Abstract
Context: Iridoids belong to a group of monoterpene compounds with cyclopentane ring and found as mostly the glycoside forms in nature. They act primarily as the defense substances and found in various medicinal plants.
Objective: Although many iridoids exhibit anti-inflammatory and anticancer activities, their molecular targets/pathways are not fully understood. Here, the antiproliferative effect of the hydrolyzed-iridoid product (H-iridoid) form through the STAT3 signaling pathways on tumor cells was investigated.
Materials and methods: H-iridoids were obtained from five iridoid glycosides with β-glucosidase treatment. The effects of several H-iridoids on cell viability and cell proliferation in tumor cells were measured by the MTT assay. The phosphorylation levels of STAT3, its regulatory molecules, and apoptosis by H-geniposide treatment in DU145 cells were investigated by immunoblots and flow cytometry.
Results: No single iridoid glycoside exerted any cytotoxicity in the tumor cells, whereas H-iridoids had significant cytotoxic, antiproliferative, and STAT3 inhibitory effects and revealed different potencies depending on their chemical structures. Among the H-iridoids tested, H-geniposide inhibited constitutive STAT3 activation through inhibiting upstream JAK1 and c-Src. Consistent with STAT3 inactivation, H-geniposide downregulated the expressions of Bcl-2, Bcl-xL, survivin, and cyclin D1; this correlated with the accumulation of cells in the sub-G1 phase of the cell cycle and the induction of apoptosis.
Discussion and conclusions: Our results indicate that the hydrolysis of the glycosidic bond from iridoid glycoside is required for exhibiting cytotoxicity in tumor cells. H-geniposide is the most potent agent and a novel blocker of STAT3 activation in DU145 cells.
Keywords::
Introduction
Many medicinal herbs containing iridoids, such as Plantago asiatica L., Cornus capitata, Cornus officinalis, Rehmannia glutinosa, Scrophularia ningpoensis, Gentiana loureirii, Gentiana pedicellata, and Harpagophytum procumbens D.C. have been used to treat inflammatory-related illnesses, including hypertension, ischials, asthma, and arthritis. Several reports have been commissioned to evaluate the pharmacological activities of the iridoids. Specifically, the iridoids have been reported to exhibit liver protective (CitationChang et al., 1983), bile acid excretion (CitationMiyagoshi et al., 1988), antimicrobial (CitationDavini et al., 1986), anti-dotal (CitationChang & Yamaura, 1993), antiviral (CitationChang, 1997), anti-inflammatory ( CitationRecio et al., 1994; CitationPark & Chang, 2004; CitationKim et al., 2009; CitationPark et al., 2010), and chemopreventive (CitationHsu et al., 1997; CitationKonoshima et al., 2000; CitationPeng et al., 2005; CitationHung et al., 2008) effects. Subsequently, iridoids are shown to have a potent antitumor property that acts in different tumor cell types, including C6 glioma cells (CitationPeng et al., 2005), non-small cell lung carcinoma (CitationHung et al., 2008), and chronic myeloid leukemia–induced apoptosis (CitationAnand et al., 2008).
Among the various transcription factors, the signal transducer and activator of transcription-3 (STAT3) are closely linked with tumorigenesis. The STAT3 is activated through tyrosine phosphorylation by a variety of cytokines (e.g., IL-6, TNFα), growth factors (e.g., epidermal growth factor, transforming growth factor-α, hepatocyte growth factor), and oncogenic kinases (e.g., Src) (CitationDarnell, 1997). The phosphorylation of STAT3 is mediated through the activation of janus-like kinase (JAK). JAK1, JAK2, JAK3, and TYK2 which have been linked with the activation of STAT3 (CitationIhle, 1996). Additionally, the role of c-Src kinase has been demonstrated in STAT3 phosphorylation (CitationSchreiner et al., 2002). Activated STAT3 can regulate the expression of genes that mediate proliferation (e.g., cyclin D1) or suppress apoptosis (e.g., Bcl-2, Bcl-xL, and survivin). Thus, STAT3 activation pathways have been closely linked with the proliferation, anti-apoptosis, and chemoresistance of tumors (CitationAggarwal et al., 2006). It is possible that iridoid glycoside and its aglycone form mediate its effects through the modulation of this pathway. However, STAT signaling, by which iridoid glycoside and its aglycone form mediate antitumor activities, remains undefined.
In the present study, we aimed to verify the antitumor activities of five H-iridoids in tumor cells. It has been shown that iridoid glycoside itself is an inactive form, whereas H-iridoid is an active form derived from the enzymatic hydrolysis of glycosidic bond (CitationPark et al., 2010). As described above, due to the critical role of STAT3 activation in tumorigenesis, including survival and proliferation, we hypothesized that H-iridoids mediate their effects in part through the suppression of STAT3 signaling. The results that follow indicate that H-geniposide, one of the H-iridoids, indeed suppressed constitutive STAT3 activation and down-regulated the expression of cell survival and proliferative gene products, leading to the suppression of the proliferation and induction of apoptosis in human prostate cancer DU145 cells.
Methods
Reagents
Five iridoid glycosides, specifically aucubin (99.5%), catalpol (>98%), geniposide (99%), geniposidic acid (> 98%), harpagoside (99%), were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan) (). The β-glucosidase (EC 3.2.1.21), 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT), propidium iodide (PI), Tris base, glycine, NaCl, sodium dodecyl sulfate (SDS), RNase A, and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO, USA). Annexin V was from BD Biosciences (Palo Alto, CA, USA). RPMI 1640, DMEM, fetal bovine serum (FBS), 0.4% trypan blue vital stain, and antibiotic–antimycotic mixture were obtained from Life Technologies (Grand Island, NY, USA). Rabbit polyclonal antibodies to STAT3 and mouse monoclonal antibodies against phospho-STAT3 (Tyr705) and Bcl-2, Bcl-xL, Survivin, cyclin D, CDK2, CDK6, procaspase-3, -8, -9, and PARP were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies of phospho-JAK1 (Tyr1022/1023), JAK1, phospho-Src (Tyr416), Src, phospho-JAK2 (Tyr1007/1008), JAK2, procaspase-8, procaspase-9, were purchased from Cell Signaling Technology. Goat anti-mouse horseradish peroxidase was purchased from Bio-Rad. Goat anti-rabbit horseradish peroxidase was purchased from Transduction Laboratories (Lexington, KY, USA).
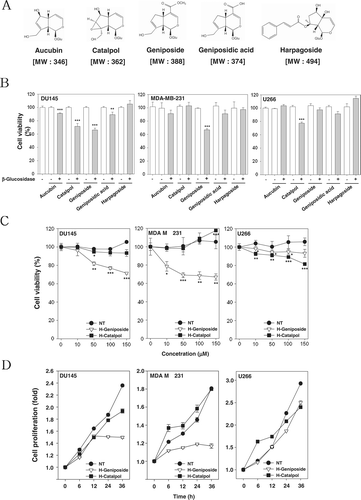
Figure 1. H-geniposide and H-catalpol exerted cytotoxicity and suppressed cell proliferation in tumor cells. (A) The chemical structures of iridoid glycosides. (B) After 1 × 104 cells were seeded on 96-well plates, various iridoid glycosides and H-iridoids were treated with 150 µM for 24 h in DU145, MDA-MB-231, and U266 cells. The representative cell viability was accessed using the MTT assay. (C) The cells were treated with various concentrations of H-geniposide (▿) and H-catalpol (▪) for 24 h. The representative cell viability was assessed using the MTT assay; ***p < 0.001 compared to non-treated (NT, •). The representative cell viability was assessed using the MTT assay. (D) After DU145, MDA-MB-231, and U266 cells (5 × 103 cells/well) were seeded onto 96-well plates, they were left non-treated (NT, •) or treated with H-geniposide (▿)and H-catalpol (▪) at 150 µM concentration for the indicated time intervals. The cell proliferation was measured using the MTT assay.
Cell lines
Human prostate carcinoma DU145, human breast carcinoma MDA-MB-231, and human multiple myeloma cell lines U266 were obtained from the American Type Culture Collection (Manassas, VA, USA). The DU145 and U266 cells were cultured in RPMI 1640 medium containing 10% FBS. MDA-MB-231 cells were cultured in DMEM supplemented with 10% FBS. All media were also supplemented with 100 U/mL of penicillin and 100 µg/mL of streptomycin.
Preparation of H-iridoids
To prepare the H-iridoid products, 1 mM of iridoid glucoside was pre-incubated with the same volume of 0.5 mM of β-glucosidase (iridoid:enzyme = 1.0:0.5 v/v), and diluted by adding distilled water to the specified concentrations. Under these conditions, almost all the iridoid glycosides were easily converted by β-glucosidase into their hydrolyzed products (CitationPark et al., 2010).
MTT assay
Cell viability was measured by an MTT assay to detect NADH-dependent dehydrogenase activity. A 50 µL MTT solution (5 mg/mL) in 1× phosphate-buffered saline (PBS) was directly added to the cells, which were then incubated for 4 h to allow the MTT to metabolize to formazan. Absorbance was measured with an automated spectrophotometric plate reader at a wavelength of 570 nm. Cell viability was normalized as relative percentages in comparison with untreated controls.
Western blot analysis
After the DU145, MDA-MB-231, and U266 cells were treated with the indicated concentrations of H-iridoids, the cells were lysed and the total protein concentrations were determined by Bradford reagent (Bio-Rad, Hercules, CA, USA). Equal amounts of lysates resolved on SDS–polyacrylamide gel electrophoresis (SDS-PAGE) were transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad), and the membrane was blocked with 1× TBS containing 0.1% Tween 20 and 5% skim milk or 2% BSA for 1 h at room temperature. After the blocking, the membranes were incubated overnight at 4°C with the respective primary antibodies. The membranes were washed thrice and incubated with diluted horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000) for 1 h at room temperature. After three washes, the membranes were detected using an enhanced chemiluminescence (ECL) kit (Millipore, Bedford, MA, USA).
Cell cycle analysis
The DU145 cells were seeded onto 6-well plates at a density of 1 × 106 cells per well and incubated for one day. After treatment with 150 µM of geniposide, β-glucosidase, or 150 µM of H-geniposide for 24 h, the cells were collected and washed with 1× PBS. Cell pellets were fixed in 70% cold ethanol overnight at −20°C. The fixed cells were resuspended in 1× PBS containing 1 mg/mL RNase A and incubated for 1 h at 37°C; the cells were stained by adding 50 µg/mL PI for 30 min at room temperature in the dark. The DNA contents of the stained cells were analyzed using CellQuest Software with a FACS Vantage SE (Becton Dickinson, Heidelberg, Germany) flow cytometry (Becton Dickinson).
Annexin V analysis
The 1 × 106 cells were treated with geniposide, β-glucosidase or H-geniposide for 24 h, fixed with 4% paraformaldehyde, and stained by Annexin V conjugated to FITC or with a 1 µg/mL DAPI solution. The cells were washed and observed accordingly with a flow cytometry (Becton Dickinson).
Statistical analysis
All numeric values are represented as the mean ± SD. Statistical significance of the data compared with the untreated control was determined using the Student’s unpaired t-test. Significance was set at p < 0.05.
Results
H-iridoids exert cytotoxicity against human prostate, breast cancer, and multiple myeloma cells
To determine cytotoxicity, cultured human prostate (DU145), breast cancer cells (MDA-MB-231), and multiple myeloma (U266) were treated with 150 µM concentrations of various iridoid glycosides () and H-iridoids for 24 h, followed by measurement by MTT assay. Here, there were some significant results as shown in : five iridoid glycosides did not exhibit any cytotoxicity in themselves and the H-iridoids had significant cytotoxic effects compared with the iridoid glycosides in all tumor cells. Among these H-iridoids, H-aucubin, H-catalpol, H-geniposide, and H-geniposidic acid significantly increased the levels of cytotoxicity in DU145 cells. The H-geniposide significantly increased the levels of cytotoxicity on MDA-MB-231 cells, and H-catalpol significantly increased the levels of cytotoxicity on U266 cells. These results indicated that the various H-iridoids had cell type specificity. Since H-catalpol and H-geniposide were found to exhibit the most potent cytotoxic effects in all tumor cells, the tumor cells were treated with the indicated concentrations of H-catalpol and H-geniposide for 24 h, and then cell viability was analyzed using a MTT assay. As shown in , H-geniposide significantly suppressed cell viability, whereas H-catalpol had a minimal effect in DU145 and MDA-MB-231 cells. But H-catalpol was found to exhibit strong cytotoxic effects in U266 cells compared with the H-geniposide. As shown in , H-catalpol and H-geniposide significantly suppressed cell proliferation in these tumor cells.
Several H-iridoids inhibit constitutive STAT3 activation in tumor cells
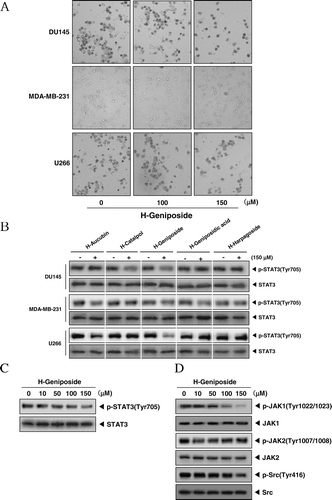
The morphological changes of DU145, MDA-MB-231, and U266 cells which were treated with 100 or 150 µM of H-geniposide for 24 h were revealed under inverted microscopy observation as shown in . We first investigated whether the five H-iridoids suppress constitutive STAT3 activation in these tumor cells. Because DU145, MDA-MB-231, and U266 cells have been shown to express constitutive STAT3 activation (CitationHeo et al., 2011; CitationLee et al., 2011a), we set out to determine whether the H-iridoids could inhibit this activation in these cells. Indeed, H-catalpol and H-geniposide did inhibit STAT3 activation in DU145 cells: maximum inhibition occurring at 150 µM of H-catalpol and H-geniposide and had no effect on the expression of STAT3 proteins (, first and second panels). H-aucubin, H-geniposide, and H-geniposidic acid repressed STAT3 activation in MDA-MB-231 cells and had no effect on the expression of STAT3 proteins (, third and fourth panels). H-aucubin and H-geniposide blocked STAT3 activation in U266 cells and had no effect on the expression of STAT3 proteins (, fifth and sixth panels). The suppression of constitutive STAT3 activation by the H-iridoids was found to be not due to loss of cell viability (data not shown).
Figure 2. H-geniposide inhibited the upstream signaling molecules of the STAT3 pathway. (A) After DU145, MDA-MB-231, and U266 cells (1 × 106 cells/well) were seeded onto 6-well plates, they were treated with 150 µM of H-geniposide for 24 h. Then, the cells were fixed and observed using a microscope. (B) DU145, MDA-MB-231, and U266 cells (1 × 106 cells/well) were treated with five H-iridoids (150 µM) for 8 h. Whole-cell extracts were prepared and immunoblotted with antibodies for phospho-STAT3 (Tyr705) and STAT3. (C) DU145 cells (1 3 106 cells/well) were treated with H-geniposide (0, 10, 50, 100 mm) for 8 h. Whole-cell extracts were prepared and immunoblotted with antibodies for phospho-STAT3 (Tyr 705) and STAT3. (D) DU145 cells (1 3 106 cells/well) were treated with H-geniposide (0, 10, 50, 100, 150 mm) for 8 h. Whole-cell extracts were prepared and immunoblotted with antibodies for phospho-JAKI (Tyr1022/1023), phospho-JAK2 (Tyr1007/1008), and JAK2 or phospho-Src (Tyr416) and Src.
H-geniposide inhibits STAT3 pathway in DU145 cells
In the next experiment, we focused on H-geniposide in the STAT3 signaling pathway. To determine whether H-geniposide suppresses constitutive STAT3 activation in DU145 cells, cells were treated with various concentrations of H-geniposide for 8 h. First, H-geniposide suppressed the phosphorylation of STAT3 (Tyr705) in a concentration-dependent manner in DU145 cells (, upper panel) and had no effect on the expression of STAT3 proteins (, lower panel). In order to know which upstream signaling molecule is involved in H-geniposide-mediated STAT3 inactivation, we examined the effects of H-geniposide on the phosphorylation of JAK2 and c-Src in DU145 cells. As shown in , JAK2 was constitutively active in DU145 cells and the treatment with H-geniposide clearly suppressed this phosphorylation in a concentration-dependent manner. We also found that H-geniposide suppressed the constitutive phosphorylation of c-Src kinase (, third panel). The levels of total c-Src kinase remained unchanged under the same conditions (, fourth panel).
H-geniposide represses the expression of proliferative products in DU145 cells
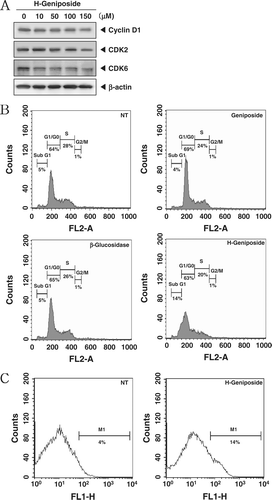
Cyclin D1, which is required for cell proliferation and for the transition from the G1 phase to the S phase of the cell cycle, is also known to be regulated by STAT3 (CitationAggarwal et al., 2006). In addition, cyclin D1 associates specifically with CDK2 and CDK6 (CitationBates et al., 1994). We demonstrated that H-geniposide treatment suppressed the expressions of cyclin D1, CDK2, and CDK6 in a concentration-dependent manner ().
Figure 3. H-geniposide-induced cell cycle arrest and apoptosis. (A) DU145 cells were treated with the indicated concentrations of H-geniposide for 24 h. Thereafter, equal amounts of lysates were analyzed by western blot analysis using antibodies against Cyclin D1, CDK2, and CDK6. The β-actin was used as a loading control (bottom panel). (B) After DU145 (1 × 106 cells/well) were seeded onto 6-well plates, they were left NT or treated with geniposide (150 µM), β-glucosidase, or H-geniposide. After 24 h incubation, the cells were harvested, washed with a cold PBS buffer, and digested with RNase. Cellular DNA staining with propidium iodide and flow cytometric analysis was done to determine the cell cycle distribution as described in the “Materials and methods”. Data are from one representative experiment of the three independent experiments that had similar results among all the results. (C) DU145 cells were treated with H-geniposide for 24 h and the cells were incubated with an FITC-conjugated Annexin V antibody and then analyzed by a flow cytometry as described in “Materials and methods”.
H-geniposide causes the accumulation of the cells in the G1 phase of the cell cycle in DU145 cells
We set out to determine the effect of H-geniposide on cell cycle phase distribution. Importantly, we also found that the sub-G1 contents of DNA standing for apoptotic portions were significantly increased in H-geniposide-treated DU145 cells, but not in geniposide- and β-glucosidase-treated cells (). H-geniposide increased the cell accumulation at the sub-G1 phase (14%) compared with the non-treated (NT) cells (5%). Taken together, these results suggest that SC induced apoptotic cell death in LNCaP and MCF-7 cells.
H-geniposide promotes apoptotic cell death in DU145 cells
To evaluate the potential activity of H-geniposide to induce apoptosis, we performed an annexin V assay. Consistent with previous results, H-geniposide increased the rate of early apoptotic cells in DU145 cells in the annexin V assay and reached the level of 14% at a concentration of 150 µM compared with the non-treated cells (NT, 4%) (). Taken together, these results strongly suggest that H-geniposide induced apoptotic cell death in the cells.
H-geniposide represses the expression of anti-apoptotic gene products
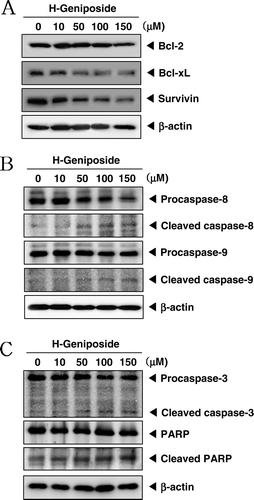
Once activated, STAT3 then becomes dimerized and translocates into the nucleus. It also regulates the expression of various gene products involved in cell survival, including Bcl-2, Bcl-xL, and survivin (CitationAggarwal et al., 2006). We found that H-geniposide treatment downregulated the expressions of these anti-apoptotic proteins in a concentration-dependent manner ().
Figure 4. H-geniposide down-regulated the expression of anti-apoptotic gene products and induced apoptosis via caspase-3 activation. (A) DU145 cells were treated with indicated concentrations of H-geniposide for 24 h. Thereafter, equal amounts of lysates were analyzed by western blot analysis using antibodies against Bcl-2, Bcl-xL, and survivin. The β-actin was used as a loading control (bottom panel). (B) Lysates from the cells were subjected to western blot analysis for procaspase-8, cleaved caspase-8, procaspase-9, cleaved caspase-9, and β-actin. (C) Lysates from the cells were subjected to western blot analysis for PARP, caspase-3, and β-actin. A representative blot is shown from the three independent experiments with identical observations.
H-geniposide induces cleavage of procaspase-8 and procaspase-9
Since procaspase-8 and procaspase-9 are linked with the apoptotic cell death pathway (CitationHakem et al., 1998; CitationLuo et al., 1998), we examined their levels in H-geniposide-treated DU145 cells. H-geniposide induced the cleavage of procaspase-8 and procaspase-9 at 100–150 µM as seen by the disappearance of the procaspase band and the appearance of its cleavage products ().
H-geniposide activates caspase-3 and causes PARP cleavage
DU145 cells were treated with various concentration of H-geniposide and then were examined for caspase-3 activation by western blotting using specific antibodies. We found a concentration-dependent activation of caspase-3 by H-geniposide. Activation of downstream caspase-3 led to the cleavage of a 116 kDa PARP protein into 87 kDa fragments. These results clearly suggest that H-geniposide induces caspase-3-dependent apoptosis in DU145 cells ().
Discussion
Although iridoids suppress proliferation in various cancer cell types and induces apoptosis, the exact mechanisms of their hydrolyzed forms are not clearly defined at present. The purpose of our study was to investigate the effects of H-iridoids on the STAT3 signaling pathway, gene products, and cellular responses. Here, we found that H-iridoids exerted cytotoxicity, suppressed constitutive STAT3 activation in parallel with the inhibition of constitutive JAK1 and c-Src activation in tumor cells (DU145, MDA-MB-231, and U266). Moreover, H-geniposide, one of the H-iridoid, also downmodulated the expression of STAT3-regulated gene products, including Bcl-xL, Bcl-2, survivin, and cyclin D1, and inhibited cell proliferation, the accumulation of cells in the G1-G0 phase, and apoptosis through caspase-3 activation.
Constitutive STAT3 activation has been closely linked with a variety of tumors, including prostate cancer, multiple myeloma, breast cancer, head and neck squamous cell carcinoma, lymphomas and leukemias, brain tumor, colon cancer, Ewing sarcoma, gastric cancer, esophageal cancer, ovarian cancer, nasopharyngeal cancer, and pancreatic cancer (CitationAggarwal et al., 2006). Thus, the suppression of the constitutively active STAT3 has emerged as a legitimate target for cancer therapy. We found for the first time that H-catalpol and H-geniposide could suppress constitutive STAT3 activation in human prostate carcinoma (DU-145) cells; H-aucubin, H-geniposide, and H-geniposidic acid repressed STAT3 activation in human breast carcinoma (MDA-MB-231) cells; H-aucubin and H-geniposide blocked STAT3 activation in multiple myeloma cells. Specifically, how H-geniposide blocks constitutive STAT3 activation was also investigated. The effects of H-geniposide on STAT3 phosphorylation correlated with the suppression of the upstream protein tyrosine kinases JAK1 and c-Src. All Src-transformed cells have persistently activated STAT3, and dominant-negative STAT3 blocks transformation (CitationBowman et al., 2000). Besides, H-geniposide did not suppress the constitutive activation of JAK2, suggesting that the blocking of STAT3 phosphorylation by H-geniposide is potentially associated with the suppression of JAK1 and c-Src.
We found, for the first time, that H-geniposide downregulated various anti-apoptotic gene products, such as Bcl-2, Bcl-xL, and survivin. The expressions of Bcl-2 and Bcl-xL have been known to be regulated through the activation of STAT3, and this protein is overexpressed in human prostate cancer cells (CitationSingh et al., 2005). The downregulation of the expressions of Bcl-2 and Bcl-xL could contribute to H-geniposide’s ability to induce apoptosis in human prostate cancer cells. Consistently, we have reported that genipin, an active compound of Gardenia fruit, downregulated STAT3-regulated gene products, such as survivin, Bcl-2, and Bcl-xL, and potentiates chemotherapeutic agent-induced apoptosis in human multiple myeloma cells (CitationLee et al., 2011b). The antiproliferative effect of H-geniposide, previously reported against a wide variety of tumor cells (CitationPeng et al., 2005; CitationAnand et al., 2008; CitationHung et al., 2008), could be due to the downregulation of these gene products.
We also found that the expression of cyclin D1, CDK2, and CDK6 were downmodulated by H-geniposide. Indeed, H-geniposide was found to induce cell cycle arrest in the G1 phase and apoptosis selectively in human prostate carcinoma cells (CitationYim et al., 2005), which could be due to the down-regulation of cyclin D1. Besides, constitutive STAT3 activation is involved in the growth promotion and apoptosis inhibition functions which result in chemo-resistance in tumor cells ( CitationCatlett-Falcone et al., 1999; CitationAggarwal et al., 2006). The reversal effect is probably mediated through the upregulation of cyclin D1, Bcl-2, and survivin (CitationDanial et al., 1995). The Bcl-2 and survivin, which are regulated by STAT3, are overexpressed in human prostate carcinoma DU145 cells and also can lead to the resistance of apoptosis by various chemotherapeutic agents, in parallel with an increase in chemo-resistance (CitationSimonian et al., 1997). In the near future, we will examine whether H-iridoids potentiate the cytotoxic and apoptotic effects of chemotherapeutic agents via the suppressing of STAT3 signaling in tumor cells.
Conclusions
Overall, our results demonstrate that H-iridoids, such as H-geniposide, inhibit constitutive STAT3 activation through the suppression of constitutive JAK1 and c-Src activation, which makes H-iridoids a potentially effective suppressor of tumor cell survival and proliferation. However, further clinical studies are needed with H-iridoids alone and in combination with standard chemotherapeutics to demonstrate the potential applications for H-iridoids.
Acknowledgments
We thank Mr. Keith Grainger for his review and feedback on the article. Hyundu Hwang and Chulwon Kim contributed equally to this study.
Declaration of interest
The authors have no conflicts of interest to disclosure. This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Ministry of Education, Science and Technology (MoEST) (No. 2011-0006220).
References
- Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. (2006). Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci, 1091, 151–169.
- Anand P, Kunnumakkara AB, Harikumar KB, Ahn KS, Badmaev V, Aggarwal BB. (2008). Modification of cysteine residue in p65 subunit of nuclear factor-κB (NF-κB) by picroliv suppresses NF-κB-regulated gene products and potentiates apoptosis. Cancer Res, 68, 8861–8870.
- Bates S, Bonetta L, MacAllan D, Parry D, Holder A, Dickson C, Peters G. (1994). CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene, 9, 71–79.
- Bowman T, Garcia R, Turkson J, Jove R. (2000). STATs in oncogenesis. Oncogene, 19, 2474–2488.
- Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, Dalton WS, Jove R. (1999). Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity, 10, 105–115.
- Chang IM. (1997). Antiviral activity of aucubin against hepatitis B virus replication. Phytother Res, 11, 189–192.
- Chang IM, Ryu JC, Park YC, Yun HS, Yang KH. (1983). Protective activities of aucubin against carbon tetrachloride-induced liver damage in mice. Drug Chem Toxicol, 6, 443–453.
- Chang IM, Yamaura Y. (1993). Aucubin: a new antidote for poisonous Amanita mushrooms. Phytother Res, 7, 53–56.
- Danial NN, Pernis A, Rothman PB. (1995). Jak-STAT signaling induced by the v-abl oncogene. Science, 269, 1875–1877.
- Darnell JE Jr. (1997). STATs and gene regulation. Science, 277, 1630–1635.
- Davini E, Iavarone C, Trogolo C, Aureli P, Pasolini B. (1986). The quantitative isolation and antimicrobial activity of the aglycone of acubin. Phytochemistry, 25, 2420–2422.
- Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW. (1998). Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell, 94, 339–352.
- Heo JY, Kim HJ, Kim SM, Park KR, Park SY, Kim SW, Nam D, Jang HJ, Lee SG, Ahn KS, Kim SH, Shim BS, Choi SH, Ahn KS. (2011). Embelin suppresses STAT3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase PTEN. Cancer Lett, 308, 71–80.
- Hsu HY, Yang JJ, Lin SY, Lin CC. (1997). Comparisons of geniposidic acid and geniposide on antitumor and radioprotection after sublethal irradiation. Cancer Lett, 113, 31–37.
- Hung JY, Yang CJ, Tsai YM, Huang HW, Huang MS. (2008). Antiproliferative activity of aucubin is through cell cycle arrest and apoptosis in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol Physiol, 35, 995–1001.
- Ihle JN. (1996). STATs: signal transducers and activators of transcription. Cell, 84, 331–334.
- Kim BH, Park KS, Chang IM. (2009). Elucidation of anti-inflammatory potencies of Eucommia ulmoides bark and Plantago asiatica seeds. J Med Food, 12, 764–769.
- Konoshima T, Takasaki M, Tokuda H, Nishino H. (2000). Cancer chemopreventive activity of an iridoid glycoside, 8-acetylharpagide, from Ajuga decumbens. Cancer Lett, 157, 87–92.
- Lee HJ, Seo NJ, Jeong SJ, Park Y, Jung DB, Koh W, Lee HJ, Lee EO, Ahn KS, Ahn KS, Lü J, Kim SH. (2011). Oral administration of penta-O-galloyl-β-D-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with JAK1-STAT3 inhibition. Carcinogenesis, 32, 804–811.
- Lee JC, Ahn KS, Jeong SJ, Jung JH, Kwon TR, Rhee YH, Kim SH, Kim SY, Yoon HJ, Zhu S, Chen CY, Kim SH. (2011). Signal transducer and activator of transcription 3 pathway mediates genipin-induced apoptosis in U266 multiple myeloma cells. J Cell Biochem, 112, 1552–1562.
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. (1998). Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell, 94, 481–490.
- Miyagoshi M, Amagaya S, Ogihara Y. (1988). Choleretic actions of iridoid compounds. J Pharmacobio-dyn, 11, 186–190.
- Park KS, Chang IM. (2004). Anti-inflammatory activity of aucubin by inhibition of tumor necrosis factor-α production in RAW 264.7 cells. Planta Med, 70, 778–779.
- Park KS, Kim BH, Chang IM. (2010). Inhibitory potencies of several iridoids on cyclooxygenase-1, cyclooxygnase-2 enzymes activities, tumor necrosis factor-a and nitric oxide production in vitro. Evid Based Complement Alternat Med, 7, 41–45.
- Peng CH, Tseng TH, Huang CN, Hsu SP, Wang CJ. (2005). Apoptosis induced by penta-acetyl geniposide in C6 glioma cells is associated with JNK activation and Fas ligand induction. Toxicol Appl Pharmacol, 202, 172–179.
- Recio MC, Giner RM, Máñez S, Ríos JL. (1994). Structural considerations on the iridoids as anti-inflammatory agents. Planta Med, 60, 232–234.
- Schreiner SJ, Schiavone AP, Smithgall TE. (2002). Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem, 277, 45680–45687.
- Simonian PL, Grillot DA, Nuñez G. (1997). Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood, 90, 1208–1216.
- Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. (2005). Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J Biol Chem, 280, 19911–19924.
- Yim D, Singh RP, Agarwal C, Lee S, Chi H, Agarwal R. (2005). A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res, 65, 1035–1044.