Abstract
Context: Lichens are composite organisms consisting of a symbiotic association of a fungus (the mycobiont) with a photosynthetic partner (the phytobiont), usually either a green alga or cyanobacterium. The morphology, physiology and biochemistry of lichens are very different from those of the isolated fungus and alga in culture. Lichens occur in some of the most extreme environments on the Earth and may be useful to scientists in many commercial applications.
Objective: Over the past 2 decades, there has been a renewed and growing interest in lichens as a source of novel, pharmacologically active biomolecules. This review summarizes the past and current research and development trends in the characterization and use of lichens and their bioactive compounds in traditional medicine and other biopharmaceutical applications of commercial interest.
Methods: The present review contains 10 illustrations and 188 references compiled from major databases including Science Direct, Chemical Abstracts, PubMed and Directory of Open Access Journals.
Results: Lichen morphology, symbiosis, diversity and bioactivities including enzyme inhibitory, antimicrobial, antifungal, antiviral, anticancer, anti-insecticidal and antioxidant actions were reviewed and summarized. Recent progress in lichens and lichen-forming fungi was discussed with emphasis on their potential to accelerate commercialization of lichen-based products.
Conclusions: Lichens are an untapped source of biological activities of industrial importance and their potential is yet to be fully explored and utilized. Lichen-derived bioactive compounds hold great promise for biopharmaceutical applications as antimicrobial, antioxidant and cytotoxic agents and in the development of new formulations or technologies for the benefit of human life.
Introduction
Lichens are symbiotic plant-like organisms, usually composed of a fungal partner, mycobiont, and one or more photosynthetic partners, phytobiont, most often either a green alga or cyanobacterium (CitationSre-Indrasutdhi, 2005). Although the dual nature of these lichens is now widely recognized and lichen products have been used in traditional medicine for centuries, they are less studied and understood than the single microorganisms (CitationNash, 1996). Lichen species comprise more than 20% of the global fungal biodiversity and as unique symbiotic organisms that occur in some of the most extreme environments on Earth—arctic tundra, hot deserts, rocky coasts, toxic slag heaps, etc. The substances that lichens produce to survive in these extreme environments are also unique but little understood. As our understanding of the bioregulatory role of different endogenous biomolecules and their mechanism of action develops, more attention is drawn to lichens as a promising source for drug discovery (CitationKarthikaidevi et al., 2009). Although bioactive phenolic compounds with new chemical structures of pharmaceutical interest have been recently reported (CitationBoustie & Grube, 2005), most research effort has been focused on the discovery of new lichen species and lichen taxonomy, and despite recent progress, only usnic acid has been used for pharmaceutical and cosmetic product development to date (CitationCansaran et al., 2006). This review is intended to summarize the past and current research and development trends in the characterization and use of lichens and their bioactive compounds in traditional medicine and other biopharmaceutical applications of commercial interest.
Lichen morphology
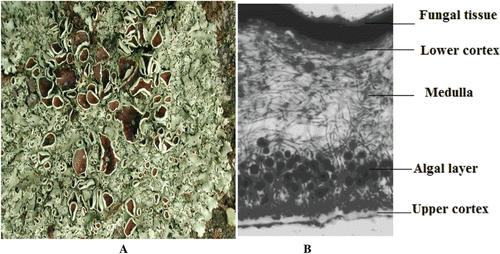
The morphology of the lichenized thallus is strongly influenced by the phytobiont and its direct contact with the mycobiont (). Lichen thalli have been grouped as: (1) crustose (phytobiont in a distinct layer below an upper mycrobiont cortical layer with no lower cortex); (2) leprose (groups of phycobiont surrounded by mycobiont); (3) foliose (leafy; phycobiont in a layer below an upper cortex with a discrete cortex below, separate from the substratum on which it grows; (4) filamentose (filamentous; phycobiont surrounded by a sheath of mycobiont); and (5) fruticose (shrubby; erect, vertical or trailing; radial in structure, often attached at the base, with the phycobiont in a layer inside the outer cortex).
Figure 1. (A) Lichen thallus, (B) Vertical section of a foliose lichen thallus, showing (bottom to top) the upper cortex of compact fungal tissue (mycobiont), the algal layer (phycobiont), medulla of loosely interwoven hyphae, and the lower cortex of compacted dark brown fungal tissue (mycobiont).
As the potential relationships of mycobionts and phytobionts may in fact be quite complex, a rigorous classification of the types of relationships between them was developed by CitationRambold and Triebel (1992). Since lichens cannot be regarded as individuals from a genetic and evolutionary perspective, this has major implications in many areas of lichen investigation such as developmental and reproductive studies (CitationNash, 1996). In culture, the unlichenized mycobionts remain relatively amorphous and initiate thallus development when they first come in contact with their phytobiont (CitationAhmadjian, 1993). There is a variation in the degree to which the symbiosis is obligatory for the partners involved. The green alga Trebouxia, which occurs in approximately 20% of all lichens, has rarely been found as a free-living organism. In contrast, other phytobiont genera such as Gleocapsa, Nostoc, Scytonema and Trentepohlia commonly occur in both lichenized and free-living state (CitationLücking et al., 2009). In some cases, the free-living populations (Nostoc and Scytonema) and their lichenized counterparts (Collema and Peltula) occur in the same habitat such as desert soils. The ability of the same phytobiont species to occur in a free-living and lichenized state at the same time is not well described (CitationBeck, 2002) because relatively few lichen algae have been identified as species, and generally, the systematics at the species level of many cyanobacteria and unicellular green algae are not well resolved. Nevertheless, it appears that most lichens are highly specific in their choice of phytobiont (CitationBeck et al., 1998; CitationRambold et al., 1998). The mycobionts growth is normally fairly slow and they are unlikely to survive well in a free-living state due to competition with other fungi and/or nutrient consumption by other organisms (CitationNash, 1996). Multiple phytobiont species (e.g., Trebouxia) have also been isolated from different lichen thalli belonging to the same lichen species (CitationFriedl, 1989; CitationIhda et al., 1993). Thus, most mycobionts are assumed to have an obligate relationship to lichenization, although the specificity of the mycobiont for a particular phytobiont may not be as great as one might assume.
Lichen symbiosis
The lichen symbiosis is a very successful one as lichens are found in almost all terrestrial habitats from the tropics to the polar-regions. As a result of the symbiosis, the lichen’s phytobiont and mycobiont have expanded into many habitats where separately they would be rare or non-existent. For example, most free-living algae and cyanobacteria normally occur in aquatic or very moist terrestrial habitats, but as lichens they also occur abundantly in habitats that are frequently dry. Lichenization is one mechanism where mycobiont enhances the water uptake and reduce the light intensity to which the phytobiont is exposed (CitationErtl, 1951). Thus, there may well be benefits to lichenization from the perspective of the phytobiont. In lichens, fungi share the photosynthetically derived carbon source from algae and in return provide water and nutrients to algae. Overall, it may be less important to evaluate lichenization from a strict cost/benefit perspective than to recognize it as a prominent example of a successful symbiosis. As a result of this symbiosis, lichens produce characteristic secondary metabolites and bioactive compounds, which seldom occur in other organisms. Additional studies will undoubtedly help elucidate further our understanding of the lichen symbiosis.
Lichen diversity
Among the terrestrial autotrophs of the world, lichens exhibit intriguing morphological variation in miniature. In color they exhibit a fantastic array of orange, yellow, red, green, gray, brown, and black (CitationWirth, 1995; CitationBrodo et al., 2001). Lichens vary in size from less than 1 mm to long, pendulous forms that hang over 2 m from tree branches. Almost all lichens are perennials, although a few ephemerals (e.g. Vezdaea) are known. At the other extreme, some lichens are estimated to survive well over 1000 years and may be useful in dating rock surfaces (CitationBeschel, 1961). Linear growth varies from imperceptible to many millimeters in a year.
Lichens occur commonly as epiphytes on trees and other plants, and in some ecosystems, epiphytic lichen biomass may exceed several hundred kg/ha (CitationCoxson & Nadkarni, 1995). In addition, they frequently colonize bare soil, where they are an important component of cryptogamic soil crusts in arid and semi-arid landscapes (CitationEvans & Johansen, 1999; CitationBelnap & Lange, 2003). Furthermore, lichens occur almost ubiquitously on rocks with the most obvious ones occurring as epiliths, either growing over the surface or embedded within the upper few millimeters. A few lichens even occur endolithically within the upper few millimeters of the rock in Antarctica (CitationFriedmann, 1982). In the tropics and subtropics, some rapidly growing lichens even colonize the surface of leaves as epiphylls (CitationLücking & Bernecker-Lücking, 2002). Although most lichens are terrestrial, a few occur in freshwater streams (e.g. Peltigera hydrothyria) and others—in the marine intertidal zone (e.g. Lichina spp. and the Verrucaria maura group). Lichens occur in most terrestrial ecosystems of the world, but their biomass contribution varies from insignificant to being a major component of the whole ecosystem (CitationKershaw, 1985). In many polar and subpolar ecosystems, lichens are the dominant autotrophs (CitationLongton, 1988). CitationLadd (2009) studied a total of 161 taxa of lichens and related fungi from the Gulf Coastal Plain in south-central Arkansas. Recently, a new lichen species of Caloplaca obamae was discovered in the Channel Island National Park of Santa Rosa Island, California (CitationKnudsen, 2009). It produced a thin thallus with orange granules (30–50 µm diameter) and discontinuous algal layer (50–100 µm thick). In comparison to the reported associated species C. ludificans, C. obamae did not produce ascospores and apothecia.
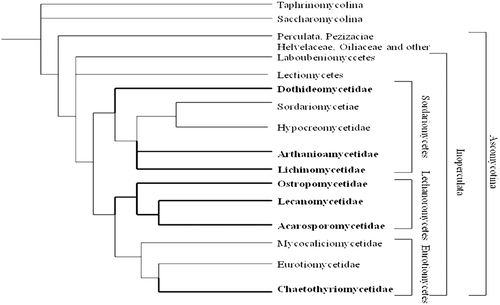
The formation of lichen associations represents one of the most successful lifestyles among fungi. Representing 20.6% of the 64,200 described fungi, the mycobionts belong to different subdivisions such as ascomycotina, basidiomycotina, deutromycotina, mastigomycotina, and myxomycotina. Out of the 13,250 lichen-forming fungal species described to date, nearly 13,000 are ascomycetes, approximately 50 are basidiomycetes and 200 are deutromycyces. Lichen-forming fungi represent 46.3% () of all described ascomycetes and are the focal point to understanding the ascomycete relationships (CitationHawksworth, 1988; CitationDePriest, 2004). Very few lichen species belong to basidiomycotina, deutromycotina, mastigomycotina, myxomycotina but not a single species belongs to the zygomycotina subdivision. Furthermore, within the ascomycetes, all lichen fungi belong to any of the three classes: the Sordariomycetes, the Lecanoromycetes, or the Eurotiomycetes (). Of these classes, the Lecanoromycetes is nearly exclusively lichenized and contains an overwhelming majority of all lichen-forming species. Before the advent of molecular studies, ascomycetes were classified on the basis of their reproductive structures. This system divided fungi into traditional classes such as apothecial Discomycetes, cleiostothecial Plectomycetes, and perithecial Pyrenomycetes, with asexual forms classified as anamorphic Deuteromycetes. Classification using molecular phylogenies has allowed researchers to modify these classes to monophyletic groups (CitationGargas & Taylor, 1995; CitationSpatafora, 1995; CitationLumbsch, 2000) and subsequently a new phylogenetic system has been proposed by CitationEriksson and Winka (1997). Recently, a report co-authored by 103 researchers from various institutions worldwide on the discovery of 100 new species of lichenized fungi representing a wide taxonomic and geographic range was published in Phytotaxa (CitationLumbsch et al., 2011). The newly described species were: Acarospora flavisparsa, A. janae, Aderkomyces thailandicus, Amandinea maritima, Ampliotrema cocosense, Anomomorpha lecanorina, A. tuberculata, Aspicilia mansourii, Bacidina sorediata, Badimia multiseptata, B. vezdana, Biatora epirotica, Buellia sulphurica, Bunodophoron pinnatum, Byssoloma spinulosum, Calopadia cinereopruinosa, C. editae, Caloplaca brownlieae, C. decipioides, C. digitaurea, C. magnussoniana, C. mereschkowskiana, C. yorkensis, Calvitimela uniseptata, Chapsa microspora, C. psoromica, C. rubropulveracea, C. thallotrema, Chiodecton pustuliferum, Cladonia mongkolsukii, Clypeopyrenis porinoides, Coccocarpia delicatula, Coenogonium flammeum, Cresponea ancistrosporelloides, Crocynia microphyllina, Dictyonema hernandezii, D. hirsutum, Diorygma microsporum, D. sticticum, Echinoplaca pernambucensis, E. schizidiifera, Eremithallus marusae, Everniastrum constictovexans, Fellhanera borbonica, Fibrillithecis sprucei, Fissurina astroisidiata, F. nigrolabiata, F. subcomparimuralis, Graphis caribica, G. cerradensis, G. itatiaiensis, G. marusa, Gyalideopsis chicaque, Gyrotrema papillatum, Harpidium gavilaniae, Hypogymnia amplexa, Hypotrachyna guatemalensis, H. indica, H. lueckingii, H. paracitrella, H. paraphyscioides, H. parasinuosa, Icmadophila eucalypti, Krogia microphylla, Lecanora mugambii, L. printzenii, L. xanthoplumosella, Lecidea lygommella, Lecidella greenii, Lempholemma corticola, Lepraria sekikaica, Lobariella sipmanii, Megalospora austropacifica, M. galapagoensis, Menegazzia endocrocea, Myriotrema endoflavescens, Ocellularia albobullata, O. vizcayensis, Ochrolechia insularis, Opegrapha viridipruinosa, Pannaria phyllidiata, Parmelia asiatica, Pertusaria conspersa, Phlyctis psoromica, Placopsis imshaugii, Platismatia wheeleri, Porina huainamdungensis, Ramalina hyrcana, R. stoffersii, Relicina colombiana, Rhizocarpon diploschistidina, Sticta venosa, Sagenidiopsis isidiata, Tapellaria albomarginata, Thelotrema fijiense, Tricharia nigriuncinata, Usnea galapagona, U. pallidocarpa, Verrucaria rhizicola, and Xanthomendoza rosmarieae (CitationLumbsch et al., 2011).
Figure 2. Distribution of lichen species in various subdivisions of fungi (Source: CitationHawksworth, 1988).
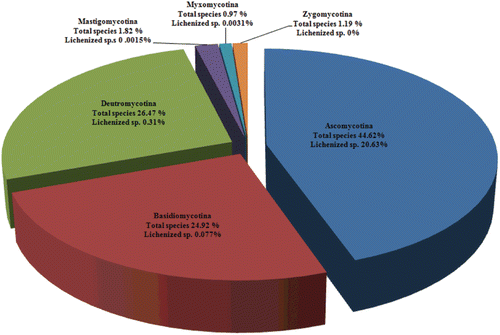
Figure 3. Phylogenetic relationship in phylum Ascomyceta (Source: CitationTehler & Wedin, 2008). Lichenized texa are marked with thick lines and with names in bold.
Lichen compounds and traditional biomedical uses
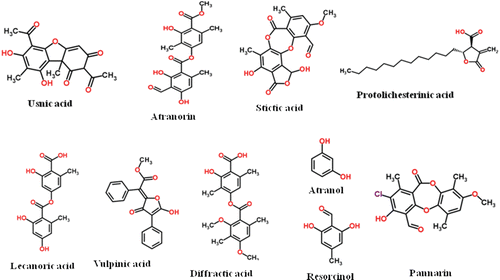
Many lichens are known to produce unique secondary metabolites and have considerable biological activities (Vartia, 1973; CitationRichardson, 1988; CitationLawrey, 1989; CitationElix, 1996). Many lichens are edible; however, some lichens contain toxic substances. According to CitationAsahina and Shibata (1971) and CitationDayan & Romagni (2001), the lichen compounds may be classified into the following groups: (1) aliphatic lichen substances (including acids, zeorin compounds, polyhydric alcohols); (2) aromatic lichen substances (including pulvic acid derivatives, depsides, depsidones, quinones, xanthone derivatives, diphenyleneoxide derivatives, nitrogen containing compounds, triterpenes, tetronic acids); and (3) carbohydrates (polysaccharides). To date, the chemistry of about a third of all lichen species has been studied and about 350 secondary metabolites have been identified. The chemical structures of approximately 200 of them have been established. Lichen’s secondary metabolites are usually insoluble in water and can be extracted into organic solvents. They amount to between 0.1 and 10% of the dry weight of the thallus, sometimes up to 30% (CitationVarita, 1973). The chemical structures of some common lichen compounds are presented in . These substances have been mostly identified as lactones (e.g., protolichessterinic acid), phenolic compounds (e.g., atranol and resorcinol), depsides (e.g., diffractic acid), pulvinic acid derivative (e.g., vulpinic acid), dibenzofurans and usnic acids (e.g., usnic acid). In addition, other lichen substances like atranorin, stictic acid, lecanoric acids and pannarin have been frequently studied (CitationKhanuja et al., 2007; CitationRanković & Mišić, 2008; CitationGomes et al., 2002).
Although lichens have been used for medical purposes since ancient times, information on the edible and medicinal uses of lichens is scattered (CitationChevallier, 1996). The medicinal use of lichens can be traced back to the 18th dynasty (1700–1800 BC) when Evernia furfuracea (L.) Mann or (Parmeliaceae) was first used as a drug (CitationLaunert, 1981). Some lichens were claimed to be good for coughs, jaundice, rabies and restoring lost hair (CitationPereira, 1853). Herbal medicine texts made account of several species of lichens including Cladonia, Evernia, Lobaria, Parmelia, Peltigera, Pertusaria, Physica, Rocella, Usnea and Xanthoria (CitationPerez-Llano, 1944a). During the middle age, lichens figured prominently in the herbals used by practitioners. However, lichens have been essentially overlooked to a great extent by the modern pharmaceutical industry, despite all the evidence of biological activity in lichen extracts provided in literature (CitationKhanuja et al., 2007). The people of Northern California used Letharia vulpina (L.) Hue. (Parmeliaceae) in stomach diseases (CitationMalhotra et al., 2008). A novel species of Dictyonema was used by the Waorani as hallucinogen (CitationDavis & Yost, 1983). In the Arabian medicine, Alectoria usneoides was used in the treatment of splenomegaly (enlarged spleen). Usnea sp. was used in the Traditional Chinese Medicine (TCM), homeopathic system of medicine and traditional medicine in the Pacific Islands and New Zealand. Usnea sp. is valued for its demulcent properties and finds use in treatments of mild inflammation of the oral and pharyngeal mucosa. Usnea filipendula Stirt was used in the former Soviet Union for cuts and wounds (CitationChevallier, 1996). The Spanish folk medicine has documented the use of lichens in various medical aliments (CitationMalhotra et al., 2008). Decoction of Pseudoevernia furfuracea (L.) Zopf. (Parmeliaceae) is used in Alfacar and Viznar in respiratory ailments. Ramalina bourgeana Mont. ex Nyl. (Ramalinaceae) is consumed for diuretic and stone-dissolving (lithontriptic) properties (CitationGonzález-Tejero, 1995). The lichen Xanthoparmelia scabrosa (Taylor) Hale (Parmeliaceae) is an ingredient in various aphrodisiac formulations sold on the international market. Traditionally, Cetraria islandica (L.) was used to treat mild inflammation of the oral and pharyngeal mucosa, dyspepsia, and loss of appetite. In the European folk medicine, Cetraria islandica (L.) was used in cancer treatment (CitationChevallier, 1996). Reindeer lichens such as Cladonia rangiferina (L.) F. H. Wigg. syn. Cladina rangiferina (L.) Nyl. (Cladoniaceae) were commonly used to treat colds, arthritis, fever (CitationPerez-Llano, 1944b) as well as jaundice constipation, convulsions, coughs, and tuberculosis (Brown, 2001). Three Parmelia sp. are contained in the Indian drug chharila used as aphrodisiac (CitationLal & Upreti, 1995; CitationKumar & Upreti, 2001). In India, Parmelia chinense finds applications as diuretic and as liniment for headache and powder to heal wounds, whereas the Tinea (ringworm) like disease is treated with Parmelia sancti-angeli. Parmelia peforatum is medically recognized in Afghanistan (CitationChandra & Singh, 1971). Parmelia nepalense (Talyor) Hale ex Sipman is used in Nepal for treatment of toothache and sore throat (CitationKumar et al., 1996). In the Western Himalayas, Thamnolia vermicularis (Schwartz) Ach. (Icmadophilaceae) is used as antiseptic (CitationNegi & Kareem, 1996). In Sikkim (India), Heterodermia diademata (Talyor) D.D. Awas., (Physciaceae) was used for cuts and wounds (CitationSaklani & Upreti, 1992). Several reviews have discussed the pharmaceutical potential and biological activities of lichen substances (CitationHuneck, 1999; CitationMuller, 2001; CitationYamamoto, 2000; CitationBoustie & Grube, 2005).
Many countries have developed commercial pharmacological products based on lichen substances. For instance, usnic acid (CitationIngolfsdottir, 2002) was used in anticeptic products in Germany (Camillen 60 Fudes spray and nail oil) and Italy (Gessato™ shaving). However, at high doses, usnic acid has been shown to exhibit toxic effects (acute oral toxicity, LD50 of 0.84 g/kg) and fatal hepatotoxicity (∼500 mg/day of usnic acid) in mice (CitationDurazo et al., 2004; CitationNeff et al., 2004). Icelandic lichens were marketed in cold remedies formulation by the trade names of Isla-Moos® (Engelhard Arzneimittel GmbH & Co. KG, Germany) and Broncholind® (MCM Klosterfrau Vertriebsgesellschaft mbH, Germany). In Japan, lichen extracts or substances were used in cosmetics, pharmaceuticals and neutraceutical products. The riminophenazine antibiotics, exemplified by clofazimine (Lamprene®), were developed as antimycobacterial drugs (CitationReddy et al., 1999). The antituberculous activity of these drugs was due to the active compounds diploicin and depsidone extracted from the Irish lichen Buellia canescens (CitationBarry, 1946; CitationBarry & Twomey, 1950; CitationNolan et al., 1948).
Biological activities of lichens
Lichens produce a wide array of biologically active primary (intracellular) and secondary (extracellular) metabolites (CitationLauterwein et al., 1995). Primary metabolites include amino acids, polyols, carotenoids, polysacharids and vitamins. Some, like the polysaccharide cell wall compounds lichenan and isolichenan, have taxonomic significance. Carotenoid compounds have also been intensely studied for dues to evolutionary relationships. Lichen’s secondary metabolites, often called lichen acids, are produced primarily by the mycobiont, secreted onto the surface of lichen’s hyphae either in amorphous forms or as crystals. Past and current studies show that lichen’s secondary metabolites exert a wide variety of biological activities that include antibiotic, antimycobacterial, antiviral, anti-inflammatory, analgesic, antipyretic, plant growth inhibitory, antiherbivore, enzyme inhibitory, antiproliferative and cytotoxic effects (CitationShawuti & Abbas, 2007).
Antibacterial activities of lichens
It is well known that pathogenic microbes pose serious threats to human health and are increasing in prevalence in institutional health care settings (James et al., 1997) due to the growing resistance that infectious agents have developed against antibiotics (CitationBabita et al., 2008). Therefore, new alternatives for combating the spread of infection through antibiotic-resistant microbes are necessary for keeping pace with the evolution of ‘super’ pathogens. Natural products are proposed as a therapeutic alternative to conventional antimicrobial treatment (CitationAli et al., 1999; CitationNimri et al., 1999). Among them, lichen-derived products and their antibiotic properties are of special interest to scientists (CitationLawrey, 1986) as up to 50 % of all lichens have been reported to possess antibiotic activities (CitationSharnoff, 1997).
Historically, Burkholder (1944) has first pioneered research on lichens as antibacterial agents. One of the most frequently reported lichen-derived products with a strong antimicrobial activity is usnic acid (CitationIngolfsdottir, 2002). Usnic acid, evernic acid and vulpinic acid inhibited the growth of the Gram-positive bacteria Staphylococcus aureus, Bacillus subtilis and Bacillus megaterium, but had no affect on the gram negative bacteria Escherichia coli or Pseudomonas aeruginosa (CitationLawrey, 1986). Acetone, chloroform, diethyl ether, methanol and petroleum ether extracts of Parmelia sulcata containing salazinic acid demonstrated antibacterial activity against Aeromonas hydrophila, Bacillus cereus, Bacillus subtilis, Listeria monocytogenes, Proteus vulgaris, Yersinia enterocolitica, Staphylococcus aureus, Streptococcus faecalis, Candida albicans and Candida glabrata (CitationCandan et al., 2007). Diethyl ether, acetone and ethanol extracts of Cetraria aculeate contained protolichesterinic acid with promising antibacterial activity against nine bacteria belonging to Gram-positive and Gram-negative groups (Türka et al., 2003). Most of the antibacterial activities were tested on Bacillus, Pseudomonas, E. coli, Staphylococcus aureus, Kleibsiella, Candida, Salmonella, Yersinia and Proteus sp. (Inglfsdottir et al., 1985; CitationYilmaz et al., 2004; CitationRanković & Mišić, 2008; CitationKarthikaidevi et al., 2009; CitationKaragöz et al., 2009; Taya et al., 2004; CitationMartins et al., 2010; CitationManojlovic et al., 2010; CitationRanković et al., 2010; CitationSantiago et al., 2010; CitationSwathi et al., 2010; CitationZambare et al., 2010).
Any bioactive compound, which is studied for antimicrobial activity, must have a specific concentration for an effective killing performance that varies with the compound’s chemical structure, the test microorganism and its resistance to the bioactive compound. Minimal inhibitory concentrations (MICs) are used to characterize the biological activity of various lichen solvent extracts. Solvents include acetone, methanol, ethanol, diethyl ether, chloroform and petroleum ether. Among these, methanol is the most commonly used solvent for extraction of bioactive compound from lichens ( and ). The antimicrobial pattern of lichen extracts varies with the microbes and their cell membrane composition which is different in Gram-positive and Gram-negative microbes. In Gram-positive bacteria, Bacillus and Staphylococcus are the most dominantly genera studied on lichen extracts, followed by Mycobacterium, Streptococcus, Listeria and Micrococcus (). Among the Bacillus species, B. sublitis was the most sensitive microorganism to lichen substances such as atranorin, protolichsterinic acid, salazinic acid, usnic acid, norstictic acid, protoacetraric acid, fumaroprotoacetraric acid, atranol, lecanoric acid, stictic acid, divericatic acids and zeorin. In addition to the above active components (except atranol), Staphylococcus sp. was also sensitive to alectosarmentin and barbatic acid (). Likewise, lichen active compounds, present in lichen extracts, were found active against various against Gram-negative microbes (). Next to E. coli as the most studied Gram-negative microorganism, pathogens like Aeromonas, Anterobacter, Helicobacter, Kleibsiella, Pseudomonas and Proteus sp. have also been proved sensitive to lichen active compounds ().
Table 1. Antibacterial activity of lichen species against Gram-positive bacteria.
Table 2. Antibacterial activity of lichen species against Gram-negative bacteria.
Antifungal activities of lichens
The acetone and methanol extracts of Lasallia pustulata (L.) Méret. (Umbilicariaceae), Parmelia sulcata Taylor and Umbilicaria crustulosa (Ach.) Frey (Umbilicariaceae) manifested a very selective antifungal activity (CitationRanković et al., 2007). Usnic acid together with isodivaricatic acid, 5-propylresorcinol, divaricatinic acid were identified as antifungal agents (CitationSchmeda-Hirschmann et al., 2008). Acetone, chloroform, diethyl ether, methanol and petroleum ether extracts of Parmelia sulcata containing salazinic acid demonstrated antifungal activity against Aspergillus niger, Aspergillus fumigatus, and Penicillium notatum (CitationCandan et al., 2007). Parietin and anthraquinone isolated from methanol extracts of Caloplaca cerina (Ehrh. ex Hedwig) Th.Fr. (Teloschistaceae) displayed a significant antifungal activity (CitationManojlovic et al., 2005). Extracts of Andean lichens Protousnea poeppigii (Nees and Flot.) Vain. (Parmeliaceae) and Usnea florida var. rigida Acharius demonstrated antimicrobial activity against the pathogenic fungi Microsporum gypseum, Trichophyton mentagrophytes and T. rubrum. Acetone extracts of three lichen species - Evernia prunastri, Hypogymnia physodes and Cladonia portentosa—were investigated for antifungal activity against eight plant pathogenic fungi: Pythium ultimum, Phytophthora infestans, Rhizoctonia solani, Botrytis cinerea, Colletotrichum lindemuthianum, Fusarium solani, Stagonospora nodorum and Ustilago maydis (CitationHalama & Van, 2004). CitationManojlovic et al. (2000) isolated anthraquinones from Xanthoria lichen species possessing antifungal activity. A potent fingitoxic compound, lecanoric acid, was isolated from Parmotrema tinctorum lichen and tested against the fungus Cladosporium sphaerospermum (CitationGomes et al., 2002). Antifungal activities have been reported for the lichen substance anthraquinone parietin from Caloplaca cerina (CitationManojlovic et al., 2005) and for divaricatinic acid, isodivaricatic acid, usnic acid, and 5-propylresorcinol compounds from Andean lichens Protousnea poeppigii and Usnea rigida (CitationSchmeda-Hirschmann et al., 2008). Antifungal activities were tested on Aspergillus, Botrytis, Fusarium, Mucor, Penicillium and Tricoderma species with low MIC values (0.00625-6.25 mg/mL) indicating high activity (specificity) of these lichen extracts against fungal pathogens. The lichen active compounds reported to possess antifungal activity—divaricatic acid, zeorin, lecanoric acid, lichenic acid, atranorin, salanizic acid, protolichesterinic acid, fumarprotoacetraric acid, protocetraric acid, stictic acid and usnic acid—are summarized in .
Table 3. Antifungal activity of lichen species.
Antiviral activities of lichens
Antiviral properties have been attributed to various lichen substances. Anthraquinones, especially the polyphenolic and/or polysulphonate substituted types, have been shown to exhibit potent antiviral properties (CitationSchinazi et al., 1990; CitationSydiskis et al., 1991). CitationCohen et al. (1996) isolated anthraquinones, bianthrones and hyperacin derivatives from lichens whose antiviral activities were positively correlated with an increasing substitution of chlorine in the anthraquinone structure. It is plausible to suggest that similar manipulations could improve the antiviral effects of the nascent compounds in crude lichen extracts. Plant polysaccharides have also been shown to exhibit potent antiviral activities, especially against enveloped viruses (CitationHosoya et al., 1991; CitationPremanathan et al., 1999). CitationEsimone et al. (2007) reported that the crude polysaccharide fraction (CPF) of a Parmelia perlata lichen extract targeted the enveloped positive-sense RNA virus (yellow fever virus) but was inactive on non-enveloped RNA viruses (poliomyelitis and IBDV). However, an empirical conclusion to this effect could only be substantiated after further screening of CPF against several other enveloped viruses and after detailed molecular elucidation studies. Usnic acid isolated from Teloschistes chrysophthalmus (L.) Th. Fr. (Teloschistaceae) and parietin isolated from Ramalina celastri demonstrated antiviral activity against the arena viruses Junin and Tacaribe (CitationFazio et al., 2007). Recently, CitationPraveen-Kumar et al. (2010a) reported an antifungal activity of microlichen Ramalina hossei H. Magn & G. Awasthi. The aqueous extracts and the ethanolic extracts prepared from the lichen species Xanthoria parietina and Xanthoparmelia tinctina were evaluated for antiviral activity against human parainuenza virus type 2(HPIV-2) and cytotoxic activity towards Vero cells. The EC50 of the ethanol extract of X. tinctina for HPIV-2 replication was 20 µg/mL, and for aqueous extract was 22.5 µg/mL (CitationKaragöz & Aslan, 2005). In vitro antiviral activities of lichen extracts were reported for human cytomegalovirus (CitationWood et al., 1990), HIV (CitationHirabayashi et al., 1989; CitationNeamati et al., 1997), HIV-RT (CitationPengsuparp et al., 1995), and Epstein-Barr virus (CitationYamamoto et al., 1995). Lichenan, a structural component of the mycosymbiont cell wall (CitationHonegger & Haish 2001), contained a linear {1→3, 1→4} β-d-glucan linkage (CitationTvaroska et al., 1983) that inhibited symptom development and virus accumulation in four greenhouse-grown Nicotiana spp. infected by a tobacco mosaic virus (CitationStubler & Buchenauer, 1996).
Anticancer activities of lichens
Some lichen substances like usnic acid, cristazarin, protolichesterinic acid, polyporic acid, depsidone and lichenin have been investigated for antitumor effects on tumor cells—melanoma B-16 (CitationKhanuja et al., 2007), P388 leukaemia (CitationTakai et al., 1979), K-562 leukaemia (CitationHirayama et al., 1980), Ehrlich solid tumor (CitationCain, 1966) and lymphocyte (Correche et al., 2002) cells. In vitro anticancer activities of lichen extracts have been evaluated according to the cell proliferation assay (CitationTokiwano et al., 2009) in three cancer cell lines: human pancreatic (PANC-1) (CitationIngolfsdottir et al., 2002), prostate (DU-145) (CitationRusso et al., 2006) and breast (MCF7) (CitationBogo et al., 2010) cancer cell lines.
The anticancer properties of lichen extracts have been studied for many years. Extracts from the lichen Collema flaccidum showed significant anticancer activity in the crown gall tumor inhibition test. The purified inhibitors were identified as colleflaccinosides and bisanthraquinone glycosides (CitationRezanka & Dembitsky, 2006). Extracts containing depsidone pannarin exhibited similar anticancer activities by inducing cell death in human prostate carcinoma DU-145 cells (CitationMaier et al., 1999; CitationRusso et al., 2006) and cell apoptosis in human melanoma M14 cells (CitationRusso et al., 2006; Citation2008). Tenuiorin (a tridepside) and methyl orsellinate extracted from Peltigera leucophlaebia inhibited cell proliferative activities on human breast (T-47D), pancreatic (PANC-1) and colon (WIDR) cancer cell lines (CitationIngolfsdottir et al., 2002). Usnic acid showed inhibitory effects on the cell growth and proliferation of two different human cancer cell lines—the breast cancer cell line T-47D and the pancreatic cancer cell line Capan-2 (Einarsdottir et al., 2010). Lecanoric acid, a secondary metabolite from Parmotrema timctorum, exerted anticancer activities against HEp-2 larynx carcinoma, MCF7 breast carcinoma, 786-0 kidney carcinoma and B16-F10 murine melanoma cell lines (CitationBogo et al., 2010).
The antitumor and cytotoxic activities of some lichen constituents in different cell systems have been reviewed by CitationHuneck (2001) and CitationIngolfsdottir et al. (1997). Crude extracts from various lichen species were screened for their cytotoxic activities and some of them were found to be cytotoxic in different cancer cell lines (CitationPerry et al., 1999; Bezivin et al., 2003). Usnic acid exhibited an antiproliferative effect on human leukemia cells (K562) and endometrial carcinoma (Ishikawa, HEC-50) cells (Carderelli et al., 1997; Kristmundsdottir et al., 2002). A lichen-derived polysaccharide CFP-2 reduced the viability of HL-60 and K562 cells due to apoptotic pathway and telomerase activity, suggesting its possible therapeutic potential against cancer (CitationLin et al., 2003). Protolichesterinic acid isolated from Cetraria islandica L. (Ach.) inhibited growth of malignant cell lines (Ogmundsdottir et al., 1998). Antiproliferative effects of several lichen compounds in human platelets were ascribed to their inhibitory activities on 12(S)-HETE which plays role in carcinogenesis and metastasation (CitationBucar et al., 2004). CitationZeytinoglu et al. (2004) reported genotoxic/antigenotoxic and cytotoxic activities of extracts from C. aculeata in bacterial and mammalian cell systems. Pannarin inhibited cell growth and induced cell death in human prostate carcinoma DU-145 cells (CitationMaier et al., 1999). The orcinol derivatives, tenuiorin and methyl orsellinate, present in extracts of Peltigera leucophlebia (Nyl.) Gyeln (Peltigeraceae), exhibited in vitro inhibitory activity against 15-lipoxygenase from soybeans. On this account, tenuiorin and methyl orsellinate were further tested for antiproliferative activity on cultured human breast, pancreatic and colon cancer cell lines. Bianthraquinone glycosides, colleflaccinosides isolated from Collema flaccidum (Ach.) Ach. (Collemataceae), collected in Israel and Russia, were reported to have antitumor activity (CitationRezanka & Dembitsky, 2006).
Anti-insecticidal activities of lichens
Killing larvae of mosquitoes is a successful way of minimizing mosquito population in breeding grounds before they reach adult stage (CitationVinayaka et al., 2009). The most commonly used insecticidal agents are currently based on synthetic chemicals; however, their repeated use has been reported for widespread development of chemical resistance and public concern over possible health problems associated with food and environment (CitationBonning & Hammock, 1992). Phytochemicals contain many bioactive ingredients which offer an alternative source of insect-control agents and that have little or no harmful effect on non-target organisms and the environment. It is observed that the methanol extract of R. conduplicans was active against mosquito larvae (CitationVinayaka et al., 2009). Extracts from lichen Letharia vulpine showed potent insecticidal activities against Spodoptera ornithogalli and S. littoralis (CitationKhanuja et al., 2007). Bioassays with (−)- and (+)-usnic acids against larvae of Culex pipiens revealed that the LC50 values were 0.8 and 0.9 ppm, respectively (CitationCetin et al., 2008).
Enzyme inhibition activities of lichens
Lichen substances like usnic acids, resorcinol derivatives and atranorin were found to be potent enzyme inhibitors of ornithine decarboxylase and arginine decarboxylase that affect the polyamine metabolism (CitationBoustie & Grube, 2005). Atranoin (from Psedevernia furfuracea) and resorcinol (from Protousnea spp.) were reported for trypsin and tyrosinase inhibition, respectively (CitationKhanuja et al., 2007). Inhibition of tyrosinase (for melanin biosynthesis) and xanthine oxidase (for hyperuricaemia) with lichen extracts were reported by various researchers (CitationHiguchi et al., 1993; CitationBehera et al., 2005; CitationKim & Cho, 2007; CitationVerma et al., 2008).
Tyrosinase or polyphenol oxidase (monophenol, odiphenol: oxygen oxidoreductase; EC 1.14.18.1) is a copper enzyme that catalyzes two different reactions using molecular oxygen (Sanchez-Ferrer et al., 1995): the hydroxylation of mono-phenols to o-diphenols (monophenolase activity) and the oxidation of the o-diphenols to o-quinones (diphenolase activity). This enzyme is widely distributed in plants, microorganisms and animals where tyrosinase is responsible for melanization. In humans, the melanization is influenced by several mechanisms such as anti-oxidation, direct tyrosinase inhibition, melanin inhibition of migrated cells and hormonal activities (CitationProta & Thomson, 1976; CitationPawelek & Korner, 1982). Tyrosinase inhibitors have been frequently used in cosmetics as depigmenting agents for hyperpigmentation (CitationFunasaka et al., 2000). A concerted effort has been made to search for naturally occurring tyrosinase inhibitors from various organisms, many of them being largely free from harmful adverse effects (CitationSasaki & Yoshizaki, 2002).
Umbilicaria esculenta extracts strongly inhibited disaccharide hydrolytic enzymes of mold and mammalian origin (CitationLee & Kim 2000). A glucosidase inhibitory activity by extracts of Parmelia austrosinensis and Parmelia praesorediosa was also reported (CitationLee & Kim, 2000). Anti-inflammatory, analgesic and antipyretic activities of lichen substances (CitationOkuyama et al., 1995) were evidenced by inhibition of lipoxygenase (CitationIngolfsdottir et al., 2002), prostaglandin (CitationSankawa et al., 1982) and leukotriene B4 biosyntheses (CitationKumar & Muller, 1999).
Antioxidant activities of lichens
Many lichen extracts have been reported for antioxidant properties due to their phenolic content. Antioxidant agents inhibit and prevent reactive oxygen species, which can cause degenerative diseases. Natural antioxidants are preferred over many synthetic antioxidants, which can be toxic, for therapeutic applications. CitationJayaprakasha and Rao (2000) examined the antioxidant properties of methyl orsellinate, atranorin, osellinic acid and lecanoric acid. CitationBhattarai et al. (2008) reported stronger antioxidant activities in extracts from Antarctic lichens than from lichens native to temperate or tropical regions. Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale (Parmeliaceae) including methyl orsenillate, orsenillic acid, atranorin and lecanoric acid showed moderate antioxidant activity (Jayapraksha & Rao, 2000). An animal study reported antioxidant activities of lichen Cetraria islandica (CitationGülçin et al., 2002).
Antioxidant activities as assessed by DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical and ABTS [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonate)] radical scavenging capacities were determined and compared with those of commercial standards BHA (butylated hydroxyanisole) and Trolox [(±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid] (CitationPaudel et al., 2008; CitationGülçin et al., 2002; CitationKekuda et al., 2009). Many researchers reported antioxidant activity of lichen extracts based on lipid peroxidation inhibition and total phenol content (CitationBehera et al., 2005; CitationOdabasoglu et al., 2005; CitationYucel et al., 2007; Islas et al., 2008; CitationÖzen & Kinalioğlu, 2008; CitationVerma et al., 2008; CitationVinayaka et al., 2009; CitationManojlovic et al., 2010; CitationPraveen-Kumar et al., 2010b). Stactic acid derivatives (β-orcinol depsodomes) were obtained from usnea articulate lichens with potential antioxidant activity (Dévéhat et al., 2007). Lichens produce a number of secondary metabolites—polysaccharides and/or phenolic compounds—that are known to exhibit such properties (CitationLiu et al., 1997; CitationHidalgo et al., 1994; CitationSanchez-Moreno et al., 1999; CitationGermano et al., 2002; CitationDuh et al., 1999; CitationOkamoto et al., 1992; CitationSuzuki et al., 1992).
The antioxidant activity has been evaluated based on DPPH free radical scavenging, reducing power, superoxide anion radical scavenging and lipid peroxidation inhibition. Methanol has been used as the most efficient and suitable solvent for extraction of bioactive compounds with antioxidant activities from lichens, hence, most antioxidant activity assays have been performed on methanol extracts (). The lichens Peltigera canina, Peltigera praetextata, Sticta nylanderiana, Ramalina conduplicans, Usnea ghttensis and Parmotrema pseudotinctorum all had more than 85% DPPH scavenging activity. Furthermore, as shown in , the methanol extracts of lichens showed the highest activities of reducing power, superoxide radical scavenging and lipid peroxidation inhibition.
Table 4. Antioxidant activity of lichen extracts in different solvents.
Bioactivities of lichen mycobionts
As described in the previous sections, lichens and their metabolites have various biological activities such as antimicrobial, antifungal, antiviral, antiprotizoal, antiproliferative, antioxidant and anti-inflammatory (CitationBehera et al., 2005; CitationHalama & Van, 2004; CitationIngolfsdottir, 2002; CitationMüller, 2001; CitationPerry et al., 1999; CitationYamamoto et al., 1998). CitationDembitsky & Tolstikov (2003) proposed that this phenomenon may be due to the presence of halogenated compounds in the lichen mycobiont. Haloginated compounds are phenol-based molecules synthesized in lichens and other organisms (CitationNeidleman & Geigert, 1986) by the enzyme haloperoxidase in presence of hydrogen peroxide and halide ions (Cl−, Br−, I−) (CitationGrifin, 1990). Hence, the use of isolated lichen mycobionts, lichen-forming fungi (LFF), may overcome, owning to their faster growth and metabolite production, disadvantages that impede commercialization of LFF-derived bioactive compounds. Examples of LFF include Pyrenula japonica, Pyrenula pseudobufonia (CitationTanahashi et al., 1999). CitationWei et al. (2008) isolated 94 LFF from lichen species, collected from China and Korea, that showed a promising antimicrobial activity against the plant pathogenic fungus Colletotrichum acutatum, a causal agent of anthracnose on hot pepper. CitationHur et al. (2003) reported on the isolation (from Heterodermia lichen species), cultivation and antifungal activity of a LFF against 14 fungal species. contains a list of LFF with promising antifungal activities. Only a few reports are available on antibacterial activities of LFF in literature. For example, an antibacterial activity of LFF from Nephromopsis pallescens lichen against Helicobacter pylori was recently reported by CitationLuo et al. (2011).
Table 5. Antifungal activities of lichen-forming fungi.
Pharmaceutical and biotechnological uses of LFF require large quantities of fungal materials for extraction. Most lichen fungi can be cultured in liquid and semiliquid media. Most of the lichen end products that are formed in the fermentation media are a mix of substances that need further purification using chemical separation methods like selective extraction, preparative chromatography, etc. shows a selection of nutrient media that have been used to induce biosynthesis of LFF-based bioactive compounds. However, to date, progress in evaluation of lichen-derived fungi for antifungal activity against plant pathogenic fungi in order to develop less harmful and safer protectants (e.g., as novel agrochemicals) has been slow. The LFF have shown promising antifungal activities, however, more research needs to be done to reveal the full potential of biological activities from LFF.
Table 6. Cultivation media for production of bioactive compounds from lichen-forming fungi (Stocker & Hager, 2008).
Conclusions
Despite their broad spectrum of biological activities, lichens have for long been overlooked by mycologists and agro-chemists, mainly due to their slow growth in nature and difficulties in their artificial cultivation. Because of that, the stage of large-scale industrial production of lichen metabolites has not been reached yet. More research and development is required to develop, optimize and scale-up promising lichen-based technologies of high industrial and national importance. The biopharmaceutical industry would benefit though the commercialization of biotechnologies aimed at production of natural anti-oxidants, anti-microbial, anti-insecticidal, antipyretic, and anti-cancer agents. Lichens hold great potential that needs to be fully explored and utilized for the benefit of human health and our society.
Declaration of interest
The authors report no conflicts of interest.
References
- Ahmadjian V. (1993). The Lichen Symbiosis. New York, USA: John Wiley.
- Ali MS, Azhar I, Amtul Z, Ahmad VU, Usmanghani K. (1999). Antimicrobial screening of some Caesalpiniaceae. Fitoterapia, 70, 299–304.
- Asahina Y, Shibata S. (1971). Chemistry of Lichen Substances. Tokyo, Japan: Japan Society for the Promotion of Science.
- Babita P, Hari DB, Jin SL, Soon GH, Hyun WS, Joung HY. (2008). Antibacterial potential of Antarctic lichens against human pathogenic Gram-positive bacteria. Phytother Res, 22, 1269–1271.
- Banerjee M, Sarkar P. (2008). In vitro callusing in Stevia rebaudiana Bertoni using cyanobacterial media- a novel approach to tissue culture. Int J Integr Biol, 3, 163–168.
- Barry VC, Twomey D. (1950). Antituberculous substances. VI. Derivatives of diploicin. Proc R Irish Acad, 53B, 55–59.
- Barry VC. (1946). The thyroid and tuberculosis. Nature, 158, 131.
- Beck A, Friedl T, Rambold G. (1998). Selectivity of photobiont choice in a defined lichen community, inferences from cultural and molecular studies. New Phytologist, 139, 709–720.
- Beck A. (2002). Photobionts, diversity and selectivity in lichen symbioses. Inter Lichenolog News, 35, 18–24.
- Behera BC, Verma N, Sonone A, Makhija U. (2005). Evaluation of antioxidant potential of the cultured mycobiont of a lichen Usnea ghattensis. Phytother Res, 19, 58–64.
- Belnap J, Lange OL. (2003). Biological Soil Crusts: Structure, Function, and Management, Ecological Studies. Berlin, Springer.
- Beschel RE. (1961). Dating rock surfaces by lichen growth and its application to glaciology and physiography (lichenometry). In: Raasch GO, ed. Geology of the Arctic. vol. 2, Toronto, Canada: University of Toronto Press, pp. 1044–1062.
- Bézivin C, Tomasi S, Lohézic-Le Dévéhat F, Boustie J. (2003). Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine, 10, 499–503.
- Bhattarai HD, Paudel B, Hong SG, Lee HK, Yim JH. (2008). Thin layer chromatography analysis of antioxidant constituents of lichens from Antarctica. J Nat Med, 62, 481–484.
- Bogo D, de Matos MF, Honda NK, Pontes EC, Oguma PM, da Santos EC, de Carvalho JE, Nomizo A. (2010). In vitro antitumour activity of orsellinates. Z Naturforsch, C, J Biosci, 65, 43–48.
- Bonning BC, Hammock BD. (1992). Development and potential of genetically engineered viral insecticides. Biotechnol Genet Eng Rev, 10, 455–489.
- Boustie J, Grube M. (2005). Lichens—a promising source of bioactive secondary metabolites. Plant Genet Resour, 3, 273–287.
- Brodo IM, Sharnoff SD, Sharnoff S. (2001). Lichens of North America. New Haven, USA: Yale University Press.
- Bucar F, Schneider I, Ogmundsdóttir H, Ingólfsdóttir K. (2004). Anti-proliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine, 11, 602–606.
- Burkholder PR, Evans AW, McVeigh I, Thornton HK. (1944). Antibiotic activity of lichens. Proc Natl Acad Sci USA, 30, 250–255.
- Cain BF. (1966). Potential anti-tumour agents. IV. Polyporic acid series. J Chem Soc Perkin Trans I, 11, 1041–1045.
- Candan M, Yilmaz M, Tay T, Erdem M, Türk AO. (2007). Antimicrobial activity of extracts of the lichen Parmelia sulcata and its salazinic acid constituent. Z Naturforsch, C, J Biosci, 62, 619–621.
- Cansaran D, Cetin D, Halici MG, Atakol O. (2006). Determination of usnic acid in some Rhizoplaca species from Middle Anatolia and their antimicrobial activities. Z Naturforsch, C, J Biosci, 61, 47–51.
- Cardarelli M, Serino G, Campanella L, Ercole P, De Cicco Nardone F, Alesiani O, Rossiello F. (1997). Antimitotic effects of usnic acid on different biological systems. Cell Mol Life Sci, 53, 667–672.
- Cetin H, Tufan-Cetin O, Turk AO, Tay T, Candan M, Yanikoglu A, Sumbul H. (2008). Insecticidal activity of major lichen compounds, (-)- and (+)-usnic acid, against the larvae of house mosquito, Culex pipiens L. Parasitol Res, 102, 1277–1279.
- Chandra S, Singh A. (1971). A lichen crude drug (chharila) from India. J Res Indian Med, 6, 209–215.
- Chevallier A. (1996). The Encyclopedia of Medicinal Plants. Dorling Kindersley. London.
- Cohen PA, Hudson JB, Towers GH. (1996). Antiviral activities of anthraquinones, bianthrones and hypericin derivatives from lichens. Experientia, 52, 180–183.
- Correché ER, Carrasco M, Giannini F, Piovano M, Garbarino J, Enriz D. (2002). Cytotoxic screening activity of secondary lichen metabolites. Acta Farmaceutica Bonaerense, 21, 273–278.
- Coxson DS, Nadkarni NM. (1995). Ecological roles of epiphytes in nutrient cycles of forest ecosystems. In: Lowman MD, Nadkarni NM, ed. Forest Canopies. London, Academic Press, pp. 495–543.
- Davis EW, Yost JA. (1983). Novel hallucinogens from Eastern Ecuador. Harvard University, Bot Museum Leaflets, 29, 291–295.
- Dayan FE, Romagni JG. (2001). Lichens as a potential source of pesticides. Pestic Outlook, 12, 229–232.
- Dembitsky VM, Tolstikov GA. (2003). Halogenated phenol compounds in lichen and fungi. Chem Sustain Develop, 11, 557–565.
- DePriest PT. (2004). Early molecular investigations of lichen-forming symbionts: 1986-2001*. Annu Rev Microbiol, 58, 273–301.
- Lohézic-Le Dévéhat F, Tomasi S, Elix JA, Bernard A, Rouaud I, Uriac P, Boustie J. (2007). Stictic acid derivatives from the lichen Usnea articulata and their antioxidant activities. J Nat Prod, 70, 1218–1220.
- Duh PD, Tu YY, Yen GC. (1999). Antioxidant activity of aqueous extract of harn jyur (Chyrsanthemum morifolium Ramat). Lebensmittel-Wissenchaft Technol, 32, 269–277.
- Durazo FA, Lassman C, Han SH, Saab S, Lee NP, Kawano M, Saggi B, Gordon S, Farmer DG, Yersiz H, Goldstein RL, Ghobrial M, Busuttil RW. (2004). Fulminant liver failure due to usnic acid for weight loss. Am J Gastroenterol, 99, 950–952.
- Einarsdóttir E, Groeneweg J, Björnsdóttir GG, Harethardottir G, Omarsdóttir S, Ingólfsdóttir K, Ogmundsdóttir HM. (2010). Cellular mechanisms of the anticancer effects of the lichen compound usnic acid. Planta Med, 76, 969–974.
- Ekong US, Mgbii AI, Adikwu MU. (2008). Evaluation of the in vitro combination effect of colloidal silver concentrate on the antifungal activity of ethanolic extract of the lichen Usnea subfloridans. Nigerian Annals Nat Sci, 8, 1–5.
- Elix JA. (1996). Biochemistry and secondary metabolites. In: Nash III TH, ed. Lichen Biology. Cambridge, UK: Cambridge University Press, pp. 154–181.
- Eriksson OE, Winka K. (1997). Supraordinal taxa of Ascomycota. Myconet, 1, 1–16.
- Ertl L. (1951). Über die Lichtverhä ltnisse in Laubflecten. Planta, 39, 245–270.
- Esimone CO, Ofokansi KC, Adikwu MU, Ibezim EC, Abonyi DO, Odaibo GN, Olaleye DO. (2007). In vitro evaluation of the antiviral activity of extracts from the lichen Parmelia perlata (L.) Ach. against three RNA viruses. J Infect Dev Ctries, 1, 315–320.
- Evans RD, Johansen JR. (1999). Microbiotic crusts and ecosystem processes. Crit Rev Plant Sci, 18, 183–225.
- Fazio AT, Adler MT, Bertoni MD, Sepúlveda CS, Damonte EB, Maier MS. (2007). Lichen secondary metabolites from the cultured lichen mycobionts of Teloschistes chrysophthalmus and Ramalina celastri and their antiviral activities. Z Naturforsch, C, J Biosci, 62, 543–549.
- Friedl T. (1989). Systematik und Biologie von Trebouxia (Microthamniales, Chlorophyta). als phycobiont der Parmeliaceae (lichenisierte Ascomyceten). Ph.D. Thesis. Bayreuth: Universtät Bayreuth.
- Friedmann EI. (1982). Endolithic microorganisms in the antarctic cold desert. Science, 215, 1045–1053.
- Funasaka Y, Komoto M, Ichihashi M. (2000). Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res, 13 Suppl 8, 170–174.
- Gargas A, Taylor JW. (1995). Phylogeny of Discomycetes and early radiations of the apothecial Ascomycotina inferred from SSU rDNA sequence data. Exp Mycol, 19, 7–15.
- Germanò MP, De Pasquale R, D’Angelo V, Catania S, Silvari V, Costa C. (2002). Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J Agric Food Chem, 50, 1168–1171.
- Gomes AT, Honda NK, Roese FM, Muzzi RM, Marques MR. (2002). Bioactive derivatives obtained from lecanoric acid, a constituent of the lichen Parmotrema tinctorum (Nyl.). Hale (Parmeliaceae). Rev Bras Farmacogn, 12, 74–75.
- González-Tejero MR, Martínez-Lirola MJ, Casares-Porcel M, Molero-Mesa J. (1995). Three lichen used in popular medicine in Eastern Andalucia (Spain). Econ Bot, 49, 96–98.
- Grifin BW. (1990). Peroxidases in Chemistry and Biology, Everse J, Everse KE, Grisham MB, eds, vol. II, Boca Raton: CRC Press, pp. 85–138.
- Gülçin I, Oktay M, Küfrevioglu OI, Aslan A. (2002). Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol, 79, 325–329.
- Gupta VK, Darokar MP, Saikia D, Pal A, Fatima A, Khanuja SPS. (2007) Antimycobacterial activity of lichens. Pharm Biol, 45, 200–204.
- Halama P, Van HC. (2004). Antifungal activity of lichen extracts and lichenic acids. Biocontrol, 49, 95–107.
- Hawksworth, DL. (1988). The fungal partner. In: Galun M, ed. Handbook of Lichennology. vol. I, Boca Raton, Florida, USA: CRC Press, pp. 35–38.
- Luo H, Yamamoto Y, Liu Y, Jung JS, Kahng HY, Koh YJ, Hur JS. (2010). The In vitro antioxidant properties of chinese highland lichens. J Microbiol Biotechnol, 20, 1524–1528.
- Hidalgo ME, Fernández E, Quilhot W, Lissi E. (1994). Antioxidant activity of depsides and depsidones. Phytochemistry, 37, 1585–1587.
- Higuchi M, Miura Y, Boohene J, Kinoshita Y, Yamamoto Y, Yoshimura I, Yamada Y. (1993). Inhibition of tyrosine activity by cultured lichen tissues and bionts. Planta Med, 59, 253–255.
- Hirabayashi K, Iwata S, Ito M, Shigeta S, Narui T, Mori T, Shibata S. (1989). Inhibitory effect of a lichen polysaccharide sulfate, GE-3-S, on the replication of human immunodeficiency virus (HIV) in vitro. Chem Pharm Bull, 37, 2410–2412.
- Hirayama T, Fujikawa F, Kasahara T, Otsuka M, Nishida N, Mizuno D. (1980). [Anti-tumor activities of some lichen products and their degradation products (author’s transl)]. Yakugaku Zasshi, 100, 755–759.
- Honegger R, Haish A. (2001). Immunocytochemical location of the (1→3), (1→4)-β-glucan lichenin in the lichen-forming ascomycete Cetraria islandica (Icelandic moss). New Phytol, 150, 739–746.
- Hosoya M, Balzarini J, Shigeta S, De Clercq E. (1991). Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob Agents Chemother, 35, 2515–2520.
- Huneck S. (1999). The significance of lichens and their metabolites. Naturwissenschaften, 86, 559–570.
- Huneck S. (2001). New results on the chemistry of lichen substances. In: Falk H, Kirby GW, Moore RE, eds. Progress in the Chemistry and Organic Natural Products. New York, USA: Springer-Verlag, Springer, pp. 1–276.
- Hur JS, Kim HJ, Lim KM, Koh YJ. (2003). Isolation, cultivation, and antifungal activity of a lichen-forming fungus. Plant Pathol J, 19, 75–78.
- Ihda TA, Nakano T, Yoshimura I, Iwatsuki Z. (1993). Phycobionts isolated from Japanese species of Anzia (lichenes). Archiv fu¨r Protistenkunde, 143, 163–172.
- Ingólfsdóttir K, Bloomfield SF, Hylands PJ. (1985). In vitro evaluation of the antimicrobial activity of lichen metabolites as potential preservatives. Antimicrob Agents Chemother, 28, 289–292.
- Ingólfsdóttir K, Gudmundsdóttir GF, Ogmundsdóttir HM, Paulus K, Haraldsdóttir S, Kristinsson H, Bauer R. (2002). Effects of tenuiorin and methyl orsellinate from the lichen Peltigera leucophlebia on 5-/15-lipoxygenases and proliferation of malignant cell lines in vitro. Phytomedicine, 9, 654–658.
- Ingolfsdottir K, Hjalmarsdottir MA, Sigurdsson A, Gudjonsdottir GA, Brynjolfsdottir A, Steingrimsson O. (1997). In vitro susceptibility of Helicobacter pylori to protolichesterinic acid from the lichen Cetraria islandica. Antimicrob Agents Chemother, 41, 215–217.
- Ingólfsdóttir K. (2002). Usnic acid. Phytochemistry, 61, 729–736.
- Valencia-Islas N, Zambrano A, Rojas JL. (2007). Ozone reactivity and free radical scavenging behavior of phenolic secondary metabolites in lichens exposed to chronic oxidant air pollution from Mexico City. J Chem Ecol, 33, 1619–1634.
- Hughes JM, Tenover FC. (1997). Approaches to limiting emergence of antimicrobial resistance in bacteria in human populations. Clin Infect Dis, 24 Suppl 1, S131–S135.
- Jayaprakasha GK, Rao LJ. (2000). Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Z Naturforsch, C, J Biosci, 55, 1018–1022.
- Jeon HS, Lökös L, Han KS, Ryu AA, Kim JA, Koh YJ, Hur JS. (2009). Isolation of lichen-forming fungi from Hungarian lichens and their antifungal activity against fungal pathogens of hot pepper anthracnose. Plant Pathol J, 25, 38–46.
- Karagöz A, Aslan A. (2005). Antiviral and cytotoxic activity of some lichen extracts. Biologia Bratislava, 60, 281–86.
- Karagöz A, Doğruöz N, Zeybek Z, Aslan A. (2009). Antibacterial activity of some lichen extracts. J Med Plants Res, 3, 1034–1039.
- Karthikaidevi G, Thirumaran G, Manivannan K, Anantharaman P, Kathiresan K, Balasubaramanian T. (2009). Screening of the antibacterial properties of lichen Roccella belangeriana (awasthi) from Pichavaram mangrove (Rhizophora sp.). Adv Biol Res, 3, 127–131.
- Kekuda TRP, Vinayaka KS, Kumar SVP, Sudharshar SJ. (2009). Antioxidant and antibacterial activity of lichen extracts, honey and their combination. J Pharm Res, 2, 1875–1878.
- Kershaw KA. (1985). Physiological Ecology of Lichens. Cambridge: Cambridge University Press.
- Khanuja SPS, Tiruppadiripuliyur RSK, Gupta VK, Srivastava SK, Verma SC, Saikia D, Darokar MP, Shasany AK, Pal A. (2007). Antimicrobial and anticancer properties of methyl-beta-orcinolcarboxylate from lichen (Everniastrum cirrhatum). US Patent No. 0099993A1.
- Kim MS, Cho HB. (2007). Melanogenesis inhibitory effects of methanolic extracts of Umbilicaria esculenta and Usnea longissima. J Microbiol, 45, 578–582.
- Knudsen K. (2009). Caloplaca obamae, a new species from Santa Rosa Island, California. Opuscula Philolichenum, 6, 37–40.
- Kosanic M, Rankovic B. (2011). Lichens as possible sources of antioxidants. Pak J Pharm Sci, 24, 165–170.
- Kristmundsdóttir T, Aradóttir HA, Ingólfsdóttir K, Ogmundsdóttir HM. (2002). Solubilization of the lichen metabolite (+)-usnic acid for testing in tissue culture. J Pharm Pharmacol, 54, 1447–1452.
- Kumar K, Upreti DK. (2001). Parmelia sp. (lichens) in ancient medicinal plant lore of India. Econ Bot, 55, 458–459.
- Kumar KC, Müller K. (1999). Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism. J Nat Prod, 62, 817–820.
- Kumar SK, Banskota AH, Manandhar MD. (1996). Isolation and identification of some chemical constituents of Parmelia nepalensis. Planta Med, 62, 93–94.
- Ladd D. (2009). Lichens and related fungi of Pine Bluff Arsenal, Arkansas. Opuscula Philolichenum, 7, 101–120.
- Lal BM, Upreti DK. (1995). Ethno botanical notes on three Indian lichens. Lichenologist, 27, 77–99.
- Launert E. (1981). Edible and Medicinal Plants. London: Hamlyn Publisher.
- Lauterwein M, Oethinger M, Belsner K, Peters T, Marre R. (1995). In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (-)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob Agents Chemother, 39, 2541–2543.
- Lawrey JD. (1986). Biological role of lichen substances. Bryologist, 89, 111–122.
- Lawrey JD. (1989). Lichen secondary compounds, evidence for a correspondence between antiherbivore and antimicrobial function. Bryologist, 92, 326–328.
- Lee KA, Kim MS. (2000). Glucosidase inhibitor from Umbilicaria esculenta. Can J Microbiol, 46, 1077–1081.
- Lilly VG, Barnett HL. (1951). Physiology of the Fungi. New York: McGraw-Hill Book Co.
- Lin X, Cai YJ, Li ZX, Chen Q, Liu ZL, Wang R. (2003). Structure determination, apoptosis induction, and telomerase inhibition of CFP-2, a novel lichenin from Cladonia furcata. Biochim Biophys Acta, 1622, 99–108.
- Liu F, Ooi VE, Chang ST. (1997). Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci, 60, 763–771.
- Longton RE. (1988). Biology of Polar Bryophytes and Lichens. Cambridge: Cambridge University Press.
- Lu¨cking R, Bernecker-Lu¨cking A. (2002). Distance, dynamics, and diversity in tropical rainforests, an experimental approach using foliicolous lichens on artificial leaves. I. Growth performance and succession. Ecotropica, 8, 1–13.
- Lücking R, Lawrey JD, Sikaroodi M, Gillevet PM, Chaves JL, Sipman HJ, Bungartz F. (2009). Do lichens domesticate photobionts like farmers domesticate crops? Evidence from a previously unrecognized lineage of filamentous cyanobacteria. Am J Bot, 96, 1409–1418.
- Lumbsch HT, Ahti T, Altermann S, Amo De Paz G, Aptroot A, Arup U, Peña AB, Bawingan PA, Benatti MN, Betancourt L, Björk CR, Boonpragob K, Brand M, Bungartz F, Cáceres MES, Candan M, Chaves JL, Clerc P, Common R, Coppins BJ, Crespo A, Dal-Forno M, Divakar PK, Duya MV, Elix JA, Elvebakk A, Fankhauser JD, Farkas E, Ferraro LI, Fischer E, Galloway DJ, Gaya E, Giralt M, Goward T, Grube M, Hafellner J, Hernández MJE, Campos MDLAH, Kalb K, Kärnefelt I, Kantvilas G, Killmann D, Kirika P, Knudsen K, Komposch H, Kondratyuk S, Lawrey JD, Mangold A, Marcelli MP, Mccune B, Messuti MI, Michlig A, González RM, Moncada B, Naikatini A, Nelsen MP, Øvstedal DO, Palice Z, Papong K, Parnmen S, Pérez-Ortega S, Printzen C, Rico VJ, Plata ER, Robayo J, Rosabal D, Ruprecht U, Allen NS, Sancho L, De Jesus LS, Vieira TS, Schultz M, Seaward MRD, Sérusiaux E, Schmitt I, Sipman HJM, Sohrabi M, Søchting U, Søgaard MZ, Sparrius LB, Spielmann A, Spribille T, Sutjaritturakan J, Thammathaworn A, Thell A, Thor G, Thüs H, Timdal E, Truong C, Türk R, Tenorio LU, Upreti DK, Boom PVD, Rebuelta MV, Wedin M, Will-Wolf S, Wirth V, Wirtz N, Yahr R, Yeshitela K, Ziemmeck F, Wheeler T, Lücking R. (2011). One hundred new species of lichenized fungi, a signature of undiscovered global diversity. In: Phytotaxa. Auckland, New Zealand: Magnolia Press, 18, pp. 1–127.
- Lumbsch HT. (2000). Phylogeny of filamentous ascomycetes. Naturwissenschaften, 87, 335–342.
- Luo H, Yamamoto Y, Jeon HS, Liu YP, Jung JS, Koh YJ, Hur JS. (2011). Production of anti-Helicobacter pylori metabolite by the lichen-forming fungus Nephromopsis pallescens. J Microbiol, 49, 66–70.
- Madamombe IT, Afolayan AJ. (2003). Evaluation of antimicrobial activity of extract from South African Usnea barabata. Pharm Biol, 41, 199–202.
- Maier MS, Gonzalez Marimon DI, Stortz CA, Adler MT. (1999). A revised structure for (-)-dihydropertusaric acid, a gamma-butyrolactone acid from the lichen Punctelia microsticta. J Nat Prod, 62, 1565–1567.
- Malhotra S, Subban R, Singh AP. (2008). Lichens - role in traditional medicine and drug discovery. The Internet J Altern Med, 5, 1–5.
- Manojlovic NT, Solujic S, Sukdolak S, Krstic LJ. (2000). Isolation and antimicrobial activity of anthraquinones from some species of lichen genus Xanthoria. J Serb Chem Soc, 65, 555–560.
- Manojlovic NT, Solujic S, Sukdolak S, Milosev M. (2005). Antifungal activity of Rubia tinctorum, Rhamnus frangula and Caloplaca cerina. Fitoterapia, 76, 244–246.
- Manojlovic NT, Vasiljevic PJ, Gritsanapan W, Supabphol R, Manojlovic I. (2010). Phytochemical and antioxidant studies of Laurera benguelensis growing in Thailand. Biol Res, 43, 169–176.
- Manojlovic NT, Vasiljevic PJ, Marković ZS. (2010). Antimicrobial activity of extracts and various fractions of chloroform extract from the lichen Laurera benguelensis. J Biol Res Thessaloniki, 13, 27–34.
- Martins MCB, Gonçalves de Lima MJ, Silva FP, Ximenes EA, Henrique da Silva N, Pereira EC. (2010). Cladia aggregata (lichen) from Brazilian northeast, chemical characterization and antimicrobial activity. Braz Arch Biol Technol, 53, 115–122.
- Müller K. (2001). Pharmaceutically relevant metabolites from lichens. Appl Microbiol Biotechnol, 56, 9–16.
- Murashige T, Skoog F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–494.
- Nash TH. (1996). Lichen Biology, 2nd edn. Cambridge, UK: Cambridge University Press.
- Neamati N, Hong H, Mazumder A, Wang S, Sunder S, Nicklaus MC, Milne GW, Proksa B, Pommier Y. (1997). Depsides and depsidones as inhibitors of HIV-1 integrase: discovery of novel inhibitors through 3D database searching. J Med Chem, 40, 942–951.
- Neff GW, Reddy KR, Durazo FA, Meyer D, Marrero R, Kaplowitz N. (2004). Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J Hepatol, 41, 1062–1064.
- Negi HR, Kareem A. (1996). Lichens, the unsung heroes. Amruth, 1, 3–6.
- Neidleman SL, Geigert J. (1986). Biohalogenation: Principles, Basic Roles and Applications. New York, USA: Ellis Horwood Ltd, J Wiley and Sons.
- Nimri LF, Meqdam MM, Alkofahi A. (1999). Antibacterial activity of Jordanian medicinal plants. Pharm Biol, 37, 196–201.
- Nolan TJ, Algar J, McCann EP, Manahan WA, Nolan N. (1948). Chemical constituents of lichens found in Ireland. Buellia canescens. III. Constitution of diploicin. Sci Proc R Dublin Soc, 24A, 319–334.
- Odabasoglu F, Aslan A, Cakir A, Suleyman H, Karagoz Y, Bayir Y, Halici M. (2005). Antioxidant activity, reducing power and total phenolic content of some lichen species. Fitoterapia, 76, 216–219.
- Ogmundsdóttir HM, Zoëga GM, Gissurarson SR, Ingólfsdóttir K. (1998). Anti-proliferative effects of lichen-derived inhibitors of 5-lipoxygenase on malignant cell-lines and mitogen-stimulated lymphocytes. J Pharm Pharmacol, 50, 107–115.
- Oh SO, Jeon HS, Lim KM, Koh YJ, Hur JS. (2006). Antifungal activity of lichen-forming fungi isolated from Korean and Chinese lichen species against plant pathogenic fungi. Plant Pathol J, 22, 381–385.
- Okamoto G, Hayase F, Kato H. (1992). Scavenging of active oxygen species by glycated proteins. Biosci Biotechnol Biochem, 56, 928–931.
- Okuyama E, Umeyama K, Yamazaki M, Kinoshita Y, Yamamoto Y. (1995). Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med, 61, 113–115.
- Özen T, Kinalioğlu K. (2008). Determination of antioxidant activity of various extracts of Parmelia saxatilis. Biologia, 63, 211–216.
- Paudel B, Bhattarai HD, Lee JS, Hong SG, Shin HW, Yim JH. (2008). Antioxidant activity of polar lichens from King George Island (Antarctica). Polar Biol, 31, 605–608.
- Pawelek JM, Körner AM. (1982). The biosynthesis of mammalian melanin. Am Sci, 70, 136–145.
- Pengsuparp T, Cai L, Constant H, Fong HH, Lin LZ, Kinghorn AD, Pezzuto JM, Cordell GA, Ingolfsdóttir K, Wagner H. (1995). Mechanistic evaluation of new plant-derived compounds that inhibit HIV-1 reverse transcriptase. J Nat Prod, 58, 1024–1031.
- Pereira J. (1853). The elements of material medica and therapeutics, Vol. 2, 3rd American ed.
- Perez-Llano GA. (1944a). Economic uses of lichens. Econ Bot, 2, 15–45.
- Perez-Llano GA. (1944b). Lichens their biological and economic significance. Bot Rev, 10, 1–65.
- Perry NB, Benn MH, Brennan NJ, Burgess EJ, Ellis G, Galloway DJ, Lorimer SD, Tangney RS. (1999). Antimicrobial, antiviral and cytotoxic activity of New Zealand lichens. Lichenologist, 31, 627–636.
- Praveen-Kumar SV, Prashith-Kekuda TR, Vinayaka KS, Swathi D, Mallikarjun N, Nishanth BC. (2010a). Studies on proximate composition, antifungal and anthelmintic activity of a macrolichen Ramalina hossei H. Magn & G. Awasthi. Int J Biotechnol Biochem, 6, 193–203.
- Praveen-Kumar SV, Prashith-Kekuda TR, Vinayaka KS, Sudharshan SJ, Mallikarjun N, Swathi D. (2010b). Studies on antibacterial, anthelmintic and antioxidant activities of a macrolichen Parmotrema pseudotinctorum (des. Abb.). Hale (Parmeliaceae). from Bhadra wildlife sanctuary, Karnataka. Int J PharmTech Res, 2, 1207–1214.
- Premanathan M, Kathiresan K, Yamamoto N, Nakashima H. (1999). In vitro anti-human immunodeficiency virus activity of polysaccharide from Rhizophora mucronata Poir. Biosci Biotechnol Biochem, 63, 1187–1191.
- Prota G, Thomson RH. (1976). Melanin pigmentation in mammals. Endeavour, 35, 32–38.
- Rambold G, Friedl T, Beck A. (1998). Photobionts in lichens, possible indicators of phylogenetic relationships? Bryologist, 101, 392–397.
- Rambold G, Triebel D. (1992). The inter-lecanoralean associations. Bibliotheca Lichenologica, Band 48, 3–201.
- Rankovic B, Misic M, Sukdolak S. (2007). Evaluation of antimicrobial activity of the lichens Lasallia pustulata, Parmelia sulcata, Umbilicaria crustulosa, and Umbilicaria cylindrica. Mikrobiologiia, 76, 817–821.
- Ranković B, Mišić M. (2008). The antimicrobial activity of the lichen substances of the lichens Cladonia furcata, Ochrolechia androgyna, Parmelia caperata and Parmelia conspresa. Biotechnol Biotechnol Eq, 22, 1013–1016.
- Ranković B, Kosanić M, Sukdolak S. (2010). Antimicrobial activity of some lichens and their components. In: Anninos P, Rossi M, Pham TD, Falugi C, Bussing A, Koukkou M, eds. Recent Advances in Clinical Medicine. Cambridge, UK: World Scientific and Engineering Academy and Society Press, pp. 279–286.
- Reddy VM, O’Sullivan JF, Gangadharam PR. (1999). Antimycobacterial activities of riminophenazines. J Antimicrob Chemother, 43, 615–623.
- Rezanka T, Dembitsky VM. (2006). The colleflaccinosides, two chiral bianthraquinone glycosides with antitumor activity from the lichen Collema flaccidum collected in Israel and Russia. Nat Prod Res, 20, 969–980.
- Richardson DHS. (1988). Medicinal and other economic aspects of lichens. In: Galun M, ed. Handbook of Lichenology. Boca Raton, Florida, USA: CRC Press, pp. 93–108.
- Russo A, Piovano M, Lombardo L, Garbarino J, Cardile V. (2008). Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells. Life Sci, 83, 468–474.
- Russo A, Piovano M, Lombardo L, Vanella L, Cardile V, Garbarino J. (2006). Pannarin inhibits cell growth and induces cell death in human prostate carcinoma DU-145 cells. Anticancer Drugs, 17, 1163–1169.
- Saklani A, Upreti DK. (1992). Folk uses of some lichens in Sikkim. J Ethnopharmacol, 37, 229–233.
- Sánchez-Ferrer A, Rodríguez-López JN, García-Cánovas F, García-Carmona F. (1995). Tyrosinase: A comprehensive review of its mechanism. Biochim Biophys Acta, 1247, 1–11.
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. (1999). Free radical scavenging capacity an inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int, 32, 407–412.
- Sankawa U, Shibuya M, Ebizuka Y, Noguchi H, Kinoshita T, Iitaka Y, Endo A, Kitahara N. (1982). Depside as potent inhibitor of prostaglandin biosynthesis: A new active site model for fatty acid cyclooxygenase. Prostaglandins, 24, 21–34.
- Santiago KAA, Borricano JNC, Canal JN, Marcelo DMA, Perez MCP, dela Cruz TEE. (2010). Antibacterial activities of fruticose lichens collected from selected sites in Luzon Island, Philippines. Philip Sci Lett 3, 18–29.
- Sasaki K, Yoshizaki F. (2002). Nobiletin as a tyrosinase inhibitor from the peel of Citrus fruit. Biol Pharm Bull, 25, 806–808.
- Schinazi RF, Chu CK, Babu JR, Oswald BJ, Saalmann V, Cannon DL, Eriksson BF, Nasr M. (1990). Anthraquinones as a new class of antiviral agents against human immunodeficiency virus. Antiviral Res, 13, 265–272.
- Schmeda-Hirschmann G, Tapia A, Lima B, Pertino M, Sortino M, Zacchino S, Arias AR, Feresin GE. (2008). A new antifungal and antiprotozoal depside from the Andean lichen Protousnea poeppigii. Phytother Res, 22, 349–355.
- Sharnoff SD. (1997). Lichens and people [Online]. Available at: http://www.lichen.com/people.html Accessed on 5 July 2011.
- Shawuti G, Abbas A. (2007). Research progress on biological activities of lichens secondary metabolites. Food Sci J, 28, 624–627.
- Spatafora JW. (1995). Ascomal evolution of filamentous ascomycetes, evidence from molecular data. Can J Bot, 73, 811–815.
- Sre-Indrasutdhi V. (2005). Isolation and optimization of lichenized fungi for bioactive compound screening. Master Thesis. Thailand: Mahidol University.
- Stocker-Wçrgçtter E, Hager A. (2008). Appendix, culture methods for lichens and lichen symbionts, Nash III TH, ed. Lichen Biology. Cambridge: Cambridge University Press, pp. 362–363.
- Stubler D, Buchenauer H. (1996). Antiviral activity of the glucan lichenan (poly-β{1→3, 1→ 4} d-anhydroglucose). 1. Biological activity in tobacco plants. J Phytopathol, 144, 37–43.
- Suzuki S, Hirahara T, Shim kuk J, Horinouchi S, Beppu T. (1992). Purification and properties of thermostable-tyrosinase from an obligately symbiotic thermophile, Symbiobacterium thermophilum. Biosci Biotechnol Biochem, 56, 84–89.
- Swathi D, Suchitha Y, Prashith Kekuda TR, Venugopal TM, Vinayaka KS, Mallikarjun N, Raghavendra HL. (2010). Antimicrobial, antihlmintic and insecticidal activity of a macrolichen Everniastrum cirrhatum (FR.). Hale. Int J Drug Develop Res, 2, 780–789.
- Sydiskis RJ, Owen DG, Lohr JL, Rosler KH, Blomster RN. (1991). Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob Agents Chemother, 35, 2463–2466.
- Takai M, Uehara Y, Beisler JA. (1979). Usnic acid derivatives as potential antineoplastic agents. J Med Chem, 22, 1380–1384.
- Tanahashi T, Takenaka Y, Ikuta Y, Tani K, Nagakura N, Hamada N. (1999). Xanthones from the cultured lichen mycobionts of Pyrenula japonica and Pyrenula pseudobufonia. Phytochemistry, 52, 401–405.
- Tay T, Türk AO, Yilmaz M, Türk H, Kivanç M. (2004). Evaluation of the antimicrobial activity of the acetone extract of the lichen Ramalina farinacea and its (+)-usnic acid, norstictic acid, and protocetraric acid constituents. Z Naturforsch, C, J Biosci, 59, 384–388.
- Tehler A, Wedin M. (2008). Systematics of lichenized fungi. In: Nash III TH, ed. Lichen Biology. Cambridge: Cambridge University Press, pp. 336–352.
- Tokiwano T, Satoh H, Obara T, Hirota H, Yoshizawa Y, Yamamoto Y. (2009). A lichen substance as an antiproliferative compound against HL-60 human leukemia cells: 16-O-Acetyl-leucotylic acid isolated from Myelochroa aurulenta. Biosci Biotechnol Biochem, 73, 2525–2527.
- Türk AÖ, Yılmaza M, Kıvanc M, Türk H. (2003). The antimicrobial activity of extracts of the lichen Cetraria aculeata and its protolichesterinic acid constituent. Z Naturforsch C, 58, 850–854.
- Tvaroska I, Ogawa K, Deslandes Y, Marchessault RH. (1983). Crystalline conformation and structure of lichenan and barley β-glucan. Can J Chem, 61, 1608–1616.
- Varita KO. (1973). Antibiotics in lichens. In: The Lichens. Academic Press New York, pp. 547–561.
- Verma N, Behera BC, Sonone A, Makhija U. (2008). Lipid peroxidation and tyrosinase inhibition by lichen symbionts grown in vitro. African J Biochem Res, 2, 225–231.
- Vinayaka KS, Praveen Kumar SV, Prashith Kekuda TR, Krishnamurthy YL, Mallikarjun N, Swathi D. (2009). Proximate pomposition, antioxidant, anthelmintic and insecticidal activity of a macrolichen Ramalina conduplicans Vain. (Ramalinaceae). Eur J Appl Sci, 1, 40–46.
- Wei X, Jeon HS, Han KS, Koh YJ, Hur JS. (2008). Antifungal activity of lichen-forming fungi against Colletotrichum acutatum on hot pepper. Plant Pathol J, 24, 202–206.
- Wirth V. (1995). Die Flechten Baden-Wu¨rtembergs. Verbreitungsatlas. Vols. I and II. Stuttgart, Eugen Ulmer.
- Wood S, Huffman J, Weber N, Andersen D, North J, Murray B, Sidwell R, Hughes B. (1990). Antiviral activity of naturally occurring anthraquinones and anthraquinone derivatives. Planta Med, 56, 65–52.
- Yamamoto Y, Kinoshita Y, Kurokawa T, Yoshimura I, Ahmadjian V, Yamada Y. (1995). Cell growth and pigment production in suspension cultures of a mycobiont isolated from the lichen Cladonia cristatella. Can J Bot, 73, 590–594.
- Yamamoto Y, Kinoshita Y, Matsubara H. (1998). Screening of biological activities and isolation of biological active compounds from lichens. Recent Res Develop Phytochem, 2, 23–34.
- Yamamoto Y. (1990). Studies of Cell Aggregates and the Production of Natural Pigments in Plant Cell Culture. Osaka: Nippon Paint Publication.
- Yamamoto Y. (2000). Screening of biological activities and isolation of biological-active compounds from lichens. Chem Regulation of plants (Shokubutsu no kagaku chosetu), 35, 169–179.
- Yilmaz M, Türk AO, Tay T, Kivanç M. (2004). The antimicrobial activity of extracts of the lichen Cladonia foliacea and its (-)-usnic acid, atranorin, and fumarprotocetraric acid constituents. Z Naturforsch, C, J Biosci, 59, 249–254.
- Yucel O, Odabasoglu F, Gullue M, Çalik ZZ, Çakir A, Aslan A, Yazici K, Halici M. (2007). Antioxidant and antimicrobial properties of a lichen species, Cladoniaragirmis growing in Turkey. Turkish J Pharm Sci 4, 101–109.
- Zambare VP, Zambare AV, Christopher LP. (2010). Antioxidant and antibacterial activity of extracts from lichen Xanthoparmelia somloensis, native to the black hills, South Dakota, USA. Int J Med Sci Technol, 3, 46–51.
- Zeytinoglu H, Incesu Z, Tuylu BA, Turk A, Barutca B. (2004). The antimutagenic and antimitotic properties of the extract of the lichen Cetraria aculeata. Toxicol Appl Pharma, 197, 296–296.