Abstract
Context: The bacterium Pseudomonas syringae pv. syringae (Pss) is a pathogen of many plant species and causes, for example, brown spot disease in bean plants (Phaseolus vulgaris). Pss excretes the syringolins, natural product molecules that act as a virulence factors and inhibit the proteasome of the host plants.
Objective: Proteasome inhibitors belong to an important class of anticancer agents and bortezomib (Velcade®) has been Food and Drug Administration-approved for the treatment of multiple myeloma (MM) and mantle cell lymphoma. Syringolins represent a new class of proteasome inhibitors and the present work was undertaken to design a potent syringolin-inspired analogue (TIR-203) for anticancer drug development.
Materials and methods: TIR-203 was tested against human MM and neuroblastoma (NB) cells. Cancer cells were treated with TIR-203 at various concentrations (0–10 µM) and the cell viability was measured using the MTS assay. To determine the effects on proteasomal activities, the cell culture-based proteasome inhibition assay was used. Syringolin A (SylA) and bortezomib were included as controls.
Results: TIR-203 inhibited the cell proliferation of MM and NB cells in a dose-dependent manner at significantly lower concentrations than SylA. In MM cells, TIR-203 effectively inhibited the chymotrypsin-like (CT-L), caspase-like (C-L), and trypsin-like (T-L) activities of the proteasome. In NB cells, TIR-203 inhibited the CT-L and C-L activities, but not the T-L activity.
Discussion and conclusions: The newly designed proteasome inhibitor TIR-203 is more potent than the natural product SylA and strongly inhibits the cell viability and proteasomal activity of MM and NB cells.
Introduction
Syringolins are produced by the plant pathogen Pseudomonas syringae pv. syringae (Pss). Syringolin A (SylA) is a small molecule natural product and represents the predominant form of syringolins produced by Pss, whereas SylB-F are syringolin variants expressed in minor quantities. Brown spot disease is one of many known diseases in bean plants (Phaseolus vulgaris) caused by Pss (CitationHirano & Upper, 2000).
We previously found that SylA inhibits cancer cell proliferation (CitationColeman et al., 2006) and, as a result of a multi-national collaboration, we discovered that SylA inhibits the proteasome (CitationArcher et al., 2010; CitationGroll et al., 2008). Importantly, this study provided the first biological evidence that SylA is a virulence factor that by means of proteasome inhibition weakens the host as part of the pathogen’s invasion strategy. Glidobactins (CitationOka et al., 1988a,b,c,d) and cepafungins (CitationShoji et al., 1990; CitationTerui et al., 1990) are structurally related molecules, and together, they form a new family of 12-membered lactam proteasome inhibitors, the syrbactins (). The total synthesis of syringolin A and syringolin B has been reported by three independent groups (CitationClerc et al., 2009b, Citation2010; CitationDai & Stephenson 2010; CitationPirrung et al., 2010) and the syrbactins were evaluated in biological systems (CitationArcher et al., 2010; CitationClerc et al., 2009a,Citationb, Citation2010, Citation2011; CitationColeman et al., 2006; CitationGroll et al., 2008).
Figure 1. The chemical structures of (A) syringolin A (SylA), (B) syringolin B (SylB), (C) glidobactin A (GlbA), and (D) syringolin B analogue TIR-203.
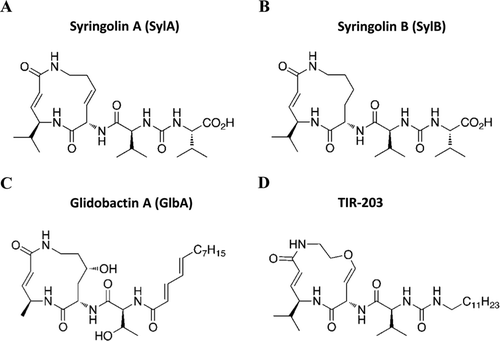
Most recently, the structural analogue TIR-203 was synthesized by the UC Riverside group, inspired by the structural properties of syringolin B (CitationIbarra-Rivera et al., 2011). Syringolin B is a favored platform for the design of structural variants because a large portion of its structure is based on lysine, analogues of which can be readily accessed. In this paper, we have analyzed the biological activity of TIR-203 in human multiple myeloma (MM) and neuroblastoma (NB) cells and measured the compounds ability to inhibit the proteolytic activity of the three catalytic subunits of the proteasome.
Materials and methods
Synthesis of TIR-203
Syringolin B (SylB) was used as a structural template and a SylB analogue was chemically synthesized and named TIR-203 (CitationIbarra-Rivera et al., 2011). The design reflects structural elements from the two strongest syrbactin proteasome inhibitors – the more hydrophobic side chain of glidobactin A (GlbA) and the two unsaturations within the macrolactam ring of SylA. SylA was used as a control for activity comparison and synthesized as previously described (CitationClerc et al., 2009b). TIR-203 is stereochemically homogeneous, as dictated by the synthetic route and shown by the spectral data, primarily NMR. The purity is greater than 95% based on chromatographic purification. Bortezomib (Velcade®) was purchased from LC Laboratories (Woburn, MA, USA). All stock solutions were prepared at 10 mM in H2O (SylA) or DMSO (TIR-203 and bortezomib), sterile-filtered, and stored frozen at −20°C. At the beginning of each experiment, aliquots were thawed and diluted to the final concentration.
Mammalian cell cultures and reagents
The MM cell line MM.1R derived from the parent cell line MM.1, which was established from peripheral blood of a MM patient who had become resistant to steroid-based therapy. MM.1R is resistant to dexamethasone and was kindly provided by N. Krett (Northwestern University) (CitationGreenstein et al., 2003). The human NB cell line MYCN2 (CitationSlack et al., 2005) was derived from line SH-EP and was kindly provided by Dr. J. Shohet (Texas Children’s Hospital). Cell lines were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (MM.1R) or Dulbecco’s Modified Eagle Medium (DMEM) (MYCN2) from Biosource (Rockeville, MD, USA) containing 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA) and cells were supplemented with penicillin (100 U/mL) and streptomycin (100 µg/mL). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 and seeded 24 h before drug treatments.
Cell viability assay
The CellTiter 96 Aqueous One solution Cell Proliferation Assay [2-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] (Promega, San Luis Obispo, CA, USA) was performed as previously described (CitationArcher et al., 2010) and used to determine the viability of cancer cells after 24 h treatment with TIR-203 at indicated concentrations (0–10 µM) by measuring the absorbance at 490 nm using the Multi-Mode Synergy™ MX Microplate Reader (BioTek, Inc., Winooski, VT, USA) and data expressed in percent (%) cell survival relative to control (untreated) cells. SylA and bortezomib were used as controls for comparison.
Proteasome inhibition assay
The cell culture-based proteasome-Glo inhibition assay was performed as previously described (CitationArcher et al., 2010). Solid white 96-well microtiter cell culture plates were seeded with cells as indicated and treated with TIR-203. Proteasome inhibition was measured using the proteasome Glo™ reagent according to the manufacturer’s instructions (Promega). In brief, MM.1R or MYCN2 cells were treated at different concentrations [0–10 µM] as indicated and incubated for 2 h, followed by incubation for 15 min with 100 µL of proteasome-Glo reagent, containing the bioluminescent substrates Suc-LLVY-aminoluciferin, Z-nLPnLD-aminoluciferin, and Z-LRR-aminoluciferin to measure the chymotrypsin-like (CT-L), caspase-like (C-L), and trypsin-like (T-L) activities, respectively. Luminescence was measured with a Multi-Mode Synergy™ MX Microplate Reader (BioTek, Inc.) and expressed as relative light units (RLU). SylA and bortezomib were used as controls for comparison.
Statistical analyses
All experiments were performed in three independent experiments (n = 3). Error bars indicate standard deviations (±SD). Data were prepared using the GraphPad Prism software from GraphPad Software, Inc. (La Jolla, CA, USA).
Results and discussion
The proteasome inhibitor, bortezomib, was first approved by the US Food and Drug Administration (FDA) for the treatment of MM and mantle cell lymphoma. Because proteasome inhibitors have been proven effective in MM and increase the chemosensitivity of cells, we tested the anti-proliferative effect of analogue TIR-203 in a MM cell culture system using MM.1R cells. This cell line was isolated from a patient that had become resistant to steroid-based (dexamethasone) therapy. As shown in , TIR-203 reduced the viability of MM.1R cells in a dose-dependent manner and was cytotoxic to cells at concentrations ≥0.1 µM. At ≥0.5 µM, TIR-203 exhibited similar cell inhibitory effects as SylA at 20 µM or bortezomib at 0.01 µM. This result reveals that the incorporated modifications of analogue TIR-203 have significantly increased its anti-proliferative activity against MM cells.
Figure 2. Syringolin B-analogue TIR-203 inhibits the proliferation of human multiple myeloma (MM) cells and human neuroblastoma (NB) cells. (A) MM.1R cells and (B) MYCN2 cells were treated over a period of 24 h with natural product derived syringolin B-analogue TIR-203 at various concentrations (0–10 µM). SylA and bortezomib (Btz) were used as controls for comparison. The viability of cells is expressed as percent (%) cell survival relative to untreated control cells and was determined by MTS assay as outlined in the material and methods.
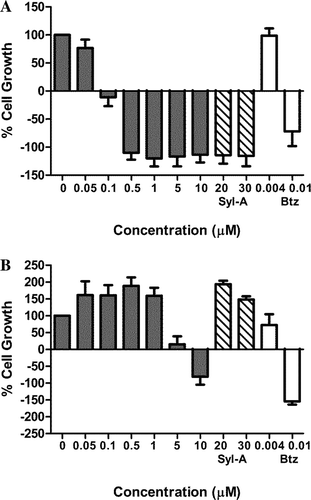
Similarly, we were curious to see if TIR-203 inhibits human NB cells. NB is a pediatric cancer of the sympathetic nervous system that our laboratory investigates intensively in order to identify novel drug therapeutics for children with NB. TIR-203 also reduced the viability of MYCN2 cells in a dose-dependent manner, but higher concentrations were required to achieve similar effects and cytotoxic effects were observed at >0.5 µM (). However, in comparison to SylA which had no effect in MYCN2 cells at 20–30 µM, TIR-203 was effective between 5–10 µM.
To further characterize the properties of analogue TIR-203, we employed a cell-based assay that allows the measurement of the proteolytic activity of the proteasome by assessing the three individual catalytic subunit activities, namely the chymotrypsin-like (CT-L), the trypsin-like (T-L), and the caspase-like (C-L) activities. As shown in , the CT-L activities in both MM1.R and MYCN2 cells were inhibited in a dose-dependent manner and a significantly lower concentration of TIR-203 (~1 µM for MM1.R and ~2.5 µM for MYCN2) was required to inhibit the CT-L proteolytic activity compared to SylA (20 µM).
Figure 3. Inhibitory effects of syringolin B-analogue TIR-203 on proteasomal activity in multiple myeloma (MM) cells and neuroblastoma (NB) cells. The (A) chymotrypsin-like (CT-L), (B) caspase-like (C-L), and the trypsin-like (T-L) proteolytic activities of MM.1R and MYCN2 cells were measured. Cells were treated over a period of 2 h with natural product derived syringolin B-analogue TIR-203 at various concentrations (0–10 µM). SylA and bortezomib (Btz) were used as controls for comparison. The proteasomal activities were measured as outlined in the material and methods section.
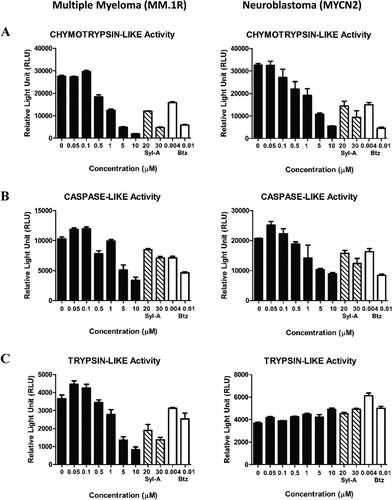
A similar pattern was observed for both cell lines when measuring the C-L activities (), although slightly higher concentrations for TIR-203 were required to achieve comparable inhibitory effects, and this was also true for both controls (SylA and bortezomib).
TIR-203 also inhibited the T-L activity in MM cells, but no effects were noted in NB cells (). Interestingly, also SylA and bortezomib did not have any inhibitory effects in this particular NB cell type. It has previously been reported that the T-L activity is not, or to a much lesser degree inhibited by bortezomib. The fact that TIR-203 inhibits the T-L activity of MM cells, but not NB cells, is interesting and will be further explored in future experiments.
In summary, TIR-203 inhibited cell proliferation in a dose-dependent manner and was most effective against the CT-L activity, which is in agreement with previous reports for SylA (CitationGroll et al., 2008) and bortezomib (CitationAdams, 2004; CitationOrlowski & Kuhn, 2008; CitationVoorhees et al., 2003).
Conclusions
The inhibitory action of drugs often varies and strongly depends on the cell type and the individual cell lines under investigation. Based on our investigation, the newly synthesized analogue TIR-203 is clearly superior to SylA and inhibits favorably the proliferation and the CT-L activity of MM and NB cells. Bortezomib is FDA-approved for the treatment of MM and mantle cell lymphoma and currently clinically evaluated in many other types of cancer, including neuroblastoma (CitationBachmann, 2008). While it is clear that this drug class has a promising future, novel proteasome inhibitor variants need to be developed in order to increase the drug portfolio available, especially in light of possible occurrences in drug resistance (CitationOerlemans et al., 2008; CitationRi et al., 2010) and to combat the multitude of different forms of cancer and potentially other diseases.
Acknowledgments
We are grateful to Prof. Dr. Markus Kaiser (University of Duisburg-Essen, Essen, Germany) for providing SylA as well as Prof. Dr. Nancy L. Krett (Northwestern University, Chicago, IL, USA) and Prof. Dr. Jason Shohet (Texas Children’s Hospital, Houston, TX, USA) for providing multiple myeloma cell line MM.1R and neuroblastoma cell line MYCN2, respectively. Prof. Dr. Dana-Lynn Koomoa (University of Hawaii College of Pharmacy, Hilo, HI, USA) is thanked for her expert technical advice.
Declaration of interest
This work was supported by the Robert C. Perry Fund of the Hawaii Community Foundation (HCF grant # 10ADVC-47862), the Hawaii Business and Acceleration Mentors (HiBEAM) and internal funds from the College of Pharmacy (Prof. Dr. André Bachmann). Further support was provided by the University of California Cancer Research Coordinating Committee (Prof. Dr. Michael Pirrung) and postdoctoral fellowships from UC-MEXUS and CONACYT (Dr. Tannya Ibarra-Rivera).
References
- Adams J. (2004). The proteasome: A suitable antineoplastic target. Nat Rev Cancer, 4, 349–360.
- Archer CR, Koomoa DL, Mitsunaga EM, Clerc J, Shimizu M, Kaiser M, Schellenberg B, Dudler R, Bachmann AS. (2010). Syrbactin class proteasome inhibitor-induced apoptosis and autophagy occurs in association with p53 accumulation and Akt/PKB activation in neuroblastoma. Biochem Pharmacol, 80, 170–178.
- Bachmann AS. (2008). Proteasome inhibitors in pediatric cancer treatment. Hawaii Med J, 67, 247–249.
- Clerc J, Florea BI, Kraus M, Groll M, Huber R, Bachmann AS, Dudler R, Driessen C, Overkleeft HS, Kaiser M. (2009a). Syringolin A selectively labels the 20 S proteasome in murine EL4 and wild-type and bortezomib-adapted leukaemic cell lines. Chembiochem, 10, 2638–2643.
- Clerc J, Groll M, Illich DJ, Bachmann AS, Huber R, Schellenberg B, Dudler R, Kaiser M. (2009b). Synthetic and structural studies on syringolin A and B reveal critical determinants of selectivity and potency of proteasome inhibition. Proc Natl Acad Sci USA, 106, 6507–6512.
- Clerc J, Schellenberg B, Groll M, Bachmann AS, Huber R, Dudler R, Kaiser M. (2010). Convergent synthesis and biological evaluation of syringolin A and derivatives as eukaryotic 20S proteasome inhibitors. Eur J Org Chem, 21, 3991–4003.
- Clerc J, Li N, Krahn D, Groll M, Bachmann AS, Florea BI, Overkleeft HS, Kaiser M. (2011). The natural product hybrid of syringolin A and glidobactin A synergizes proteasome inhibition potency with subsite selectivity. Chem Commun (Camb), 47, 385–387.
- Coleman CS, Rocetes JP, Park DJ, Wallick CJ, Warn-Cramer BJ, Michel K, Dudler R, Bachmann AS. (2006). Syringolin A, a new plant elicitor from the phytopathogenic bacterium Pseudomonas syringae pv. syringae, inhibits the proliferation of neuroblastoma and ovarian cancer cells and induces apoptosis. Cell Prolif, 39, 599–609.
- Dai C, Stephenson CR. (2010). Total synthesis of syringolin A. Org Lett, 12, 3453–3455.
- Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T, Anderson KC, Rosen ST. (2003). Characterization of the MM.1 human multiple myeloma (MM) cell lines: A model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol, 31, 271–282.
- Groll M, Schellenberg B, Bachmann AS, Archer CR, Huber R, Powell TK, Lindow S, Kaiser M, Dudler R. (2008). A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature, 452, 755–758.
- Hirano SS, Upper CD. (2000). Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae-a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev, 64, 624–653.
- Ibarra-Rivera TR, Opoku-Ansah J, Ambadi S, Bachmann AS, Pirrung MC. (2011). Syntheses and cytotoxicity of syringolin B-based proteasome inhibitors. Tetrahedron. (In Press).
- Oerlemans R, Franke NE, Assaraf YG, Cloos J, van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova K, Lemos C, van der Heijden JW, Ylstra B, Peters GJ, Kaspers GL, Dijkmans BA, Scheper RJ, Jansen G. (2008). Molecular basis of bortezomib resistance: Proteasome subunit β5 (PSMB5) gene mutation and overexpression of PSMB5 protein. Blood, 112, 2489–2499.
- Oka M, Nishiyama Y, Ohta S, Kamei H, Konishi M, Miyaki T, Oki T, Kawaguchi H. (1988a). Glidobactins A, B and C, new antitumor antibiotics. I. Production, isolation, chemical properties and biological activity. J Antibiot, 41, 1331–1337.
- Oka M, Numata K, Nishiyama Y, Kamei H, Konishi M, Oki T, Kawaguchi H. (1988b). Chemical modification of the antitumor antibiotic glidobactin. J Antibiot, 41, 1812–1822.
- Oka M, Ohkuma H, Kamei H, Konishi M, Oki T, Kawaguchi H. (1988c). Glidobactins D, E, F, G and H; minor components of the antitumor antibiotic glidobactin. J Antibiot, 41, 1906–1909.
- Oka M, Yaginuma K, Numata K, Konishi M, Oki T, Kawaguchi H. (1988d). Glidobactins A, B and C, new antitumor antibiotics. II. Structure elucidation. J Antibiot, 41, 1338–1350.
- Orlowski RZ, Kuhn DJ. (2008). Proteasome inhibitors in cancer therapy: Lessons from the first decade. Clin Cancer Res, 14, 1649–1657.
- Pirrung MC, Biswas G, Ibarra-Rivera TR. (2010). Total synthesis of syringolin A and B. Org Lett, 12, 2402–2405.
- Ri M, Iida S, Nakashima T, Miyazaki H, Mori F, Ito A, Inagaki A, Kusumoto S, Ishida T, Komatsu H, Shiotsu Y, Ueda R. (2010). Bortezomib-resistant myeloma cell lines: A role for mutated PSMB5 in preventing the accumulation of unfolded proteins and fatal ER stress. Leukemia, 24, 1506–1512.
- Shoji J, Hinoo H, Kato T, Hattori T, Hirooka K, Tawara K, Shiratori O, Terui Y. (1990). Isolation of cepafungins I, II and III from Pseudomonas species. J Antibiot, 43, 783–787.
- Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, Shohet JM. (2005). The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proc Natl Acad Sci USA, 102, 731–736.
- Terui Y, Nishikawa J, Hinoo H, Kato T, Shoji J. (1990). Structures of cepafungins I, II and III. J Antibiot, 43, 788–795.
- Voorhees PM, Dees EC, O’Neil B, Orlowski RZ. (2003). The proteasome as a target for cancer therapy. Clin Cancer Res, 9, 6316–6325.