of all existing plants and micro-organisms. However, it is estimated that no more than 25 000 plant species have been the object of any sort of scientific investigation (CitationCalixto, 2005). Indeed, herbal therapies may be effective alternatives in the treatment of depression, as in the case of St John’s wort, by acting through a mechanism which does not differ significantly with respect to that of classical antidepressants (CitationBilia et al., 2002; CitationZhang, 2004).
Polygala paniculata Linnaeus (Polygalaceae) grows abundantly in the Brazil’s Atlantic coast. It is a small arbust that has been used as a folk medicinal herb in the treatment of different inflammatory diseases such as asthma, bronchitis, arthritis, and other diseases, including disorders of the kidney (CitationNewall et al., 1996). Plants from the genus Polygala have been shown to possess protective effects against neuronal death and cognitive impairments in neurodegenerative disorders related to excitotoxicity (CitationPark et al., 2002; CitationLee et al., 2004), and produce several biological effects, such as: anticonvulsant and anxiolytic effects (Duarte et al., 2007), antipsychotic (CitationChung et al., 2002), anti-inflammatory (CitationWang et al., 2008) and antioxidant activities (CitationCervellati et al., 2004; CitationWu et al., 2007). In addition, the hydroalcoholic extract from P. paniculata has shown protective effects against MeHg-induced neurotoxicity (CitationFarina et al., 2005). Recent studies have demonstrated that the hydroalcoholic extract of this plant and its isolated compound, rutin, possess antinociceptive properties (CitationLapa et al., 2009) and gastroprotective effect (CitationLapa et al., 2007). This flavonoid found in P. paniculata (CitationCristiano et al., 2003) is essential for the antidepressant activity of Hypericum perforatum Linnaeus (Hypericaceae) in the forced swimming test (FST) (Noldner & Schotz, 2002) and for the antidepressant activity of Schinus molle Linnaeus (Anacardiaceae) in the tail suspension test as demonstrated by our group (CitationMachado et al., 2008).
This study investigated the effect of the hydroalcoholic extract from P. paniculata in the forced swimming test, a test widely used to screen antidepressant compounds, and the possible involvement of the monoaminergic systems in its effect.
Materials and methods
Plant material and preparation of the hydroalcoholic extract
P. paniculata was collected in the spring 2006 on Daniela beach (Santa Catarina State, Brazil) and was classified by Olavo Araújo Guimarães (Universidade Federal do Paraná, Curitiba, Brazil). A voucher specimen (UPCNB 26027) of this plant was deposited in the herbarium of the Department of Botany, Universidade Federal do Paraná, Brazil.
The dried whole plant (500 g) was minced and extracted with 50% ethanol-water (1:3), while being stirred and macerated at room temperature (22 ± 3°C) for 14 days. The ethanol was evaporated and the extract (yield 135 g) was concentrated to the desired level and stored at −20°C until use. The extract was dissolved in 150 mM NaCl solution to the desired concentration just before use.
Phytochemical studies carried out by our group with P. paniculata extract have demonstrated the presence of many classes of constituents. Using chemical and spectroscopic methods (EIMS, IR, 1H and 13C NMR, NOE difference spectroscopy), the structures of two xanthones (1-hydroxy-5-methoxy-2,3-methylenedioxyxanthone and 1,5-dihydroxy-2,3-dimethoxyxanthone) were determined, together with coumarin, murragatin and flavonol rutin. In addition, using gas chromatography coupled with mass spectrometry (HRGC-MS), it was possible to characterize two sterols (spinasterol and δ-25-spinasterol) and the minor 1-hydroxy-2,3,5-trimethoxyxanthone (CitationCristiano et al., 2003).
Animals
Female Swiss mice (35–45 g) were maintained at constant room temperature (22–24°C) with free access to water and food, under a 12:12 h light:dark cycle (lights on at 07:00 h). All manipulations were conducted in the light phase, between 11:00 and 16:00 h, with each animal used only once (n = 5–12 animals per group).
All the procedures in this study were approved by the Institutional Ethics Committee and all efforts were made to minimize animals suffering and to reduce the number of animals used in the experiments.
Drugs and treatment
The hydroalcoholic extract from P. paniculata was dissolved in saline with 5% of Tween 80 and administered acutely by oral route (p.o.) 60 min before the FST or open-field test (dose-response curve). A control group received saline with 5% of Tween 80 as vehicle and the positive control group was administered with fluoxetine (20 mg/kg) diluted in saline.
To investigate the mechanisms underlying the antidepressant-like effect of P. paniculata the following drugs were used: ketanserin tartarate, 1-(2-methoxyphenyl)-4[-(2-phthalimido) butylpiperazine) (NAN-190), prazosin, yohimbine, propranolol, (R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390), sulpiride (all from Sigma-Aldrich Chemical Company, St Louis, MO, USA). Drugs were dissolved in saline except NAN-190, that was diluted in saline with 1% Tween 80 and sulpiride that was diluted in saline with 5% dimethylsulfoxide (DMSO). Control animals received appropriate vehicle.
In order to investigate the possible involvement of the serotonergic system in the antidepressant-like effect of the hydroalcoholic extract, mice were pretreated with NAN-190 (0.5 mg/kg i.p., a 5-HT1A receptor antagonist), ketanserin (5 mg/kg, i.p., a preferential 5-HT2A receptor antagonist), or vehicle and after 30 min they received the hydroalcoholic extract from P. paniculata (30 mg/kg, p.o.) or vehicle injection before being tested in the FST 60 min later.
In another set of experiments, to investigate the possible involvement of the noradrenergic system in the antidepressant-like effect of the hydroalcoholic extract in the FST, mice were pretreated with prazosin (1 mg/kg, i.p., an α1-adrenoreceptor antagonist), yohimbine (1 mg/kg, i.p., an α2-adrenoreceptor antagonist) or propranolol (1 mg/kg, i.p., a β-adrenoreceptor antagonist) and after 30 min they received the hydroalcoholic extract from P. paniculata (30 mg/kg, p.o.) or vehicle and were tested in the FST 60 min later.
In order to investigate the involvement of dopaminergic system in the antidepressant-like effect of the hydroalcoholic extract from P. paniculata, mice were pretreated with SCH23390 (0.05 mg/kg, s.c., a dopamine D1 receptor antagonist), sulpiride (50 mg/kg, i.p., a dopamine D2 receptor antagonist), or vehicle and after 30 min they received the hydroalcoholic extract from P. paniculata (30 mg/kg, p.o.) or vehicle and were tested in the FST 60 min later.
The administration schedule and the doses of the drugs used were chosen on the basis of experiments previously performed in our laboratory and literature data confirm the efficacy of the above-mentioned protocols (CitationO’Neill & Conway, 2001; Yamada et al., 2004; CitationKaster et al., 2007; CitationBrocardo et al., 2008; CitationCardoso et al., 2009).
Forced swimming test (FST)
The test was conducted using the method of CitationPorsolt et al. (1977) with some modifications. Mice were individually forced to swim in an open cylindrical container (diameter 10 cm, height 25 cm), containing 19 cm of water at 25 ± 1°C; the total duration of immobility was recorded during 6 min period. Each mouse was judged to be immobile when it ceased struggling and remained floating motionless in the water, making only those movements necessary to keep its head above water. A decrease in the duration of immobility is indicative of an antidepressant-like effect. Classical antidepressants are reported to decrease immobility time in this paradigm (CitationDhir & Kulkarni, 2007; CitationBrocardo et al., 2008; CitationRosa et al., 2008).
Open-field test
To assess the possible effects of the hydroalcoholic extract from P. paniculata on locomotor activity, mice were evaluated in the open-field paradigm as previously described (CitationHall, 1934, 1936). Mice were individually placed in a wooden box (40 × 60 × 50 cm) with the floor divided into 12 squares. The number of crossing in the squares with the four paws was registered during a period of 6 min. The floor of the open-field apparatus was cleaned with 10% ethanol between tests.
Statistical analysis
Comparisons between experimental and control groups were performed by one-way (dose-response curves) or two-way ANOVA (study of the mechanism of action) followed by Newman Keuls test when appropriate. A value of p < 0.05 was considered to be significant.
Results
Effect of the hydroalcoholic extract from P. paniculata in the immobility time in the FST and open-field test
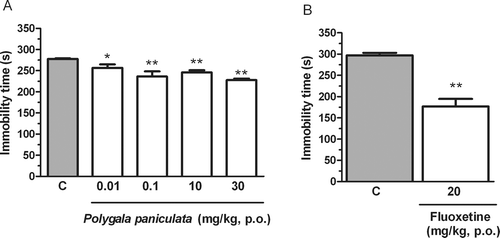
The effects of the p.o. administration of the hydroalcoholic extract from P. paniculata in the immobility time in the FST were shown in . The one-way ANOVA revealed a significant effect of the hydroalcoholic extract from P. paniculata treatment on the immobility time in the FST [F(4,34) = 9.33; p < 0.01]. The hydroalcoholic extract significantly reduced the immobility time in FST at dose of 0.01, 0.1, 10, 30 mg/kg as compared to the control group (. The classic antidepressant fluoxetine (20 mg/kg, p.o.) was used as positive control in the FST and had significant effect compared to the control group as demonstrated in (p < 0.01). The administration of the hydroalcoholic extract from P. paniculata by p.o. route at the dose of 0.1, 10 and 30 mg/kg did not alter the locomotor activity in the open-field test (), as revealed by one-way ANOVA [F(3,21) = 1.05; p = 0.39]. Considering this result, the dose of 30 mg/kg was chosen to be used in the investigation of the involvement of the monoaminergic systems in the antidepressant-like effect of hydroalcoholic extract from P. paniculata.
Table 1. Effect of the acute treatment of mice with Polygala paniculata (0.01–30 mg/kg, p.o.) on the locomotor activity in the open-field test.
Involvement of serotonergic system
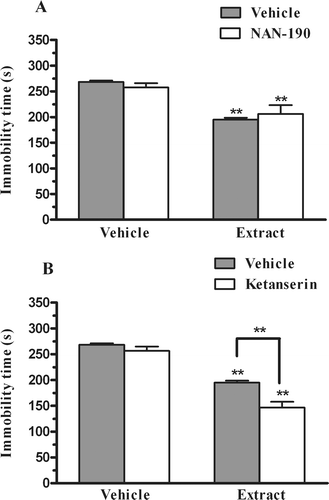
shows that the pretreatment of mice with NAN-190 (0.5 mg/kg, i.p.) did not prevent the antidepressant-like effect elicited by the extract. A two-way ANOVA showed a main effect of extract treatment [F(1,27) = 40.04, p < 0.01], but not with NAN-190 pretreatment [F(1,27) = 0.00, p = 0.99] and NAN-190 X extract interaction [F(1,27) = 1.24, p = 0.27]. shows that the pretreatment of mice with ketanserin (5 mg/kg, i.p.) also did not prevent the antidepressant like-action of the extract in the FST. Indeed, the pretreatment of mice with ketanserin produced a synergistic antidepressant-like effect with the extract. The two-way ANOVA revealed a main effect of extract treatment [F(1,30) = 144.29, p < 0.01], ketanserin pretreatment [F(1,30) = 15.72, p < 0.01] and ketanserin X extract interaction [F(1,30) = 5.69, p < 0.05].
Figure 2. Effect of pretreatment of mice with NAN-190 (0.5 mg/kg, i.p., panel A) or ketanserin (5 mg/kg, i.p., panel B) on the P. paniculata extract (30 mg/kg, p.o.) induced reduction in immobility time in the FST. Each column represents the mean + SEM of 6–9 animals. **p < 0.01 compared with the vehicle-treated control or with the group indicated by descriptive line, #p < 0.01 as compared to the extract alone.
Involvement of noradrenergic system
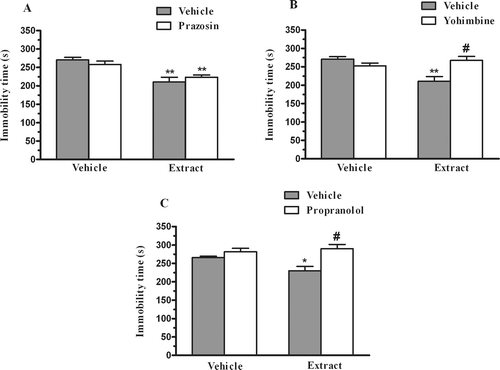
The results depicted in shows that pretreatment of mice with prazosin (1 mg/kg, i.p) was not able to reverse the antidepressant-like effect of the extract from P. paniculata (30 mg/kg, p.o.) in the FST. A two-way ANOVA revealed a main effect of the extract treatment [F(1,26) = 22.31, p < 0.01], prazosin pretreatment [F(1,26) = 0.00, p = 0.97], but not with extract X prazosin interaction [F(1,26) = 1.60, p = 0.21]. However, the pretreatment of mice with yohimbine (1 mg/kg, i.p.) was able to prevent the anti-immobility effect the extract from P. paniculata (30 mg/kg, p.o.) in the FST as shown in . The two-way ANOVA revealed a main effect of the extract treatment [F(1,30) = 5.17, p < 0.01], extract X yohimbine interaction [F(1,30) = 14.31, p < 0.01], but not with yohimbine pretreatment [F(1,30) = 3.79, p = 0.06]. The pretreatment with propranolol (1 mg/kg, p.o.) prevented the antidepressant-like effect of the extract from P. paniculata (30 mg/kg, p.o.) in the FST (. The two-way ANOVA revealed significant differences for propranolol pretreatment [F(1,25) = 15.79, p < 0.01] and the extract X propranolol interaction [F(1,25) = 5.41, p < 0.05], but not for extract treatment [F(1,25) = 2.11, p = 0.16].
Figure 3. Effect of pretreatment of mice with prazosin (1 mg/kg, i.p., panel A), yohimbine (1 mg/kg, i.p., panel B) or propranolol (1 mg/kg, i.p., panel C) on the P. paniculata extract (30 mg/kg, p.o.)-induced reduction in immobility time in the FST. Each column represents the mean + SEM of 6–9 animals. *p < 0.05; **p < 0.01 compared with the vehicle-treated control. #p < 0.01 as compared to the extract alone.
Involvement of dopaminergic system
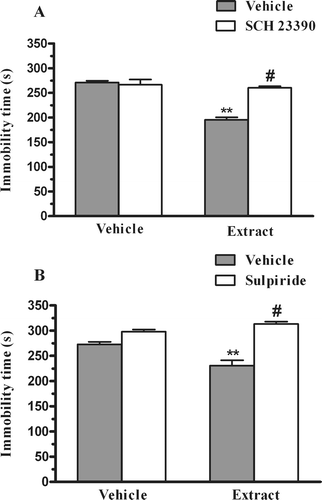
The anti-immobility effect of the extract from P. paniculata (30 mg/kg, p.o.) was significantly prevented by pretreatment of mice with SCH23390 (0.05 mg/kg, s.c., . The two-way ANOVA revealed a main effect of SCH23390 pretreatment [F(1,27) = 22.79, p < 0.01], extract treatment [F(1,27) = 42.10, p < 0.01] and extract X SCH23390 interaction [F(1,27) = 29.63, p < 0.01]. The pretreatment of mice with sulpiride (50 mg/kg, i.p., was also able to prevent the anti-immobility effect of the extract from P. paniculata in the FST. The two-way ANOVA revealed a main effect of sulpiride pretreatment [F(1,30) = 63.25, p < 0.01], and extract X sulpiride interaction [F(1,30) = 18.06, p < 0.01], but not with the extract treatment [F(1,30) = 4.11, p = 0.051].
Figure 4. Effect of pretreatment of mice with SCH23390 (0.05 mg/kg, s.c., panel A) or sulpiride (50 mg/kg, i.p., panel B) on the P. paniculata extract (30 mg/kg, p.o.)-induced reduction in immobility time in the FST. Each column represents the mean + SEM of 6–9 animals. **p < 0.01 compared with the vehicle treated control. #p < 0.01 as compared to the extract alone.
Discussion
Animal models predictive of antidepressant action have been used extensively in the development of novel therapeutic compounds and for understanding the neural substrates underlying depressive behavior (CitationMcKinney & Bunney, 1969; CitationCryan et al., 2002, Citation2005). The FST is the most widely used test for assessing pharmacological antidepressant activity. It is based upon the observation that rodents develop immobility when they are placed in a cylinder of water after they stop active escape behaviors, such as swimming. Antidepressant treatments reduce the amount of immobility, or delay its onset, and increase or prolong active escape behaviors displayed during the FST (CitationPorsolt et al., 1977).
The present study shows that the hydroalcoholic extract from P. paniculata was able to decrease the immobility time of the animals in the FST, which is consistent with an antidepressant-like action. This effect was found after the acute administration of the hydroalcoholic extract at the dose range 0.01−30 mg/kg. However, the FST has some drawbacks represented by the possibility of obtaining some false positives or negatives. Drugs enhancing motor activity may give a “false” positive effect in the FST, whereas drugs decreasing locomotion may give a “false” negative result (CitationBorsini & Meli, 1988). The reduction in the immobility time elicited by the hydroalcoholic extract from P. paniculata cannot be attributable to a psychostimulant action. This conclusion derives from the fact that in our study, the hydroalcoholic extract from P. paniculata administered by p.o. route at doses that produced a significant decrease in the immobility time in the FST did not alter the locomotor activity in open-field test, as compared to control animals. Therefore, our result indicates that the hydroalcoholic extract from P. paniculata produces a specific antidepressant-like effect. Indeed, there is an increasing interest in the study of the antidepressant effect of herbs, since treatment of depression with conventional antidepressants (monoamine oxidase inhibitors, tricyclics, selective serotonin reuptake inhibitors, selective noradrenaline reuptake inhibitors) provides a complete remission just for approximately 50% of the individuals (CitationNestler et al., 2002). Our result is somewhat similar to some pre-clinical studies that report the antidepressant-like effect of several herbal compounds in the FST including Hypericum perforatum, Curcuma longa Linnaeus (Zingiberaceae), Ginkgo biloba Linnaeus (Ginkgoaceae), Salvia elegans Vahl. (Lamiaceae), Siphocampylus verticillatus G. Don. (Campanulaceae) (CitationRodrigues et al., 2002; CitationZhang, 2004; CitationHerrera-Ruiz et al., 2006; CitationMcGarry et al., 2007).
The monoamine hypothesis based on the deficiency of one or several monoamines is commonly evoked to explain the physiopathology of depression. This hypothesis initially based on noradrenaline (CitationSchildkraut et al., 1965) and serotonin deficiency (CitationCoppen, 1967) has been extended to dopamine (CitationRandrup et al., 1975). Considering that the monoaminergic systems are implicated in the pathophysiology and treatment of human depression, the present study was aimed at investigating the influence of pharmacological agents that modulate the monoaminergic systems on the antidepressant-like activity of the hydroalcoholic extract from P. paniculata (30 mg/kg, p.o.) in the FST.
In our study, the antidepressant-like effect of the hydroalcoholic extract from P. paniculata was not prevented by the pretreatment of the animals with the 5-HT1A antagonist NAN-190, suggesting that the anti-immobility effect of the hydroalcoholic extract from P. paniculata is not mediated by a modulation of 5-HT1A receptors. These receptors are located both in the postsynaptic region of 5-HT neurons in the forebrain and on the cell bodies and dendrites of 5-HT neurons located in the raphe nuclei. The 5-HT1A autoreceptor is thought to be primarily involved in the inhibition of 5-HT release from the forebrain (CitationBarnes & Sharp, 1999). Indeed, several studies have shown that 5-HT1A receptors mediate the antidepressant-like effects of conventional antidepressants (CitationRedrobe & Bourin, 1997; CitationBerrocoso et al., 2006) and compounds that exhibit antidepressant effects (CitationKaster et al., 2005; CitationBerrocoso et al., 2006; CitationCardoso et al., 2009) in the FST. However, similar to the results found in the present study, an absence of involvement of 5-HT1A receptors in the antidepressant-like action of ebselen in the FST was recently reported (CitationPosser et al., 2009).
In our study evidence that indicates that a modulation of the 5-HT2A/2C receptors is implicated in the antidepressant-like effect of the hydroalcoholic extract from P. paniculata was given by the finding that the pretreatment of mice with the 5-HT2A/2C receptor antagonist ketanserin was able to produce a synergistic antidepressant-like effect with the extract. Noteworthy, increased 5-HT2C receptors observed in the prefrontal cortex of depressed subjects (CitationGurevich et al., 2002) and in animals after stress exposure was shown to be normalized by antidepressant treatment (CitationEnglander et al., 2005). Our result is similar to the one reported by Redrobe (1997), in which the effect of imipramine and desipramine in the FST, but not fluoxetine, was potentiated by ketanserin. Furthermore, the pretreatment with a non-selective 5-HT2 antagonist (LY53857) reduces immobility time in mice treated with active and sub-active doses of imipramine (CitationYamada & Sugimoto, 2001). Moreover experimental data demonstrated that ritanserin, a 5-HT2A/2C receptor antagonist, inhibited dopamine reuptake in the rat frontal cortex (CitationRuiu et al., 2000). Our results suggest that the antidepressant-like effect of the hydroalcoholic extract from P. paniculata is dependent on an interaction with serotonergic system, likely through the involvement of 5-HT2A/2C receptors, but not 5-HT1A receptors, similarly to the effect of tricyclic antidepressants. Reinforcing the notion that the mechanism underlying the antidepressant-like effect of the hydroalcoholic extract from P. paniculata is different from the one elicited by selective serotonin re-uptake inhibitors, but similar to the effect of tricyclics, is the fact that the effect of tricyclic antidepressant imipramine, in the FST, is not dependent on the 5-HT1A receptors (CitationBorsini et al., 1991), in contrast to the anti-immobility effects of the selective serotonin reuptake inhibitors in the FST, which seem to be mediated by presynaptic 5-HT1A receptors (CitationRedrobe & Bourin, 1997).
The noradrenergic system is also a valuable target for antidepressants since depression is associated with a hypofunction of this system (CitationElhwuegi, 2004; CitationTaylor et al., 2005). The pharmacological antagonists yohimbine and prazosin are extremely used in the investigation of the participation of noradrenergic system in the mechanism of action of compounds and plant extracts with antidepressant action (CitationDhingra & Sharma, 2006; CitationDhingra & Kumar, 2008; CitationMachado et al., 2007, 2009). In our experiments, the pretreatment of mice with prazosin, an α1-adrenoceptor antagonist, was not able to reverse the antidepressant-like effect of the hydroalcoholic extract from P. paniculata, whereas the α2-adrenoceptor antagonist yohimbine reversed its effect in the FST. It was recently reported that the pretreatment of mice with prazosin did not alter the antidepressant-like effect of the desipramine (a tricyclic antidepressant that acts as a preferential noradrenaline reuptake inhibitor) in an animal model of depression (CitationZhang et al., 2009), whereas yohimbine was able to reduce the antidepressant-like effect of the desipramine in the chronic mild stress (CitationYalcin et al., 2005). Further corroborating the notion that the noradrenergic system is implicated in the mechanisms of action of the hydroalcoholic extract from P. paniculata in the FST is the fact that propranolol was able to reverse its anti-immobility effect in the FST. It is also interesting to note that propranolol antagonized the antidepressant-like effect of desipramine in rats in the FST (CitationMancinelli et al., 1991). Therefore, the reversal of the extract’s effect in the FST by yohimbine further reinforces the notion that the extract shares similar mechanisms of action with tricyclic antidepressants.
The dopaminergic system is strongly implicated in the regulation of mood (CitationDailly et al., 2004). A deficiency of mesolimbic dopamine is a leading candidate for the etiology of certain symptoms of depression (e.g., anhedonia and loss of motivation; CitationDunlop & Nemeroff, 2007) and the selective dopamine reuptake inhibitor, bupropion, has demonstrated similar efficacy to the selective serotonin reuptake inhibitors and tricyclics in the treatment of depression (CitationCroft et al., 1999; CitationWeihs et al., 2000). Moreover, it was demonstrated that the treatment with tricyclic antidepressants potentiated the dopaminergic neurotransmission, which may contribute to therapeutic effect of these drugs (CitationChiodo & Antelman, 1980; CitationD’Aquila et al., 2000; CitationPapakostas, 2006). In the present work we observed that both the selective dopamine D1 receptor antagonist (SCH23390) and the dopamine D2 receptor antagonist (sulpiride) significantly antagonized the anti-immobility effects of hydroalcoholic extract from P. paniculata in the FST. This result indicates that an activation of dopamine D1 and D2 receptors may underlie the anti-immobility effect of the hydroalcoholic extract from P. paniculata in the FST. Some studies have shown that sulpiride (CitationTakamori et al., 2001) and SCH23390 (CitationGambarana et al., 1995) were able to reverse the imipramine-induced decrease in the number of escape failures, an antidepressant profile in learned helplessness in rats. Furthermore, sulpiride was shown to reverse the antidepressant-like effect of imipramine in the FST (CitationBorsini et al., 1991). Similarly to imipramine, the antidepressant-like effect of the hydroalcoholic extract from P. paniculata seems to be mediated by an activation of dopamine D1 and D2 receptors.
Finally, the involvement of the monoaminergic receptors observed in our experiments possibly converges in an intracellular response that leads to the effect observed in the FST. A possibility to explain the antidepressant-like effect of the extract is that the inhibition of the enzyme GSK3β is implicated in the behavioral responses. Increased 5HT2 receptors occur in major depression and the activation of these receptors cause activation of GSK3β by promoting its dephosphorylation. Inhibition of GSK3 isoforms, either through pharmacologic or genetic means, has been shown to produce antidepressant-like effects (CitationJope & Roh, 2006; CitationRosa et al., 2008; Beaulieu, 2010). Further studies are needed to establish the intracellular pathways involved in the antidepressant-like effect of hydroalcoholic extract from P. paniculata.
Altogether, our results firstly indicate that the hydroalcoholic extract from P. paniculata causes a specific antidepressant-like effect that seems to be mediated by an interaction with the serotonergic (5-HT2A receptors), noradrenergic (α2 and β-receptor) and dopaminergic D1 and D2 receptors) systems. Therefore, it may be a useful approach for the treatment of depression and further studies are necessary to elucidate which isolated compounds are responsible for the antidepressant-like effect of the hydroalcoholic extract from P. paniculata.
Acknowledgments
The present study was supported by grants from FAPESC, CNPq, CAPES, and FINEP-IBN-Net (01.06.0842-00), Brazil.
Declaration of Interest
The authors declared no conflict of interest.
References
- Barnes NM, Sharp T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology, 38, 1083–1152.
- Beaulieu JM. (2011). A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci, 36, 110011.
- Berrocoso E, Rojas-Corrales MO, Mico JA. (2006). Differential role of 5-HT1A and 5-HT1B receptors on the antinociceptive and antidepressant effect of tramadol in mice. Psychopharmacology (Berl), 188, 111–118.
- Berton O, Nestler EJ. (2006). New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci, 7, 137–151.
- Bilia AR, Gallori S, Vincieri FF. (2002). St. John’s wort and depression: Efficacy, safety and tolerability-an update. Life Sci, 70, 3077–3096.
- Borsini F, Meli A. (1988). Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl), 94, 147–160.
- Borsini F, Cesana R, Vidi A, Mennini T. (1991). Evidence that imipramine activates 5-HT1C receptor function. Eur J Pharmacol, 203, 359–363.
- Brocardo PS, Budni J, Kaster MP, Santos AR, Rodrigues AL. (2008). Folic acid administration produces an antidepressant-like effect in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology, 54, 464–473.
- Calixto JB. (2005). Twenty-five years of research on medicinal plants in Latin America: A personal view. J Ethnopharmacol, 100, 131–134.
- Cardoso CC, Lobato KR, Binfaré RW, Ferreira PK, Rosa AO, Santos AR, Rodrigues AL. (2009). Evidence for the involvement of the monoaminergic system in the antidepressant-like effect of magnesium. Prog Neuropsychopharmacol Biol Psychiatry, 33, 235–242.
- Cervellati R, Innocenti G, Dall’Acqua S, Costa S, Sartini E. (2004). Polyphenols from Polygala spp. and their antioxidant activity. Chem Biodivers, 1, 415–425.
- Chiodo LA, Antelman SM. (1980). Repeated tricyclics induce a progressive dopamine autoreceptor subsensitivity independent of daily drug treatment. Nature, 287, 451–454.
- Chung IW, Moore NA, Oh WK, O’Neill MF, Ahn JS, Park JB, Kang UG, Kim YS. (2002). Behavioural pharmacology of polygalasaponins indicates potential antipsychotic efficacy. Pharmacol Biochem Behav, 71, 191–195.
- Coppen A. (1967). The biochemistry of affective disorders. Br J Psychiatry, 113, 1237–1264.
- Cristiano R, Pizzolatti MG, Delle Monache F, Rezende CM, Branco A. (2003). Two xanthones from Polygala paniculata and confirmation of the 1-hydroxy-2,3,5-trimethoxy-xanthone at trace level by HRGC-MS. Z Naturforsch, C, J Biosci, 58, 490–494.
- Croft H, Settle EJr, Houser T, Batey SR, Donahue RM, Ascher JA. (1999). A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther, 21, 643–658.
- Cryan JF, Markou A, Lucki I. (2002). Assessing antidepressant activity in rodents: Recent developments and future needs. Trends Pharmacol Sci, 23, 238–245.
- Cryan JF, Valentino RJ, Lucki I. (2005). Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev, 29, 547–569.
- Dailly E, Chenu F, Renard CE, Bourin M. (2004). Dopamine, depression and antidepressants. Fundam Clin Pharmacol, 18, 601–607.
- D’Aquila PS, Collu M, Gessa GL, Serra G. (2000). The role of dopamine in the mechanism of action of antidepressant drugs. Eur J Pharmacol, 405, 365–373.
- Dhingra D, Kumar V. (2008). Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of garlic extract in mice. Indian J Pharmacol, 40, 175–179.
- Dhingra D, Sharma A. (2006). Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuropsychopharmacol Biol Psychiatry, 30, 449–454.
- Dhir A, Kulkarni SK. (2007). Effect of addition of yohimbine (alpha-2-receptor antagonist) to the antidepressant activity of fluoxetine or venlafaxine in the mouse forced swim test. Pharmacology, 80, 239–243.
- Duarte FS, Marder M, Hoeller AA, Duzzioni M, Mendes BG, Pizzolatti MG, De Lima TC. (2008). Anticonvulsant and anxiolytic-like effects of compounds isolated from Polygala sabulosa (Polygalaceae) in rodents: In vitro and in vivo interactions with benzodiazepine binding sites. Psychopharmacology (Berl), 197, 351–360.
- Duman RS, Malberg J, Nakagawa S, D’Sa C. (2000). Neuronal plasticity and survival in mood disorders. Biol Psychiatry, 48, 732–739.
- Dunlop BW, Nemeroff CB. (2007). The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry, 64, 327–337.
- Elhwuegi AS. (2004). Central monoamines and their role in major depression. Prog Neuropsychopharmacol Biol Psychiatry, 28, 435–451.
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. (2005). How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci, 25, 648–651.
- Farina M, Franco JL, Ribas CM, Meotti FC, Missau FC, Pizzolatti MG, Dafre AL, Santos AR. (2005). Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol, 57, 1503–1508.
- Gambarana C, Ghiglieri O, Tagliamonte A, D’Alessandro N, de Montis MG. (1995). Crucial role of D1 dopamine receptors in mediating the antidepressant effect of imipramine. Pharmacol Biochem Behav, 50, 147–151.
- Gurevich I, Englander MT, Adlersberg M, Siegal NB, Schmauss C. (2002). Modulation of serotonin 2C receptor editing by sustained changes in serotonergic neurotransmission. J Neurosci, 22, 10529–10532.
- Hall CS. (1934). Emotional behavior in the rat: I. Defecation and urination as measures of individual differences in emotionality. J Comp Psychol, 18, 385–403.
- Hall CS. (1936). Emotional behavior in the rat III. The relationship between emotionality and ambulatory activity. J Comp Psychol, 22, 345–52.
- Herrera-Ruiz M, García-Beltrán Y, Mora S, Díaz-Véliz G, Viana GS, Tortoriello J, Ramírez G. (2006). Antidepressant and anxiolytic effects of hydroalcoholic extract from Salvia elegans. J Ethnopharmacol, 107, 53–58.
- Jope RS, Roh MS. (2006). Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr Drug Targets, 7, 1421–1434.
- Kaster MP, Santos AR, Rodrigues AL. (2005). Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull, 67, 53–61.
- Kaster MP, Raupp I, Binfaré RW, Andreatini R, Rodrigues AL. (2007). Antidepressant-like effect of lamotrigine in the mouse forced swimming test: Evidence for the involvement of the noradrenergic system. Eur J Pharmacol, 565, 119–124.
- Lapa Fda R, Gadotti VM, Missau FC, Pizzolatti MG, Marques MC, Dafré AL, Farina M, Rodrigues AL, Santos AR. (2009). Antinociceptive properties of the hydroalcoholic extract and the flavonoid rutin obtained from Polygala paniculata L. in mice. Basic Clin Pharmacol Toxicol, 104, 306–315.
- Lapa Fda R, Freitas CS, Baggio CH, Missau FC, Pizzolatti MG, Santos AR, Marques MC. (2007). Gastroprotective activity of the hydroalcoholic extract obtained from Polygala paniculata L. in rats. J Pharm Pharmacol, 59, 1413–1419.
- Lee HJ, Ban JY, Koh SB, Seong NS, Song KS, Bae KW, Seong YH. (2004). Polygalae radix extract protects cultured rat granule cells against damage induced by NMDA. Am J Chin Med, 32, 599–610.
- Machado DG, Kaster MP, Binfaré RW, Dias M, Santos AR, Pizzolatti MG, Brighente IM, Rodrigues AL. (2007). Antidepressant-like effect of the extract from leaves of Schinus molle L. in mice: Evidence for the involvement of the monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry, 31, 421–428.
- Machado DG, Bettio LE, Cunha MP, Santos AR, Pizzolatti MG, Brighente IM, Rodrigues AL. (2008). Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur J Pharmacol, 587, 163–168.
- Machado DG, Bettio LE, Cunha MP, Capra JC, Dalmarco JB, Pizzolatti MG, Rodrigues AL. (2009). Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: Involvement of the monoaminergic system. Prog Neuropsychopharmacol Biol Psychiatry, 33, 642–650.
- Mancinelli A, D’Aranno V, Stasi MA, Lecci A, Borsini F, Meli A. (1991). Effect of enantiomers of propranolol on desipramine-induced anti-immobility in the forced swimming test in the rat. Pharmacol Res, 23, 47–50.
- McGarry H, Pirotta M, Hegarty K, Gunn J. (2007). General practitioners and St. John’s Wort: A question of regulation or knowledge? Complement Ther Med, 15, 142–148.
- McKinney WTJr, Bunney WEJr. (1969). Animal model of depression. I. Review of evidence: Implications for research. Arch Gen Psychiatry, 21, 240–248.
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. (2002). Neurobiology of depression. Neuron, 34, 13–25.
- Newall AC, Anderson AL, Phillipson DJ. (1996). Herbal Medicines: A Guide for Health-care Professionals, 3rd edn. London: The Pharmaceutical Press.
- Nöldner M, Schötz K. (2002). Rutin is essential for the antidepressant activity of Hypericum perforatum extracts in the forced swimming test. Planta Med, 68, 577–580.
- O’Neill MF, Conway MW. (2001). Role of 5-HT(1A) and 5-HT(1B) receptors in the mediation of behavior in the forced swim test in mice. Neuropsychopharmacology, 24, 391–398.
- Páez-Pereda M. (2005). New drug targets in the signaling pathways activated by antidepressants. Prog Neuropsychopharmacol Biol Psychiatry, 29, 1010–1016.
- Papakostas GI. (2006). Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol, 16, 391–402.
- Park CH, Choi SH, Koo JW, Seo JH, Kim HS, Jeong SJ, Suh YH. (2002). Novel cognitive improving and neuroprotective activities of Polygala tenuifolia Willdenow extract, BT-11. J Neurosci Res, 70, 484–492.
- Piato AL, Rizon LP, Martins BS, Nunes DS, Elisabetsky E. (2009). Antidepressant profile of Ptychopetalum olacoides Bentham (Marapuama) in mice. Phytother Res, 23, 519–524.
- Porsolt RD, Bertin A, Jalfre M. (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther, 229, 327–336.
- Posser T, Kaster MP, Baraúna SC, Rocha JB, Rodrigues AL, Leal RB. (2009). Antidepressant-like effect of the organoselenium compound ebselen in mice: Evidence for the involvement of the monoaminergic system. Eur J Pharmacol, 602, 85–91.
- Randrup A, Munkvad I, Fog R, Gerlach J, Molander L, Kjellberg B, ScheelKruger J. (1975). Mania, depression and brain dopamine. Curr Dev Psychopharmacol, 2, 205–48.
- Redrobe JP, Bourin M. (1997). Partial role of 5-HT2 and 5-HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol, 325, 129–135.
- Rodrigues AL, da Silva GL, Mateussi AS, Fernandes ES, Miguel OG, Yunes RA, Calixto JB, Santos AR. (2002). Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci, 70, 1347–1358.
- Rosa AO, Kaster MP, Binfaré RW, Morales S, Martín-Aparicio E, Navarro-Rico ML, Martinez A, Medina M, García AG, López MG, Rodrigues AL. (2008). Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog Neuropsychopharmacol Biol Psychiatry, 32, 1549–1556.
- Ruiu S, Marchese G, Saba PL, Gessa GL, Pani L. (2000). The 5-HT2 antagonist ritanserin blocks dopamine re-uptake in the rat frontal cortex. Mol Psychiatry, 5, 673–677.
- Schildkraut JJ, Gordon EK, Durell J. (1965). Catecholamine metabolism in affective disorders. I. Normetanephrine and VMA excretion in depressed patients treated with imipramine. J Psychiatr Res, 3, 213–228.
- Takamori K, Yoshida S, Okuyama S. (2001). Repeated treatment with imipramine, fluvoxamine and tranylcypromine decreases the number of escape failures by activating dopaminergic systems in a rat learned helplessness test. Life Sci, 69, 1919–1926.
- Taylor C, Fricker AD, Devi LA, Gomes I. (2005). Mechanisms of action of antidepressants: From neurotransmitter systems to signaling pathways. Cell Signal, 17, 549–557.
- Wang H, Gao J, Kou J, Zhu D, Yu B. (2008). Anti-inflammatory activities of triterpenoid saponins from Polygala japonica. Phytomedicine, 15, 321–326.
- Weihs KL, Settle ECJr, Batey SR, Houser TL, Donahue RM, Ascher JA. (2000). Bupropion sustained release versus paroxetine for the treatment of depression in the elderly. J Clin Psychiatry, 61, 196–202.
- Wong ML, Licinio J. (2001). Research and treatment approaches to depression. Nat Rev Neurosci, 2, 343–351.
- World Health Organization. (2001). The World Health Report− Mental Health: New Understanding. New Hope.
- Wu JF, Chen SB, Gao JC, Song HL, Wu LJ, Chen SL, Tu PF. (2007). Antioxidants and a new dihydroisocoumarins from Polygala hongkongensis Hemsl. Nat Prod Res, 21, 580–584.
- Yalcin I, Aksu F, Belzung C. (2005). Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol, 514, 165–174.
- Yamada J, Sugimoto Y. (2001). Effects of 5-HT(2) receptor antagonists on the anti-immobility effects of imipramine in the forced swimming test with mice. Eur J Pharmacol, 427, 221–225.
- Zhang HT, Whisler LR, Huang Y, Xiang Y, O’Donnell JM. (2009). Postsynaptic alpha-2 adrenergic receptors are critical for the antidepressant-like effects of desipramine on behavior. Neuropsychopharmacology, 34, 1067–1077.
- Zhang ZJ. (2004). Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci, 75, 1659–1699.