Abstract
Context: Eucalyptus has been a source of a number of biologically active compounds. The anti-leishmanial activity of terpenoids from Eucalyptus loxophleba (Benth.) ssp. lissophloia (Myrtaceae) has not yet been investigated.
Objective: Isolation of the terpenoidal constituents for evaluation of in vitro anti-leishmanial activity against the Leishmania donovani (Dd8 strain) promastigotes.
Materials and methods: The chloroform–methanol (8:2) extract of dried leaves of Eucalyptus loxophleba was used to isolate terpenoidal constituents employing solvent partitioning, column chromatography and preparative high performance liquid chromatography and characterized from spectral data. The anti-leishmanial activity of the isolated compounds was tested in vitro using an Alamar blue assay against a culture of L. donovani (Dd8 strain) promastigotes.
Results: Two new naturally occurring triterpenes, named loxanic acid and 3-acetyl loxanic acid together with four known ursane triterpenoids and one bis-monoterpene glycoside, cuniloside B isolated from the leaves showed anti-leishmanial activity (IC50 133 to 235 μM) against the promastigotes of the tested strain.
Conclusion: The terpenes isolated from the leaves of E. loxophleba showed moderate anti-leishmanial activity.
Introduction
Eucalyptus (Myrtaceae), contains many species exhibiting a wide range of biological activities. In Australia, some species [particularly a group known as oil mallees including Eucalyptus loxophleba (Benth.) ssp. lissophloia] are being promoted as a means to control dryland salinity and if valuable natural products could be produced from these species, then it would help promote their use for addressing land degradation (CitationBell et al., 2001). Apart from mono- and sesquiterpene constituents of essential oils which are particularly rich in the leaves of oil mallees, an array of secondary metabolites with diverse biological and pharmacological activities have been reported from Eucalyptus (CitationGhisalberti, 1996).
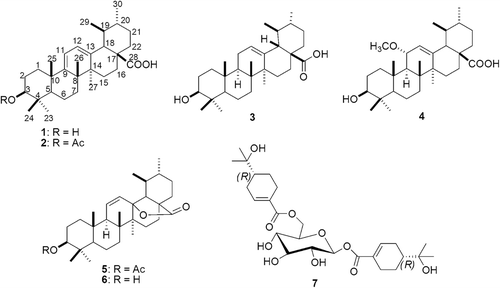
Recently, we have reported large scale isolation process for sideroxylonals and isolation of several other formylated phloroglucinol compounds from this species (CitationSidana et al., 2010, Citation2011). In continuation of our study on the chemical constituents of E. loxophleba, we now report the isolation and structure elucidation of two new triterpenoids, loxanic acid (1) and 3-acetyl loxanic acid (2) from chloroform–methanol (8:2) extract of E. loxophleba leaves together with four other triterpenoids namely ursolic acid (3) (CitationMoghaddam et al., 2007), robustanic acid (4) (CitationKhare et al., 2002), ursolic acid lactone acetate (5) (CitationKatai et al., 1983), ursolic acid lactone (6) (CitationHongcheng & Fujimoto, 1993; CitationSavian et al., 1988) and a bis-monoterpene glycoside, cuniloside B (7) (CitationHakki et al., 2010) ().
Materials and methods
General
All the solvents used for extraction were of analytical grade. High performance liquid chromatography (HPLC) grade methanol (JT Baker), ultra pure water (Elga®) and acetic acid were used for sample preparation and in HPLC mobile phases. The HPLC analysis was carried out on Phenomenex C18 column (250 × 4.6 mm) connected to a Shimadzu HPLC system consisting of a model LC-10AT VP fitted with a SIL-20AC autosampler and SPD-M10A VP photodiode array detector. Princeton SPHER-C18 column (250 × 10 mm, 5 μ) was used for preparative isolation of compounds.
All chromatographic purifications were performed on silica gel #60–120 and silica gel G and GF 254 from CDH India whereas all thin layer chromatography (TLC) (silica gel) development was performed on silica gel coated plates (Merck Kieselgel 60 F254, 0.2 mm thickness). IR spectra of neat samples were taken on an FT-IR spectrometer (Nicolet, USA). Mass spectra were recorded on GCMS-QS (Shimadzu, Japan) or an LCMS (Waters, USA.) in APCI mode. 1H and 13C NMR spectra were recorded on 400 and 100 MHz spectrometers (Bruker), respectively. Samples were dissolved in CDCl3 or C5D5N and tetramethylsilane (TMS) was used as an internal standard.
Plant material
The plant material was collected in March 2004 from a provenance trial of E. loxophleba ssp. lissophloia growing at Toolbin Western Australia managed by the Western Australian Department of Environment and Conservation and authenticated by Dr Peter Grayling. A voucher specimen has been deposited in the Gauba Herbarium at the Australian National University (WJF 09/03).
Extraction and isolation
The shade-dried leaves (10 kg) of E. loxophleba were coarsely ground and extracted with chloroform–methanol (8:2) in a Soxhlet extractor for 48 h. The crude extract (2.7 kg) obtained after evaporation of solvent in vacuo was used to isolate 180 g of a mixture of the three sideroxylonals (A, B and C) (CitationSidana et al., 2010). The mother liquor was used for further phytochemical investigations. A portion (500 g) of this mother liquor was subjected to solvent–solvent partitioning to yield chloroform (200 g) and ethyl acetate (76 g) soluble fractions. The chloroform fraction (50 g) was loaded on a vacuum liquid chromatography assembly, set by packing 600 g of silica gel G in a G-4 sintered glass funnel of 1 L capacity to obtain a column bed height of 8 cm and i.d. of 13 cm. The column was eluted with hexane–ethyl acetate and then with chloroform–methanol gradients to obtain fractions A to N pooled on basis of TLC. Fractions I and J (5.0 g) eluted with 30 to 50% ethyl acetate in hexane were found to be rich in terpenoidal constituents (blue–violet spots on normal phase silica gel TLC on charring with anisaldehyde–sulphuric acid reagent). The pooled fractions I and J were subjected to charcoal treatment to remove the coloring pigments and the enriched fraction so obtained was further sub-divided into individual components by prep-TLC over silica gel G and GF254 using chloroform–methanol (29:1) as solvent. The individual compounds were finally purified by semi-preparative HPLC using methanol–water–acetic acid (90:9:1) to afford 1 (18 mg), 3 (21 mg) and 4 (55 mg).
Fraction E (300 mg) was subjected to further fractionation by vacuum liquid chromatography over octadecyl silica. The column was eluted with a methanol–water gradient (50 to 100% methanol) to yield ten sub-fractions. The fractions revealing blue/violet spots after silica gel TLC (chloroform–methanol, 29:1) with anisaldehyde–sulphuric acid reagent were pooled and subjected to prep-HPLC (Column: C18, Luna, 250 × 30 mm, Mobile phase: MeOH: Water (2% AcOH) (90:10), Flow rate: 40 mL/min, Detection: 225 nm) to yield four sub-fractions. The first sub-fraction yielded compound 5 (51 mg) as colorless needles. The second sub-fraction was further purified by preparative TLC over silica gel G + GF254 using chloroform: methanol:acetic acid (19:0.5:0.5) as solvent to yield compounds 2 (18 mg) and 6 (21 mg).
Fractions L (7.9 g) and M (12.0 g) were separately re-chromatographed over silica gel (#60–120) using a hexane–ethyl acetate gradient. The polar sub-fractions of fractions L and M (showing grayish–black spots on silica gel TLC after charring with 10% sulphuric acid–methanol) were pooled and subjected to chromatography on Sephadex LH-20 using methanol as an eluent. Compound 7 (460 mg) was separated from sugar components and re-crystallized from methanol.
Loxanic acid (1)
Light brown powder, [α]D20 + 307.7° (c 0.05, CHCl3) IR νmax 3434, 2929, 2862, 2623, 1690, 1457, 1374, 1252, 1033 cm−1; APCI-MS: m/z 437, 391, 203; 1H and 13C NMR data are presented in .
Table 1. 1H and 13C NMR data of compounds 1 (in C5D5N) and 2 (in CDCl3).a
3-Acetyl-loxanic acid (2)
Light brown powder, [α]D20 + 171.8° (c 0.05, CHCl3) IR νmax 3267, 2927, 2865, 1723, 1632, 1456, 1372, 1275, 1221, 1165, 1141 cm−1; APCI-MS: m/z 437, 391; 1H and 13C NMR data are presented in .
Synthesis of 1 from 6
Ursolic acid lactone (6) (5 mg) was adsorbed on silica gel (25 mg, 100-200 mesh). The mixture was heated in an oven at 160°C for 3.5 h (CitationDayal, 1990). The product was identical to 1 by TLC. Similarly, the reaction of ursolic acid lactone acetate (5) under the same conditions gave 2 by TLC.
In vitro anti-leishmanial activity and cytotoxicity
Anti-leishmanial activity of the isolated compounds was tested in vitro using Alamar blue assay against a culture of L. donovani (Dd8 strain) promastigotes grown in phenol red free RPMI-1640 (Sigma, USA), supplemented with 10% fetal calf serum (Sigma, St. Louis, MO, USA) at 26°C. L. donovani (1 × 105 cells/mL) promastigotes from logarithmic phase culture were grown in 96-well plate for 48 h before treatment with compounds. Dilutions were prepared in dimethyl sulphoxide and concentration (75–300 µM) of each compound was used in triplicate. The standard Miltefosine (hexadecyl phosphocholine) was used at reported IC50 value (CitationSidana et al., 2011; CitationVermeersch et al., 2009). After treatment with compounds, the plate was kept at 26°C for 48 h. After this incubation, 20 µl of Alamar–Blue reagent (Invitrogen) was added and kept for 5 h at 37°C. Thereafter, absorbance was measured at 570 and 600 nm. The concentration of compounds that produced 50% reduction in growth (IC50) of promastigotes as compared to control (untreated) was determined (CitationMikus & Steverding, 2000; CitationOrdonez-Gutierrez et al., 2007).
In vitro cytotoxicity of the active compounds was determined against PBMC (peripheral blood mononuclear cells) separated from heparinized blood of a normal healthy individual by Ficoll–Hypaque (Sigma, USA) density gradient centrifugation. Alamar blue was used for in vitro cytotoxicity assessment. Briefly, the assay was performed in 96-well tissue culture plates. Cells were seeded to the wells of 96-well plate (1 × 105 cells/well) and were exposed to compounds (IC50 concentration and twice the concentration of IC50 were used) in triplicate. After treatment with compounds, the plate was kept at 37°C for 48 h. After this incubation, 20 µL of Alamar-Blue reagent (Invitrogen) was added and kept for 5 h at 37°C. Thereafter, absorbance was measured at 570 and 600 nm. The mean percentage of cytotoxicity was calculated relative to control (unexposed to compounds) (CitationMikus & Steverding, 2000; CitationOrdonez-Gutierrez et al., 2007).
Results and discussion
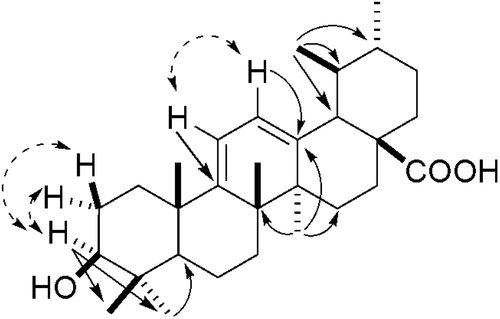
The chloroform–methanol extract of E. loxophleba leaves was fractionated by vacuum liquid chromatography followed by open column chromatography over silica gel to afford a terpenoid-rich fraction that was further sub-fractionated by preparative TLC over silica gel G and GF254 followed by semi-preparative HPLC to afford compound 1 along with two other known compounds. Compound 1 exhibited a [M-OH]+ fragment ion at m/z 437. Its molecular formula C30H46O3 was confirmed by combined application of 1H and 13C NMR (DEPT). Its UV spectrum showed an absorption band at 281 nm indicating the presence of conjugation in the molecule (homoannular diene system). The 1H and 13C NMR data of 1 indicated that it is an ursane type of triterpenoid. The 1H NMR spectrum showed five tertiary methyl signals at δ 1.33, 1.28, 1.22, 1.14 and 1.07 and two secondary methyl signals at δ 1.01 (d, J = 6.4 Hz) and 0.95 (d, J = 6.3 Hz). A downfield triplet accounting for one proton centered at δ 3.49 in 1H NMR spectrum indicated the presence of a hydroxyl group at C-3. The stereochemistry of the hydroxyl group was assigned to be β on the basis of the observed chemical shift and coupling constant (J = 8.3 Hz, suggesting axial α-H). The 1H NMR further showed two olefinic one-proton doublets at δ 5.79 (d, J = 5.8 Hz) and 5.75 (d, J = 5.8 Hz) correlated with δC 124.5 (C-12) and 116.8 (C-11), respectively. The correlations of H-11 with C-8 (42.4), C-10 (40.8) and C-13 (141.9) and H-12 with C-9 (156.9), C-14 (44.4) and C-18 (53.2) in hetero-nuclear multiple bond correlation (HMBC) () spectrum confirmed the presence of a cisoid diene at C-9 (11):12 in 1. Another one proton doublet at δH 2.76 (J = 6.0) δC 53.2 showed HMBC correlation with C-12 (δ 124.5). The quaternary carbon δ 181.2 supported the presence of a carboxyl moiety and was located at C-17 on the basis of comparison of the chemical shift value with those of structurally similar triterpenes (CitationIkuta et al., 2003; CitationBegum et al., 1997). The 1H and 13C NMR values and HMBC correlations coincided with those of eucalyptanoic acid except for the two secondary methyl groups in the spectrum (CitationBegum et al., 2002). Thus, compound 1 was established as 3β-hydroxyursa-9(11), 12-dien-28-oic acid. This is the first report of the isolation of 1 from a natural source though it has been earlier synthesized from ursolic acid lactone (CitationDayal, 1990). The structure was also confirmed by its synthesis from ursolic acid lactone (6).
The spectroscopic data of compound 2 was very similar to that of compound 1. The molecular mass, i.e., 496 [for molecular formula C32H48O4, as deduced by 1H and 13C NMR (DEPT)] of compound 2 was 42 units more than that of compound 1. This indicated that 2 is an acetyl derivative of 1. The IR spectrum of 2 displayed carboxyl (3267–2865 cm−1), and olefinic (1632 cm−1) absorption bands. The 1H NMR showed a three proton singlet at δ 2.07 correlating to carbon resonance at δ 21.3 (CH3CO) in HMQC and δ 171.1 (CH3CO) in HMBC. In 1H NMR spectrum, the triplet for 3α-H was shifted downfield to δ 4.50 as compared with δ 3.49 in the case of compound 1. Based on the above observations the acetyl group was placed at position 3 of ursa-9(11), 12-dien-28-oic acid skeleton and compound 2 was characterized as 3β-acetoxyursa-9(11), 12-dien-28-oic acid. The structure and stereochemistry of 2 were confirmed by its synthesis from ursolic acid lactone acetate (5) following the procedure of CitationDayal (1990).
In vitro anti-leishmanial activity and cytotoxicity
Compounds 1–7 were tested for anti-leishmanial activity in vitro against L. donovani (Dd8 strain) promastigotes (). Loxanic acid, 3-acetyl loxanic acid, ursolic acid lactone and ursolic acid lactone acetate showed moderate anti-leishmanial activity. The active compounds were subjected to in vitro cytotoxicity assay and were found to be non-cytotoxic towards PBMCs.
Table 2. In vitro anti-leishmanial activity and cytotoxicity assay of compounds 1–7.
Conclusion
Two new naturally occurring triterpenes, named loxanic acid and 3-acetyl loxanic acid, were isolated from the chloroform–methanol (8:2) extract of the leaves of Eucalyptus loxophleba ssp. lissophloia, together with four known compounds namely ursolic acid, robustanic acid, ursolic acid lactone, ursolic acid lactone acetate and cuniloside B. Compounds 1, 2, 5, and 6 showed moderate activity against promastigotes of Leishmania donovanii.
Acknowledgement
The authors are thankful to the director, NIPER for support. We also thank Mr. John Bartle, Dr. Richard Mazenec and Dr. Peter Grayling for assistance in collecting the Eucalyptus loxophleba leaf. Funding was provided by the Australian Government, Rural Industries Research and Development Corporation to WJF.
Declaration of interest: Authors declare no conflict of interest.
References
- Begum S, Farhat Siddiqui, BS. (1997). Triterpenoids from the leaves of Eucalyptus camaldulensis var. obtusa. J Nat Prod, 60, 20–23.
- Begum S, Sultana I, Siddiqui BS, Shaheen F, Gilani AH. (2002). Structure and spasmolytic activity of eucalyptanoic acid from Eucalyptus camaldulensis var. obtusa and synthesis of its active derivative from oleanolic acid. J Nat Prod, 65, 1939–1941.
- Bell SJ, Barton AFM, Stocker LJ. (2001). Agriculture for health and profit in Western Australia: The Western Oil Mallee Project. Ecosyst Health, 7, 116–121.
- Dayal R. (1990). Two new compounds from ursolic acid lactone. Indian J Chem B Org, 29, 156–157.
- Ghisalberti EL. (1996). Bioactive acylphloroglucinol derivatives from Eucalyptus species. Phytochemistry, 41, 7–22.
- Hakki Z, Cao B, Heskes AM, Goodger JQ, Woodrow IE, Williams SJ. (2010). Synthesis of the monoterpenoid esters cypellocarpin C and cuniloside B and evidence for their widespread occurrence in Eucalyptus. Carbohydr Res, 345, 2079–2084.
- Hongcheng W, Fujimoto Y. (1993). Triterpene esters from Eucalyptus tereticornis. Phytochemitry, 33, 151–153.
- Ikuta A, Tomiyasu H, Morita Y, Yoshimura K. (2003). Ursane- and oleanane-type triterpenes from Ternstroemia gymnanthera callus tissues. J Nat Prod, 66, 1051–1054.
- Katai M, Terai T, Meguri H. (1983). Triterpenoids of the bark of Pieris japonica D. DON (Japanese name: Asebi). II. 13C nuclear magnetic resonance of the γ-lactones of ursane- and oleanane-type triterpenoids. Chem Pharm Bull, 31, 1567–1571.
- Khare M, Srivastava SK, Singh AK. (2002). A new triterpenic acid from Eucalyptus robusta. Indian J Chem, 41B, 440–445.
- Mikus J, Steverding D. (2000). A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar blue. Parasitol Int, 48, 265–269.
- Moghaddam FM, Farimani MM, Salahvarzi S, Amin G. (2007). Chemical constituents of dichloromethane extract of cultivated Satureja khuzistanica. Evid Based Complement Alternat Med, 4, 95–98.
- Ordonez-Gutierrez L, Espada-Fernandez R, Auxiliadora DM, Jose TJ, Bolas-Fernandez F, Maria AJ. (2007). In vitro effect of new formulations of amphotericin A on amastigote and promastigotes forms of Leishmania infantum. Int J Antimicrobial Agents, 30, 325–329.
- Savian AA, Sokolskaya T, Zakharov VF. (1988). 11, 12-Dehydroursolic acid lactone from leaves of Eucalyptus viminalis. Chem Nat Comp, 24, 253–254.
- Sidana J, Rohilla RK, Roy N, Barrow RA, Foley WJ, Singh IP. (2010). Antibacterial sideroxylonals and loxophlebal A from Eucalyptus loxophleba foliage. Fitoterapia, 81, 878–883.
- Sidana J, Singh S, Arora SK, Foley WJ, Singh IP. (2011). Formylated phloroglucinols from Eucalyptus loxophleba foliage. Fitoterapia, 82, 1118–1122.
- Vermeersch M, da Luz RI, Toté K, Timmermans JP, Cos P, Maes L. (2009). In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: Practical relevance of stage-specific differences. Antimicrob Agents Chemother, 53, 3855–3859.