Abstract
Context: The leaves of Spondias tuberosa Arr. Cam. (Anacardiaceae) and Spondias mombin L. have been traditionally used for medicinal purposes. Some studies reveal their antibacterial, antimicrobial, and antiviral properties.
Objective: Determine the chemical composition, antioxidant, and antimicrobial activities of Spondias species to justify its ethnopharmacological use.
Materials and methods: Spondias species extracts were prepared with methanol:water 80:20 and analyzed by silica gel column chromatography and reversed phase liquid chromatography (HPLC). The antioxidant activity was evaluated by scavenging the radicals 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) and 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS•+) and measuring antimicrobial activity (agar well diffusion method, minimum inhibitory concentration and minimum bactericidal concentrations).
Results: The HPLC analysis of Spondias extracts demonstrated the occurrence of high yield of flavonoids. Found in S. mombin were quercetin (2.36 ± 0.01 mg/g) and ellagic acid (41.56 ± 0.01 mg/g) and in S. tuberosa species rutin (53.38 ± 1.71 mg/g), quercetin (24.46 ± 0.87 mg/g), and ellagic acid (169.76 ± 0.17 mg/g). The antibacterial activity of the extracts against the various bacteria strains varied from 8.8 to 20.1 mm. MIC values from 62.5 to 125 µg/mL were satisfactory when compared with other plant products. Medium DPPH scavenging activity IC50 for Spondias extracts varied from 0.042 to 0.558 mg/mL and for ABTS from 0.089 to 0.465 mg/mL. DPPH scavenging activity for constituent ellagic acid IC50 = 0.042 mg/mL and for quercetin IC50 = 0.081 mg/mL.
Discussion and conclusion: The chemical study of Spondias leaf extracts showed the occurrence of quercetin, rutin and ellagic acid, substances with relevant antioxidant and antimicrobial activities.
Introduction
The genus Spondias (Anacardiaceae) consists of about 8–12 species distributed across tropical regions in the world (CitationNarain et al., 2004), including the species Spondias mombin L., known as cajazeira, and Spondias tuberosa Arr. Cam., known as umbuzeiro. S. mombin is native to the Amazon region in Peru, Brazil, Venezuela, Bolivia, Colombia, the three Guianas, as well as the southern Mexico, Belize, Costa Rica, and the west Indies (CitationAdedokun et al., 2010) and S. tuberosa is a tropical plant that plays an important role in the Caatinga biome of Northeastern Brazil, since it blooms even in dry period to furnish edible fruits, largely appreciated by the population (CitationBraga, 2001). In folk medicine, the S. mombin leaf tea is used for wound healing, demonstrating anti-inflammatory action (CitationVillegas et al., 1997), against acute diarrhea (CitationGonçalves et al., 2005), in the treatment of diabetes mellitus (CitationFred-Jaiyesimi et al., 2009) and anemia (CitationMorais et al., 2005). The leaves and branches of S. mombin contain ellagitannins (geraniin and galoilgeraniin) with antiviral activity against Coxsachie B2 and Herpes simplex type 1 (CitationCorthout et al., 1991, Citation1992), as well as antimicrobial phenolic acids (CitationCorthout et al., 1994). The cattle appreciate the acid taste of S. tuberosa leaves, which are popularly used as an anti-inflammatory, to combat diarrhea, dysentery and worms (CitationMatos, 1999; CitationBraga, 2001). The wound healing and anti-inflammatory properties of these Spondias species could be correlated with the antimicrobial and radical scavenging activity of their extracts and constituents.
The reactive oxygen species (ROS) are deleterious to the wound healing process due to the harmful effects on cells and tissues. Repair of injured tissues occurs as a sequence of events, which includes inflammation, proliferation, and migration of different cell types. Topical applications of compounds with free-radical-scavenging properties in patients have shown to significantly improve wound healing and protect tissues from oxidative damage (CitationUmachigi et al., 2007). For example, polar extracts of several plants used for wound healing in Oman were examined for antioxidant and antimicrobial properties and the most promising were those which combine both actions (CitationMarwah et al., 2007).
Phenolic compounds such as flavonoids, tannins, and anthocyanins are considered important antioxidants and antimicrobial agents (CitationNascimento et al., 2000; CitationElzaawely et al., 2005). In this sense, the objectives of this study were to evaluate the antioxidant and antibacterial activities of extracts from two Spondias species native to Northeastern Brazil to validate their popular use, and analyze the chemical composition to investigate possible active principles.
Material and methods
Reagents
DPPH (1,1-diphenyl-2-picrylhydrazyl), ABTS+• [2,2-azino-bis-(3-ethylbenzothiazolin-6-sulfonic acid)], BHT, ellagic acid, quercetin and rutin were purchased from Sigma Chemical Co. Other chemicals such as dimethylsulphoxide (DMSO) and solvents were obtained from Vetec (Brazil). All solvents used for extraction were of analytical grade.
Plant material
Plant leaves were collected in May 2008 from their natural habitats in the state of Ceará (Northeastern Brazil). Voucher specimens were deposited in the Prisco Bezerra Herbarium at the Department of Biology, Federal University of Ceará (UFC), and identified by botanist Ligia Queiroz Matias, with numbers 34,826 for S. mombin and 34,887 for S. tuberosa.
Preparation of extracts
Leaves of S. mombin (3.59 kg) and S. tuberosa (3.23 kg) were macerated in methanol:water (80:20) at room temperature (25–28°C) for 7 days. The solution was filtered through a paper filter and evaporated in a rotary evaporator. The extract was concentrated in a water bath with the temperature held below 60°C. S. tuberosa and S. mombin extract yields were 1.5 and 5.0%, respectively.
Chemical analysis of main secondary metabolite classes
Chemical tests were performed using specific reagents, observing color changes or precipitate formation, following the protocols described by CitationMatos (1997).
Phytochemical analysis of plant extracts
The methanol:water (80:20) extract was subjected to silica gel column chromatography and eluted with hexane, chloroform, and ethyl acetate in mixtures of increasing polarity. Several 10 mL fractions were collected and, after solvent evaporation, the resultant material was compared by silica gel thin layer chromatography (TLC) plates. Similar samples were joined, purified by recrystallization and analyzed by infrared (IR) and nuclear magnetic resonance (NMR). TLC also compared the main purified compounds with standard compounds. IR spectra were recorded on a PerkinElmer FTIR spectrum 100 Spectrometer and the values are expressed in cm−1. NMR spectra were recorded on a Brucker Avance DRX-500 spectrometer in MeOD.
HPLC quantification of main compounds from extracts
General
HPLC analysis was performed on a Shimadzu LC-10AD pump system equipped with a Shimadzu SPD-M10A photodiode array detector with the detection wavelength set at 350 nm. A fingerprint of main compounds contained in the methanol:water (80:20) extract of S. mombin and S. tuberosa was obtained.
Sample preparation
Quantitative analysis of rutin and quercetin in the Spondias species was carried out by HPLC analysis. The concentrated extracts of S. mombin (60 mg) and S. tuberosa (30 mg) were diluted with methanol to 10 mL. The afforded solutions were filtered through a 0.45 μm syringe filter prior to HPLC use.
Chromatographic system
HPLC analysis was performed using a reversed-phase column (Hibar Lichrosopher 100RP18 4.6 mm × 25 cm − particle 5 mm) eluted at a rate of 1.25 mL/min with a solvent mixture I:II (I: aqueous solution of H3PO4 (pH 2.8); II: acetonitrile) starting with 20% II in I until 12 min, then installing a gradient to reach 40% II at 17 min, maintaining this concentration until 23 min, following with a gradient to reach 80% II at 25 min, with a detection wavelength set at 350 nm and 20 mL injection. A rutin standard was used to obtain calibration curves. This compound was dissolved in methanol in different concentrations (1.14, 0.57, 0.228, 0.114 and 0.0456 mg/mL). Ellagic acid (standard) is also used to obtain calibration curves and it was dissolved in methanol for obtaining different concentrations (0.46, 0.92 and 1.84 mg/mL). The follow-up extractions and HPLC analysis were accomplished with the same procedure as for rutin. The recovery was determined as follows: recovery (%) = (A − B)/C × 100% where, A is the amount of detections, B is the amount of sample without added standard, C is the added amount of the standard. The relative standard deviations (RSD) of recoveries of the ellagic acid was 2.1 (n = 5; mean = 98). The identification of rutin, ellagic acid and quercetin () in Spondias extracts were evaluated by HPLC-PDA, observing the retention time (Rt) and UV-VIS spectra. The standards were prepared in methanol in a concentration of 0.1 mg/mL.
Determination of anti-free radical activity of Spondias extracts and constituents
Scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH): The capacity to scavenge the DPPH free radical, standard techniques, was monitored according to a method reported by CitationYepez et al. (2002). The diluted extract (0.1 mL) was mixed with 3.9 mL of methanol solution containing DPPH radicals (6.5 × 10−5 mol/L) for 1 h. The reduction of the DPPH radical was measured by continuously monitoring the decrease in absorption at 515 nm. The IC50 (Medium effective concentration) was calculated.
Scavenging activity against 2,2-azinobis-(3-ethyl-benzothiazolin-6-sulfonic acid (ABTS+•): The ABTS is standard technique. The ABTS+• solution (7 mM, 5 mL) was mixed with 88 µL of potassium persulfate (140 mM). The mixture was shaken and kept in the dark at room temperature for 16 h. Then, 1 mL of this solution was added to 99 mL ethanol. The absorbance is read at 734 nm and should be approximately 0.715. Several solutions of decreasing concentrations of plant extracts were prepared and 3.0 mL of ABTS+• solution was added to 30 µL of these solutions after 6 min, readings were taken at 734 nm (CitationRe et al., 1999). The IC50 (medium effective concentration) was calculated.
Antimicrobial activity
Microbial strains
The in vitro antimicrobial activity of S. mombin and S. tuberosa extracts was evaluated using isolation strains obtained from Walter Cantidio University Hospital, Ceará, Brazil and supplied by the Laboratory of Microbiology of the UFC: Gram negative bacteria Serratia marcescens, Serratia liquefaciens, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii and Enterobacter cloacae. Cultures of these bacteria were grown in brain heart infusion (BHI) broth at 37°C and maintained in Mueller-Hinton agar (MHA) at 4°C.
Antibacterial screening
Two different methods were employed for determining antibacterial activities: agar-well diffusion method (CitationCeliktas et al., 2007) and minimum inhibitory concentrations (MIC) with protocol established by the CitationCLSI (2009). Also was determined the minimum bactericidal concentrations (MBCs). All tests were performed in triplicate. All extracts were weighed and dissolved in dimethylsulphoxide (125 mg/mL), followed by sterilization using a 0.45 µm membrane filter. Each microorganism was taken in sterile saline at a density adjusted to a 0.5 McFarland turbidity standard [108 colony-forming units (CFU)/mL].
Agar-well diffusion assay
Twenty mL nutrient agar plates were inverted and dried at 37°C for 30 min. An overnight culture of bacteria (0.5 mL) was spread over the surface of the agar plate using a sterile glass spreader. Inoculated plates were inverted and incubated at 37°C for a further 30 min. Wells (6 mm diameter) were made in the center of each agar plate into which 0.2 mL of each extract was added. The agar plates were incubated for 24 h at 37°C and the diameter (in mm) of zones of bacterial inhibition (ID) were recorded using a caliper rule. All tests were performed in triplicate.
Broth dilution assay: determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC)
The minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of Spondias extract against bacterial strains was measured by broth dilution method using Mueller Hinton Broth. An overnight broth culture of each strain was prepared and the final concentration in each well was adjusted to 108 CFU/mL. The 96-well plates were prepared with 95 µL of Müeller-Hinton (MH), and 5 µL of the inoculum into each well. Hundred µL from Spondias extracts initially prepared at the concentration of 500 µg/mL was added into the first wells. Then, 100 µL from their serial dilutions was transferred into 10 consecutive wells. The last well containing 195 µL of nutrient without compound and 5 µL of the inoculum on each strip was used as a negative control. The final volume in each well was 200 µL. Ciprofloxacin (CIP) at a concentration range of 500–7.8 µg/mL was prepared in a nutrient medium and used as a standard drug. Contents of each well were mixed on a plate shaker for 20 min and then incubated at appropriate temperatures for 24 h. Microbial growth was determined by absorbance at 620 nm using an ELISA instrument (CitationOzturk & Ercisli, 2006) and confirmed by plating 5 µL samples from clear wells on nutrient agar medium to determine as minimal bactericidal concentration (MBC).
Statistical analysis
All the experiments on the antioxidant effect were calculated as means ± standard deviation (SD). The one way analysis of variance (ANOVA) test was used to determine the statistical differences followed by Tukey’s Multiple Comparison in GraphPad Prism 3.0. The significance level was set at p < 0.05.
Results and discussion
Chemical study of S. mombin and S. tuberosa leaves: S. tuberosa and S. mombin extract yields were 1.5 and 5.0%, respectively. Both extracts contain phenols, hydrolysable tannins, flavones, flavonoids, leucoanthocyanidins and saponins. These results are in accordance with CitationCorthout et al. (1992) who demonstrated the presence of hydrolysable tannins in S. mombin. CitationSatpathy et al. (2011) demonstrated the presence of alkaloids, tannins and saponins in Spondias pinnata K. The presence of flavonoids and tannins in Spondias species led us to investigate their chemical structures by HPLC-PDA, comparing retention time (Rt) and UV-VIS spectra. The identity of constituents was established by overlapping their peaks (retention time) and absorption spectra with those of rutin (1), ellagic acid (2) and quercetin (3) standards. Fingerprint chromatograms of S. tuberosa and S. mombin leaf extracts are shown in . Retention times of rutin, ellagic acid and quercetin were 5.11, 6.24 and 23.46 min, respectively. The occurrence of these compounds in S. mombin and S. tuberosa species was not previously described.
Figure 2. HPLC Chromatograms of the methanol:water extracts of S. tuberosa (A) and S. mombin (B). Compounds 1. rutin, 2. ellagic acid, 3. quercetin.
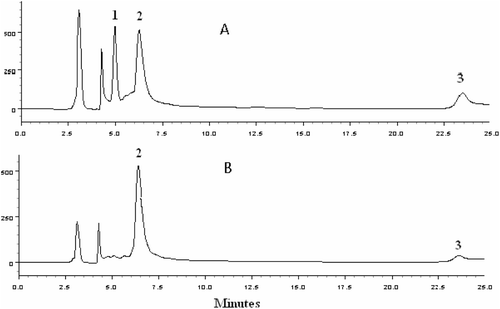
The regression equation for rutin is Y = 25,150,017.88X + 203,139.00 (R2 = 1.000); limit of quantification 0.1 μg/mL; limit of detection 0.04 μg/mL; relative standard deviations (RSD) less than 2%. The regression equation for ellagic acid is: Y = 25,916,407.45X + 309,482.00 (R2 = 0.9998); limit of quantification 0.1 μg/mL; limit of detection 0.04 μg/mL; relative standard deviations less than 2%. Each flavonoid peak was quantified using the rutin and ellagic acid linear regression equations. In these Spondias species, rutin, ellagic acid and quercetin were detected in different yields (). It was found in S. mombin, quercetin (2.36 ± 0.01 mg/g) and ellagic acid (41.56 ± 0.01 mg/g) and in S. tuberosa species rutin (53.38 ± 1.71 mg/g), quercetin (24.46 ± 0.87 mg/g) and ellagic acid (169.76 ± 0.17 mg/g). The differences in antioxidant and antimicrobial action of two species could be related to the concentration of active compounds in each plant.
Table 1. Content of flavonoids (quercetin and rutin) and ellagic acid in extracts of Spondias species from Northeastern Brazil determined by HPLC.
Phenolic acids and O-glycoside flavonols were extracted from the peels of jocote fruits (Spondias purpurea L.,) harvested in Costa Rica and characterized using ultra high-performance liquid chromatography coupled with diode array and electrospray ionization mass spectrometric detection (UHPLC–DAD–ESIMSn). The analytical system allowed the separation of 21 phenolic compounds. In addition to phenolic acids, several O-glycosides of quercetin, kaempferol, kaempferide and rhamnetin were detected (CitationEngels et al., 2011).
Antioxidant activity measured by the DPPH and ABTS+• radical scavenging system: DPPH is used as a free radical to evaluate antioxidant activity of plant extracts, and the degree of its discoloration is attributed to the hydrogen donating ability of these products, which is indicative of their scavenging potential (CitationShimada et al., 1992). shows the results of antiradical tests, where IC50 is defined as the concentration required to inhibit 50% of DPPH radical present in the solution. Both Spondias extracts exhibited high DPPH scavenging potential with IC50 = 0.417 ± 0.01 mg/mL for S. mombin and IC50 = 0.558 ± 0.008 mg/mL for S. tuberosa. When antioxidant activity was evaluated using the radical ABTS+• results were statistically equal, S. mombin 0.451 ± 0.029 and S. tuberosa 0.465 ± 0.029. CitationRufino et al. (2010) comment that it is not always a simple task to choose the most appropriate method to determine antioxidant capacity and the ABTS+• method is generally indicated for hydrophilic compounds. The DPPH method may be employed routinely with aqueous-organic extracts containing hydrophilic and lipophilic compounds. In the evaluation of antioxidant activities of aqueous, methanol and alkaloid extracts from Mitragyna speciosa leaves, the DPPH IC50 values were 0.213, 0.104 and 0.037 mg/mL, respectively (CitationParthasarathy et al., 2009). A study performed on other Spondias species indicate the presence of antioxidants but failed to provide information about the profile of phenolic compounds (CitationHazra et al., 2008). Antioxidant activity of different Spondias pinnata K. extracts, the DPPH scavenging activity expressed as IC50 ranged from 0.73 to 0.59 mg/mL (CitationSatpathy et al., 2011).
Table 2. Antioxidant capacities measured by DPPH and ABTS free radical scavenging activity expressed as IC50 (mg/mL ± SD) of methanol:water (80:20) extracts and constituents of Spondias species.
The values found of DPPH IC50 for Spondias species extracts are considered mild in relation to BHT (DPPH IC50 = 0.165 mg/mL). However the DPPH scavenging activity for constituent ellagic acid with IC50 = 0.042 mg/mL and for quercetin with IC50 = 0.081 mg/mL, show that both constituents of Spondias extracts are better antioxidants than BHT. Therefore the presence of compounds with good antioxidant activity should contribute to overall activity of extracts.
The present life style of individuals causes over production of free radicals and reactive oxygen species. Our body has a natural defense mechanism to cope with this oxidative stress but the problem arises when the free radicals outweigh the defense mechanism, damaging essential biomolecules such as proteins, DNA and lipids. Natural antioxidants play an important role in health care as they provide protection from oxidative stress and associated diseases (CitationLopez et al., 2007). Phenolic compounds widely distributed in medicinal plants, spices, vegetables, fruits, grains, and seeds are an important group of natural antioxidants with possible beneficial effects on human health. CitationTurkmen et al. (2005) demonstrated that extracts from phenolic rich plants present strong antioxidant activity. Quercetin and its glycoside rutin have shown in vitro antioxidant action (CitationSoares et al., 2005). Over the past years, both quercetin and rutin have gained tremendous interest because of their free radical scavenging activities (CitationTeresita et al., 2001), their protective effects on DNA damage (CitationUndeger et al., 2004), and their physiological effects such as anti-tumor (CitationRen et al., 2003) and anti-bacteria (CitationDall’Agnol et al., 2003) potential. Ellagic acid is a known polyphenol antioxidant (CitationHassoun et al., 1997, Citation2004; CitationHayes et al., 2010) found in numerous fruits and vegetables and has a variety of biological activities, including anticancer (CitationWhitley et al., 2003), antiproliferative (CitationSeeram et al., 2005) and antimutagen activities (CitationLoarca-Piña et al., 1998). The presence of these three phenolic compounds in Spondias species extracts guarantees their medicinal properties including antioxidant and antibacterial activities.
The antibacterial activities of S. mombin and S. tuberosa extracts were assayed in vitro by agar well diffusion method against eight Gram negative bacteria. summarizes the microbial growth inhibition of Spondias species extracts. According to results, the S. mombin extract was found to be active against three Gram negative bacteria strains and the S. tuberosa extract was found to be active against seven Gram negative bacteria strains.
Table 3. Antibacterial activities of methanol:water (80:20) extracts of Spondias species.
The MIC values were studied for the bacterial strains, being sensitive to the extracts in the agar diffusion method. The standard antibiotic was ciprofloxacin (CIP) which showed MIC values of 7.8 µg/mL. S. mombin extract presented MIC = 125 µg/mL for Serratia marcescens, Proteus mirabilis and Enterobacter cloacae. S. tuberosa extract presented MIC = 62.5 µg/mL against Serratia marcescens, Serratia liquefaciens, Proteus mirabilis and Enterobacter cloacae. Against Klebsiella pneumoniae, Pseudomonas aeruginosa and for Morganella morganii the MIC was 125 µg/mL (). CitationMeot-Duros et al. (2008) tested polar and apolar fractions of Crithmum maritimum L., Eryngium maritimum L. and Cakile maritima Scop leaves and found MIC values about 100 µg/mL. Antimicrobial activity is relevant in concentrations below 100 µg/mL for extracts and 10 µg/mL for isolated compounds (CitationRíos & Recio, 2005). CitationSatpathy et al. (2011) showed antimicrobial activity against Gram negative and Gram positive with MIC ranged between 1.8 and 2.9 mg/mL of Spondias pinnata extracts.
According to CitationNatarajan et al. (2005) the MIC method is the most suitable to evaluate antibacterial activity of natural products. Although the found activities of Spondias species were lower when compared to standard antibiotic, the increasing resistance of microorganisms to antibiotics stimulates the search for new drugs. CitationNikaido and Vaara (1985) point out that higher resistance of Gram negative bacteria to external agents has been earlier reported, and it is attributed to the presence of lipopolysaccharides in their outer membranes, which make them inherently resistant to antibiotics, detergent, and hydrophilic dyes. The antibacterial activity of quercetin and rutin was already demonstrated (CitationCushnie & Lamb, 2005) and probably contributes to Spondias species extracts antimicrobial action.
Conclusion
The chemical study of leaf extracts from Spondias species shows the occurrence of quercetin, rutin, and ellagic acid. The antioxidant and antimicrobial activities of extracts and constituents were evaluated showing relevant results. The constituents’ activities can be directly correlated to wound healing and anti-inflammatory properties of the plants then this study confirms the ethnopharmacological use of two Spondias species.
Declaration of interest
The authors thank Brazilian governmental agencies CAPES and FUNCAP (Ceará State Research Funding) for financial support.
References
- Adedokun MO, Oladoye AO, Oluwalana AS. (2010). Socio-economic importance and utilization of Spondias mombin in Nigéria. Asian Pacific J Trop Med, 3, 232–234.
- Braga R. (2001). Plantas do Nordeste, especialmente do Ceará. Fundação Guimarães Duque, Coleção Mossoroense, C, 1204.
- Celiktas OY, Hames KEE, Bedir E, Vardar F, Ozk T, Baser KHC. (2007). Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem, 100, 553–559.
- Clinical and Laboratory Standards Institute (CLSI) (2009). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard, eighth edition M07-A8. CLSI, Wayne, PA.
- Corthout J, Pieters L, Claeys M, Geerts S, Vanden Berghe D, Vlietinck A. (1991). Antiviral ellagitannins from Spondias mombin. Phytochemistry, 30, 1129–1130.
- Corthout J, Pieters L, Claeys M, Geerts S, Vanden Berghe D, Vlietinck A. (1994). Antibacterial and molluscicidal phenolic acids from Spondias mombin. Planta Med, 60, 460–463.
- Corthout J, Pieters L, Claeys M, Geerts S, Vanden Berghe D, Vlietinc A. (1992). Antiviral caffeoyl esters from Spondias mombin. Phytochemistry, 31, 1979–1981.
- Cushnie TP, Lamb AJ. (2005). Antimicrobial activity of flavonoids. Int J Antimicrob Agents, 26, 343–356.
- Dall’Agnol R, Ferraz A, Bernardi AP, Albring D, Nör C, Sarmento L, Lamb L, Hass M, von Poser G, Schapoval EE. (2003). Antimicrobial activity of some Hypericum species. Phytomedicine, 10, 511–516.
- Elzaawely AA, Xuan TD, Tawata S. (2005). Antioxidant and antibacterial activities of Rumex japonicus Houtt. Aerial parts. Biol Pharm Bull, 28, 2225–2230.
- Engels C, Gräter D, Esquivel P, Jiménez VM, Ganzle MG, Schieber A. (2011). Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res Inter (April 2011) doi:10.1016/j.foodres.2011.04.003 Key: citeulike:9197990.
- Fred-Jaiyesimi A, Kio A, Richard W. (2009). α-Amylase inhibitory effect of 3β-olean-12-en-3-yl (9Z)-hexadec-9-enoate isolated from Spondias mombin leaf. Food Chem, 116, 285–288.
- Gonçalves JL, Lopes RC, Oliveira DB, Costa SS, Miranda MM, Romanos MT, Santos NS, Wigg MD. (2005). In vitro anti-rotavirus activity of some medicinal plants used in Brazil against diarrhea. J Ethnopharmacol, 99, 403–407.
- Hassoun EA, Vodhanel J, Abushaban A. (2004). The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J Biochem Mol Toxicol, 18, 196–203.
- Hassoun EA, Walter AC, Alsharif NZ, Stohs SJ. (1997). Modulation of TCDD-induced fetotoxicity and oxidative stress in embryonic and placental tissues of C57BL/6J mice by vitamin E succinate and ellagic acid. Toxicology, 124, 27–37.
- Hayes JE, Stepanyan V, Allen P, O’Grady MN, Kerry JP. (2010). Effect of lutein, sesamol, ellagic acid and olive leaf extract on the quality and shelf-life stability of packaged raw minced beef patties. Meat Sci, 84, 613–620.
- Hazra B, Biswas S, Mandal N. (2008). Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med, 8, 63.
- Loarca-Piña G, Kuzmicky PA, de Mejía EG, Kado NY. (1998). Inhibitory effects of ellagic acid on the direct-acting mutagenicity of aflatoxin B1 in the Salmonella microsuspension assay. Mutat Res, 398, 183–187.
- López V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI. (2007). In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum Nutr, 62, 151–155.
- Marwah RG, Fatope MO, Mahrooqi RA, Varma GB, Abadi HA, Khamis S, Al-Burtamani SKS. (2007). Antioxidant capacity of some edible and wound healing plants in Oman. Food Chem, 101, 465–470.
- Matos FJA. (1999). Plantas da Medicina Popular do Nordeste. Edições UFC, Fortaleza.
- Matos FJA. (1997). Introdução à Fitoquímica Experimental, 2nd ed.,Edições UFC, Fortaleza.
- Meot-Duros L, Le Floch G, Magné C. (2008). Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J Ethnopharmacol, 116, 258–262.
- Morais SM, Dantas JDP, Silva ARA, Magalhães EF. (2005). Plantas medicinais usadas pelos índios Tapebas do Ceará. Rev Bras de Farmacognosia, 15, 169–77.
- Narain N, Almeida JN, Galvão MS, Madruga MS, Brito ES. (2004). Compostos voláteis dos frutos de maracujá (Passiflora edulis forma flavicarpa) e de cajá (Spondias mombin L.) obtidos pela técnica de Headspace dinânico. Ciência Tec Alimentos, 24, 212–16.
- Nascimento GGF, Locatelli J, Freitas PC, Silva GL. (2000). Antibacterial activity of plants extracts and phytochemicals on antibiotic resistant bacteria. Braz J Microbiol, 31, 247–56.
- Natarajan D, Britto SJ, Srinivasan K, Nagamurugan N, Mohanasundari C, Perumal G. (2005). Anti-bacterial activity of Euphorbia fusiformis – a rare medicinal herb. J Ethnopharmacol, 102, 123–126.
- Nikaido H, Vaara M. (1985). Molecular basis of bacterial outer membrane permeability. Microbiol Rev, 49, 1–32.
- Ozturk S, Ercisli S. (2006). The chemical composition of essential oil and in vitro antibacterial activities of essential oil and methanol extract of Ziziphora persica Bunge. J Ethnopharmacol, 106, 372–376.
- Parthasarathy S, Bin Azizi J, Ramanathan S, Ismail S, Sasidharan S, Said MI, Mansor SM. (2009). Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (Rubiaceae family) leaves. Molecules, 14, 3964–3974.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med, 26, 1231–1237.
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. (2003). Flavonoids: Promising anticancer agents. Med Res Rev, 23, 519–534.
- Ríos JL, Recio MC. (2005). Medicinal plants and antimicrobial activity. J Ethnopharmacol, 100, 80–84.
- Rufino MSM, Alves RE, Brito E, Pérez-Jiménez J, Saura-Calixto F, Mancini-Filho J. (2010). Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem, 121, 996–1002.
- Satpathy G, Tyagi YK, Gupta RK. (2011). Preliminary evaluation of nutraceutical and therapeutic potential of raw Spondias pinnata K., an exotic fruit of India. Food Res Inter, 44, 2076–2087.
- Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, Heber D. (2005). In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem, 16, 360–367.
- Shimada K, Fujikama K, Yahara R, Nakamura T. (1992). Antioxidant properties of xanthan on autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem, 40, 945–948.
- Soares DG, Andreazza AC, Salvador M. (2005). Avaliação de compostos com atividade antioxidante em células da levedura Saccharomyces cerevisiae. Braz J Pharm Sci, 41, 95–100.
- Teresita G, Ester RA, Osvaldo JA, Eugenia PL. (2001). Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmacol, 56, 683–687.
- Turkmen N, Sari F, Velioglu YU. (2005). The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem, 93, 713–718.
- Umachigi SP, Kumar GS, Jayaveera K, Kishore KD, Ashok KC, Dhanapal R. (2007). Antimicrobial, wound healing and antioxidant activities of Anthocephalus cadamba. Afr J Tradit Complement Altern Med, 4, 481–487.
- Undeger U, Aydin S, Basaran AA, Basaran N. (2004). The modulating effects of quercetin and rutin on the mitomycin C induced DNA damage. Toxicol Lett, 151, 143–149.
- Villegas LF, Fernández ID, Maldonado H, Torres R, Zavaleta A, Vaisberg AJ, Hammond GB. (1997). Evaluation of the wound-healing activity of selected traditional medicinal plants from Perú. J Ethnopharmacol, 55, 193–200.
- Whitley AC, Stoner GD, Darby MV, Walle T. (2003). Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid–extensive binding to protein and DNA. Biochem Pharmacol, 66, 907–915.
- Yepez B, Espinosa M, López S, Bolaños G. (2002). Producing antioxidant fractions from herbaceous matrices by supercritical fluid extraction. Fluid Phase Equil, 194, 879–884.