Abstract
Context: Prenylated caged xanthones are “privileged structure” characterized by the presence of the unusual 4-oxo-tricyclo[4.3.1.03,7]dec-8-en-2-one scaffold. The natural sources of these compounds confines mainly in the Garcinia genus in the family of Guttiferae. Gambogic acid is the most abundant substance and most of the studies have been done on this compound, particularly as a new potential antitumor agent. The history, sources, structural diversity, and biological activities of these compounds are covered.
Objective: This review is written with the intention to provide additional aspects from what have been published of prenylated caged xanthones, including history, sources, structural diversity, and biological activities.
Methods: This review has been compiled using information from a number of reliable references mainly from major databases including SciFinder, ScienceDirect, and PubMed.
Results: More than 120 prenylated caged xanthones have been found in the plant genera Garcinia, Cratoxylum, and Dascymaschalon. These compounds exhibited various potentially useful biological activities such as anticancer, anti-HIV-1, antibacterial, anti-inflammatory, and neurotrophic activities.
Conclusions: Prenylated caged xanthones, both naturally occurring and synthetic analogues, have been identified as promising bioactive compounds, especially for anticancer agents. Gambogic acid has been demonstrated to be a highly valuable lead compound for antitumor chemotherapy. The structure activity relationship (SAR) study of its analogues is still the subject of intensive research. Apoptosis cytotoxic mechanism has been identified as the major pathway. Research on the delineation of the in-depth mechanism of action is still on-going. Analogues of gambogic acid had been identified to be effective against a rare and special form of liver cancer, cholangiocarcinoma for which currently there is no chemotherapeutic treatment available.
Introduction
The special class of xanthones, prenylated caged xanthones, is emerging as a new class of both naturally occurring and synthetic analogues that have been identified as promising bioactive compounds with wide ranging and potentially useful biological activities such as anticancer, anti-HIV-1, anti-inflammatory, antibacterial, and neurotrophic activities. Reviews covering aspects of these compounds have been published (CitationChantarasriwong et al., 2010; CitationEl-Seedi et al., 2010; CitationHan & Xu, 2009). The information in this review is intended to supplement the previous information and highlight novel features of these class of compounds; the major publications up until June 2011 are included. It should be emphasized at the outset that the key structural feature that is crucial for biological activities of these compounds is the unique 4-oxo-tricyclo[4.3.1.03,7]dec-8-en-2-one scaffold, containing a highly substituted tetrahydrofuran ring with three quaternary carbons (CitationRen et al., 2011a). The early history of this class of compounds is from the resin of two species of trees Garcinia morella (Gaertn.) Desr. and Garcinia hanburyi Hook. f. (Guttiferae). G. morella is found mainly on the Indian subcontinent and the research work in this area was first carried out on this species found in India. G. hanburyi, which can grow up to the height of 50 feet, is found in the tropical rain forest of Southern China, Cambodia, Thailand, and part of Malaysia. The trade of the resin in the form of the orange–yellow gum, known as “gamboge” is derived almost solely from G. hanburyi originated from Thailand and Cambodia. The resin is collected by making an incision on the bark and the resin is collected into a hollowed bamboo container. The cultivation of the resin is similar to the present-day cultivation of the rubber latex. The gamboge was used for lowering fever and the treatment of tapeworm. It was also used for cloth dying due to its bright orange color. The gamboge was first introduced into England by the Dutch about the middle of 17th century; it is highly esteem as a pigment. It is official in the U.S. Pharmacopoeia (CitationGrieve, 1931). Before the Second World War, the trade of gamboge was centered in Chanthaburi Province in Thailand and Koh Kong (now part of Cambodia). Subsequent research has demonstrated that the gamboge is one of the important main sources of the prenylated caged xanthone and in particular, gambogic acid ().
Biosynthesis
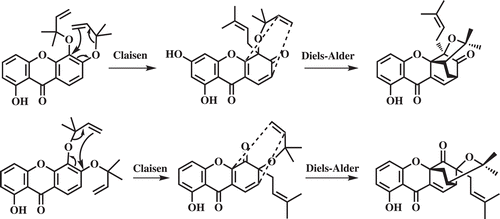
The biosynthesis of normal xanthones has been well studied (CitationEl-Seedi et al., 2010). The xanthone carbon array of the naturally occurring caged xanthone is proposed to be derived from the common benzophenone intermediates of a mixed shikimate and acetate pathway. The widely accepted biosynthetic pathway of the caged scaffold is that proposed in 1971 by Quillinan and Scheinmann involved a Claisen rearrangement followed by intramolecular Diels-Alder reaction as shown in (CitationQuillinan & Scheinmann, 1971). The Claisen rearrangement was not regioselective, allylation can occur at both C5 and C6 to give two intermediates which subsequently generated two different caged scaffold products (CitationThoison et al., 2000). The proposal was substantiated about 30 years later by biomimetic synthetic studies by CitationNicolaou and Li (2001), and Theodorakis and coworkers (CitationTisdale et al., 2004).
Sources and structure diversity of prenylated caged xanthones
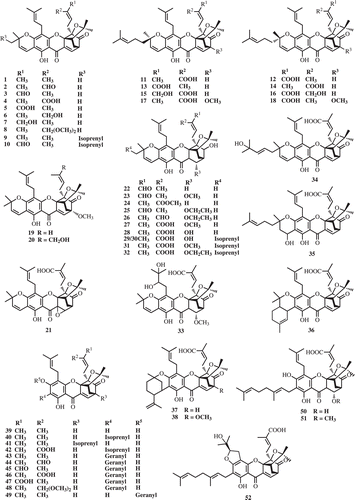
The rich sources of prenylated caged xanthones are the plants in the family Gutttiferae, confined mainly in the genus Garcinia such as G. morella, G. hanburyi, G. forbesii King, G. gaudichaudii Planch. & Triana, G. scortechinii King, G. bracteata C. Y. Wu ex Y. H. Li, G. cantleyana Whitmore, G. lateriflora Blume and G. urophylla Scort. ex King. Moreover, this type of compound is also found in Cratoxylum cochinchinense (Lour.) Bl. Interestingly, only one prenylated caged xanthone was found in a plant, Dascymaschalon sootepense Craib, which belong to the Annonaceae family. Since 1937, approximately 120 caged xanthones have been reported. represents the compounds isolated from the major sources.
Table 1. Natural sources of prenylated caged xanthones.
Figure 6. Prenylated caged xanthones from G. cantleyana, G. lateriflora, G. urophylla, and Cratoxylum cochinchinense.
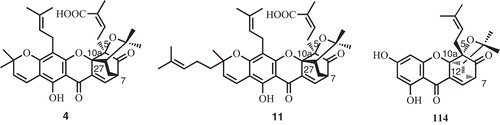
Morellin (2) was the first caged xanthone which was isolated from an alcoholic extract of the pericarp of the seeds of G. morella in 1937 (CitationRao, 1937). However, the right structure of morellin was revealed in 1963, determined by the NMR spectroscopic and x-ray crystallographic studies of its p-bromobenzenesulphonyl ester (CitationKartha et al., 1963). The other caged xanthones which were further isolated from the seeds are desoxymorellin (1), dihydroisomorellin (22) (CitationBhat et al., 1964), morellinol (6) (CitationAdawadkar et al., 1976), moreollin (25), and isomoreollin (26) (CitationSubba et al., 1978). The resin of G. morella were also investigated and found that it contained isomorellin (3) (CitationNair et al., 1964), morellic acid (4), and isomorellic acid (5) (CitationKaranjgaonkar et al., 1966).
Garcinia hanburyi has been reported as a rich source of cytotoxic caged xanthones. Its orange gum-resin from stem bark, gamboge, is used in Thai traditional medicine as a purgative and an anthelmintic and in the treatment of chronic dermatitis, hemorrhoids, and fresh and infected wounds. A major constituent in gamboge is gambogic acid (11), the chemical structure of which was reported by CitationOllis et al. (1965). Gambogic acid was found not only in the resin but also the whole plant (CitationLu et al., 1984) and fruits (CitationReutrakul et al., 2007). More than 40 caged xanthones were discovered from the resin of this plant as shown in and .
Isomorellinol (7), gambogic acid (11), and isogambogic acid (13) were found to be biologically active against KB (ED50 = 0.7, 0.9, and 2.3 μg/mL, respectively) and drug-resistant KB-V1 cell lines (ED50 = 2.3, 3.0, and 3.1 μg/mL, respectively; CitationLin et al., 1993).
Fifteen caged xanthones, namely, desoxymorellin (1), isomorellin (3), morellic acid (4), morellin dimethyl acetal (8), gambogin (9), gambogic acid (11), isomoreollin B (23), moreollic acid (27), gambogellic acid (37), hanburin (41), desoxygambogenin (43), gambogenin (44), isogambogenin (45), gambogenic acid (46), and gambogenin dimethyl acetal (48) showed the cytotoxicities against HeLa and HEL cells. Desoxymorellin (1) showed highest activity at 0.39 μg/mL (CitationAsano et al., 1996).
Caged xanthones derivatives, namely desoxymorellin (1), morellic acid (4), isomorellic acid (5), isomorellinol (7), gambogic acid (11), epigambogic acid (12), isogambogic acid (13), hydroxygambogic acid (15), hydroxyepigambogic acid (16), gambogoic acid A (31), gambogoic acid B (32), desoxygaudichaudione A (40), gaudichaudic acid (42), desoxygambogenin (43), gambogenic acid (46) and isogambogenic acid (47) showed cytotoxicities against human leukemia K562 (K562/S) and doxorubicin-resistant K562 (K562/R) cell lines (CitationHan et al., 2006a, Citation2006b, Citation2006c).
Deoxymorellin (1), morellic acid (4), isomorellic acid (5) isomorellinol (7), gambogic acid (11), and isogambogic acid (13) exhibited cytotoxicity against six human cancer cell lines, MCF-7 (breast cancer), HT-29 (colon cancer), HL-60 (leukemia), Hep G2 (lever cell), A549 (lung cell), and normal human umbilical venal epithelial cell (HUVERC; CitationLee & Chen, 2006).
In recent reports, new 16 cytotoxic prenyllated caged xanthones, gambogic aldehyde (10), 7-methoxygambogic acid (17), 7-methoxyepigambogic acid (18), 7-methoxyisomorellinol (20), methyl 8,8a-dihydromorellate (24), 8,8a-dihydro-8-hydroxymorellic acid (28), 8,8a-dihydro-8-hydroxygambogic acid (29/30), oxygambogic acid (34), gambogefic acid (36), 7-methoxygambogellic acid (38), isogambogenic acid (47), 3-O-geranylforbesione (49), 8,8a-dihydro-8-hydroxygambogenic acid (50), 10-methoxygambogenic acid (51), gambogenific acid (52) were reported from the resin of G. hanburyi. These caged xanthones showed cytotoxicity against many of cancer cell lines such as HL-60, SMMC-7221, BGC-83, P-388, P388/ADR, and HeLa cells (CitationFeng et al., 2007; CitationTao et al., 2009; CitationWang et al., 2008a, Citation2008b).
Five caged xanthones, namely isomorellin (3), morellic acid (4), isomoreollin B (23), moreollic acid (27), and hanburione (33) were found from the fruits of G. hanburyi. Among those, morellic acid (4) was found to exhibit moderately antibacterial activity against methicillin-resistant Staphylococcus aureus with a MIC value of 25 μg/mL (CitationSupondma et al., 2005a).
Twelve caged xanthones, desoxymorellin (1), isomorellin (3), morellic acid (4), isomorellinol (7), gambogic acid (11), 7-methyoxydesoxymorellin (19), dihydroisomorellin (22), 8,8a-epoxymorellic acid (21), forbesione (39), hanburin (41), 2-isoprenylforbesione or desoxyqaudichaudione A (40) and desoxygambogenin (43), were reported from ethyl acetate extracts of the resin and fruits. Most of the isolated compounds showed significant cytotoxicity against a panel of mammalian cancer cell lines, P-388, KB, Col-2, BCA-1, Lu-1, and ASK, as well as some of them exhibited anti-HIV-1 activities (CitationReutrakul et al., 2007). This publication represents the first report on the anti-HIV-1 activity of prenylated caged xanthones. These compounds also exhibited significance anti-inflammatory activity in the rat ears edema model (Reutrakul et al., unpublished results).
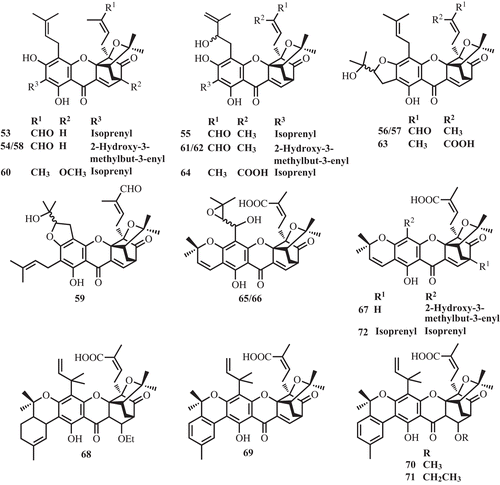
The phytochemical study of the chloroform extract of the leaves of G. gaudichaudii, the Malaysian medicinal plant, led to the isolation of 15 isoprenylated caged xanthones, namely morellic acid (4), forbesione (39), gaudichaudiones A–H (53–60), gaudichaudiones I-J (61–62), and gaudichaudiic acids A–E (63–67). Compounds 53–59 were evaluated for cytotoxicity against a panel of several cancer cell lines (P388, WEHI1640, MOLT4, HePG2 and LL/2). Most of them showed potent activities to all tested cell lines except for gaudichaudiic acid B, C (CitationCao et al., 1998a, Citation1998b; CitationWu et al., 2000). Moreover, gaudichaudiic acids F-I (68–71) (CitationXu et al., 2000), 7-isoprenylmorellic acid (72) (CitationWu et al., 2001), the polyprenylated heptacyclic xanthonoids, were reported from the bark of G. gaudichaudii ().
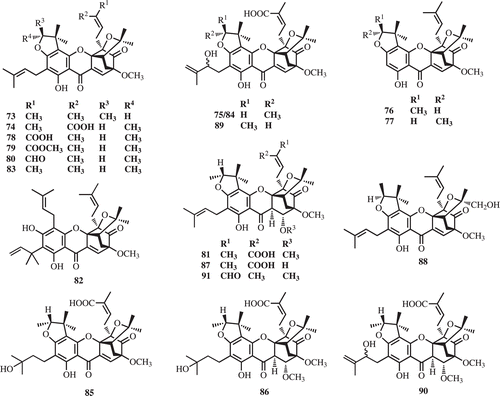
Caged-prenylated xanthones, scortechinones A-T (73–91) were reported from the twigs, latex, stem barks, and fruits of Garcinia scortechinii (CitationRukachaisirikul et al., 2000, Citation2003, Citation2005; CitationSukpondma et al., 2005b) (). Antibacterial activities of all polyprenylated xanthones, isolated from the latex, stem bark, and fruits, were evaluated. Scortechinone B (74) was found to exhibit significant antibacterial activity against a methicillin resistant Staphylococcus aureus strain with an MIC value of 2 μg/mL (CitationRukachaisirikul et al., 2005; CitationSukpondma et al., 2005b).
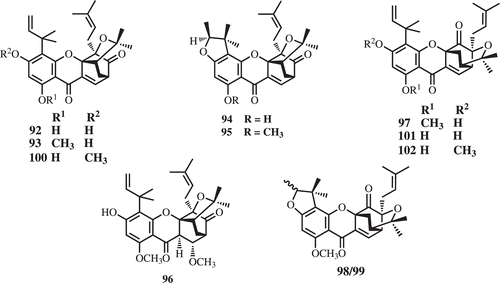
Caged-prenylated xanthones, bractatin (92), 1-O-methylbractatin (93), isobractatin (94), 1-O-methylisobractatin (95), 1-O-methyl-8-methoxy-8,8a-dihydrobractatin (96) and 3-O-methylbractatin (100), together with irregular neo caged scaffold, 1-O-methylneobractatin (97), neoisobractatin A (98), neoisobractatin B (99), neobractatin (101), 3-O-methylneobractatin (102) were isolated from the leaves, barks, and twigs of G. bracteata (). These compounds were found to exhibit significant cytotoxicities against the KB cell lines with IC50 range of 0.2–1.5 μg/mL (CitationNa et al., 2010a, Citation2010b; CitationThoison et al., 2000, Citation2005).
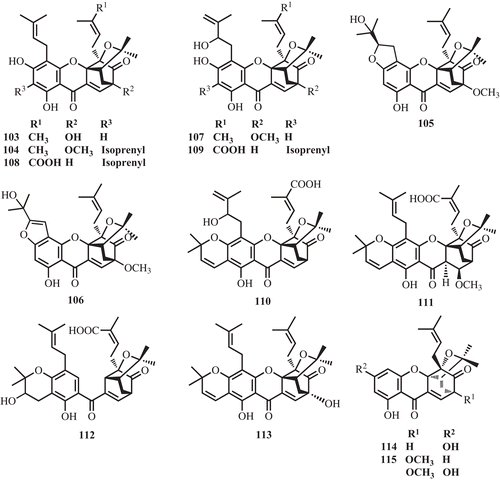
The investigation of leaves and trunk bark of G. cantleyana yielded five caged xanthones, deoxygaudichaudione A (40), gaudichaudione H (60), 7-hydroxyforbesione (103) and cantleyanones A–D (104–107) which exhibited cytotoxicity against MDA-MB-231, CaOV-3, MCF-7, and HeLa cancer cell lines with IC50 values ranging from 0.22–17.17 μg/mL (CitationShadid et al., 2007) ().
Stem bark of G. lateriflora have been reported recently to contain (−)-morellic acid (4), (−)-isogaudichaudiic acid (108), (−)-isogaudichaudiic acid B (109), (−)-isogaudichaudiic acid E (110), (−)-isomoreollic acid (111), (−)-11,12-dihydro-12-hydroxymorellic acid (112) (). The caged xanthones showed potent cytotoxicity toward HT-29 cells with the ED50 values ranging from 0.36–3.2 μM (CitationRen et al., 2010).
Phytochemical study of G. forbesii yielded one caged xanthone, forbesione (39) (CitationLeong et al., 1996). Moreover, the investigation of G. urophylla led to two caged xanthones, gaudichaudione H (60) and 7-hydroxydesoxymorellin (113) (CitationKhalid et al., 2007) ().
Besides the genus Garcinia, prenylated caged xanthones could be isolated from other genus. Sootepenseone (isobractatin) (94) was also obtained from the methanol extract of leaves of Dascymaschalon sootepense. The caged xanthone sootepenseone has been found to have antitumor activity against KB solid tumor cells and less toxic than the standard compounds (actinomycin D, vinblastin, adriamycin, and bleomycin). To the best of our knowledge, this novel caged xanthone is the only compound to have been found to have antitumor activity and was the only caged xanthone isolated from the Annonaceae family and outside the more common occurrence in the Guttiferea family (CitationReutrakul et al., 2001). In recent reports, three prenylated caged xanthones, cochinchinoxanthone (114) and cochinchinones C–D (115–116) were found from the roots and stems of Cratoxylum cochinchinense (CitationMahabusarakam et al., 2006; Ren et al., 2011; ).
In the recent reports, the absolute configuration of quaternary carbon in the bridge head of the caged scaffold (carbon 5, 7, 10a, and 27) of (−)-morellic acid (4) and (−)-gambogic acid (11) were determined by electronic circular dichroism (ECD) and NOESY. The absolute configuration at C5 and C7 were determined as 5S and 7R via the ECD spectra which displayed a positive and a negative Cotton effect near 290 and 360 nm, respectively. Based on the NOESY and CD spectroscopic data the absolute configuration of C10a and C27 were deduced as 10aS and 27S (CitationRen et al., 2010, Citation2011b). The CD spectrum of cochinchinoxanthone (114) showed negative and positive Cotton effect at 292 and 353 nm. In comparison of CD spectrum of morellic acid and NOESY correlations data, the absolute configuration of the caged structure of compound 114 were deduced as 5S, 7R, 10aR, and 12R (CitationRen et al., 2011a; ).
Biological activities
Prenylated caged xanthones were reported to have many biological activities, including antibacterial, anti-HIV-1, neurotrophic, antiatherosclerosis and anticancer activities. Gambogic acid is the prenylated caged xanthone that has been most extensively studied, in particular, with respect to the anticancer activity.
Antibacterial activity
Some prenylated caged xanthones isolated from G. hanburyi and G. scortechinii were evaluated for the antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Among those, scortechinone B (74) isolated from G. scortechinii exhibited the best activity with a MIC value of 2 μg/mL (CitationRukachaisirikul et al., 2005; CitationSukpondma et al., 2005a, Citation2005b). Some structure activity relationships on the antibacterial of the caged xanthones were discussed that the C2 prenyl substituent and carboxyl group at the terminal side chain of C5 prenyl substituent are important for the antibacterial activity (CitationRukachaisirikul et al., 2005).
Anti-HIV-1 activity
The anti-HIV-1 activity of the caged xanthone was reported only recently. Anti-HIV-1 activities of the caged xanthones isolated from the resin and fruits of G. hanburyi were established. Morellic acid (4), gambogic acid (11), and dihydroisomorellin (22) showed potent HIV-1 reverse transcriptase inhibition activity with the IC50 value less than 50 μg/mL. Moreover, deoxygambogenin and dihydroisomorellin (22) could inhibit HIV-1-mediated syncytium formation with the ED50 values of 3.0 and 1.2 μg/mL and TI values of 1.7 and 4.7, respectively, whereas the others were toxic to 1A2 cells employed in the syncytium assay (CitationReutrakul et al., 2007).
Neurotrophic activity
To discover small molecules that mimic nerve growth factor (NGF) functions, gambogic acid and its derivatives were identified for transmembrane tyrosine kinase TrkA agonists. It was revealed that gambogic amide and gambogic amines, semisynthetic compounds derived from naturally occurring gambogic acid, could selectively bind to the cytoplasmic juxtamembrane region of TrkA receptor, stimulate receptor dimerization, phosphorylation of cytoplasmic tyrosine residues on the receptor, and activate phosphatidylinositol 3-kinase (PI 3-kinase)/Akt and mitogen-activated protein (MAP) kinase. These signaling cascades play critical roles in neuronal plasticity, survival, and neurite outgrowth via the prevention of apoptotic cell death, promotion of cellular differentiation and axon elongation, and upregulation of choline acetyl transferase. Therefore, gambogic amide and gambogic amines can mimic NGF and acts as a robust TrkA to possess potent neurotrophic activities which may be useful for the treatment of neurodegenerative disorders such as Alzheimer’s disease and stroke (CitationJang et al., 2007; CitationYe et al., 2011).
Antiatherosclerosis activity
Recent report revealed that gambogic acid could inhibit vascular smooth muscle cell (VSMC) proliferation and migration induced by platelet-derived growth factor BB (PDGF-BB) and epithelial growth factor (EGF). The inhibitory effects of gambogic acid are mediated by the inhibition of PDGF receptor β and EGF receptor tyrosine phosphorylations and Rac1 activity in rat aortic smooth muscle cells (CitationLiu et al., 2010a, Citation2010b). Moreover, gambogic acid also inhibited NF-κB activation and endothelial adhesion molecule expression induced by tumor necrosis factor-alpha (TNF-α) or lipopolysaccharide (LPS) in cultured human aortic endothelial cells (HAEC; CitationZhang et al., 2010a, Citation2010b). These results indicated that gambogic acid is a potent anti-inflammatory agent which may be used to prevent or treat vascular inflammation and atherosclerosis.
Cytotoxic activity
Prenylated caged xanthones isolated from resin of G. hanburyi showed cytotoxicities against many cell lines such as Henrietta Lacks cervical carcinoma (HeLa), human embryonic lung fibroblasts (HEL), human epidermoid carcinoma (KB), drug-resistant human cervical carcinoma (KB-V1), human leukemia K562 (K562/S), doxorubicin-resistant K562 (K562/R), human hepatoma (SMMC-7221), human hepatoma (HepG2), human gastric cancer (BGC-823), human leukemia (HL-60), human leukemic monocyte (U937), lymphoma human breast cancer (MCF-7), human lung cancer (Lu-1), human lung cancer (A549), human colon cancer (Col-2), human colon cancer (HT-29), rat glioma (ASK), murine lymphocytic leukemia (P-388), adriamycin-resistant murine lymphocytic leukemia (P388/ADR) cell lines. Among those prenylated caged xanthones, desoxymorellin (1) showed the highest cytotoxic activity against HEL and HeLa cells with a minimum inhibitory concentration (MIC) of 0.39 μg/mL (CitationAsano et al., 1996) and gaudichaudic acid showed the strongest cytotoxicity against K562/S and K562/R with the IC50 values of 0.41 and 0.61 μg/mL, respectively (CitationHan et al., 2006a, Citation2006b, Citation2006c). Gambogic acid (11) exhibited the most potent cytotoxicity against KB, KB-V1 (CitationLin et al., 1993), Col-2, Lu-1, ASK (CitationReutrakul et al., 2007), HL-60, U937, MCF-7, and HepG2 with the IC50 values of 0.70, 2.30, 0.45, 2.06, 0.24, 0.50, 0.65, 0.83, and 0.83 μg/mL, respectively, whereas, morellic acid displayed the highest cytotoxicity against A549 and HT-29 with the IC50 values of 0.76 and 2.8 μg/mL, respectively (CitationLee & Chen, 2006). Gambogic aldehyde (10) inhibited the growth of P388 and P388/ADR cell with IC50 values of 0.243 and 7.60 mM (CitationWang et al., 2008a).
Cytotoxicity of gaudichaudiones A–G (53–59) and gaudichaudiic acids A–E (63–67), prenylated caged xanthones isolated from the leaves of G. gaudichaudii, against a panel of several cancer cell lines, including murine lymphocytic leukemia (P388), mouse fibrosarcoma (WEHI1640), human lymphoblastic leukemia (MOLT4), human hepatocellular carcinoma (HePG2), and mouse Lewis lung carcinoma, (LL/2) were reported. Most of them except for gaudichaudiic acid B, C showed potent activities with to all tested cell lines with the ED50 value are mostly in range of 0.5–8.0 μg/mL (CitationCao et al., 1998a, Citation1998b).
Prenylated caged xanthones isolated from G. bracteata, bractatin (92) and its derivatives, 1-O-methylbractatin (93), isobractatin (sootepenseone) (94), 1-O-methylisobractatin (95), 1-O-methyl-8-methoxy-8,8a-dihydrobractatin (96) and 3-O-methylbractatin (100), 1-O-methylneobractatin (97), neoisobractatin B (99) exhibited significant cytotoxicities against KB cell line with IC50 range of 0.2–1.5 μg/mL (CitationThoison et al., 2000, Citation2005).
Deoxygaudichaudione A (40), 7-hydroxyforbesione (103) and cantleyanones A-D (104–107) found from leaves and trunk bark of G. cantleyana showed cytotoxicities against human breast cancer (MDA-MB-231), human ovarian cancer (CaOV-3), MCF-7, and HeLa cell lines with IC50 values ranging from 0.22 to 17.17 μg/mL (CitationShadid et al., 2007).
Prenylated caged xanthones 108–112 of G. lateriflora showed potent cytotoxicity toward HT-29 cells (human colon cancer cells) with the ED50 values of 0.36–3.2 μM (CitationRen et al., 2010).
Gaudichaudione H (60) which was found in G. gaudichaudii, G. cantleyana, and G. urophylla showed growth-inhibition activity on MCF-7, DU-145 (human prostate cancer) and NCI-H460 (human lung cancer cells) with the ED50 values of 6.6–7.6 μM (CitationKhalid et al., 2007).
In vivo antitumor test, sootepenseone (isobractatin) (94) which was obtained from G. bracteata and D. sootepense showed cytotoxicity against the KB and MCF-7 cell lines and less toxic than the standard compounds (actinomycin D, vinblastin, adriamycin, and bleomycin; CitationReutrakul et al., 2001).
The cytotoxic effect of prenylated caged xanthones against sensitive and drug-resistant cell lines were studied. Gambogic acid (11) and epigambogic acid (12) had similar cytotoxicity on K562/S and K562/R with the IC50 of 1.32 and 0.89 μM for gambogic acid and 1.11 and 0.86 μM for epigambogic acid (CitationHan et al., 2006b). Moreover, gaudichaudione A (53) exhibited similar cytotoxic activity against P388 and doxorubicin-resistant P388 cell lines (CitationWu et al., 2002). It was reported that synthetic caged xanthones showed cytotoxicity in a variety of tumor cell lines at low micromolar concentrations as gambogic acid and they exhibited similar cytotoxic effect to HL-60 and adriamycin-resistant HL-60 (HL-60/ADR) cells (CitationBatova et al., 2007). These results indicated that the caged xanthones are nonsubstrate of multidrug resistance (MDR) transporter P-glycoprotein, an ATP-dependent drug efflux pump for decreasing drug accumulation in cells mediating the failure of cancer chemotherapy (CitationCallaghan et al., 2006; CitationVelinga & Dendekar, 2010). Moreover, treatment of docetaxel-resistant gastric cancer (BGC-823/Doc) cells with gambogic acid (11) at nontoxic concentrations could increase cytotoxicity, apoptosis, and G2/M arrest effects of docetaxel. Analysis of apoptotic associated gene revealed that gambogic acid (11) singly or in combination with docetaxel significantly downregulate the mRNA expression of surviving (an apoptosis inhibitor) with no effect on bcl-2. Therefore, gambogic acid (11) can reverse docetaxel resistance in BGC-823/Doc cells (CitationWang et al., 2008b).
The inhibitory effects to BGC-823 human gastric carcinoma cells and proapoptotic activity of two drugs combination, 5-fluorouracil (5-FU) and gambogic acid (11) were much stronger than single. This synergistic effect was due to gambogic acid-regulated the metabolic enzymes of 5-FU. Gambogic acid (11) decreased the mRNA levels of thymidine synthetase and dihydropyrimidine dehydrogenase, whereas it increased the mRNA level of orotate phosphoribosyltransferase. Moreover, in vivo study revealed that combined treatment caused significantly growth inhibition of human tumor xenografts (CitationWang et al., 2009a).
The selective anticancer activity of Gambogic acid (11) and its derivatives were reported. Gambogic acid showed significant apoptotic induction on human hepatoma SMMC-7721 cells, whereas had relatively less effect on human normal embryonic hepatic L02 cells. Moreover, the study on the drug distribution in cultured cells and in tumor-bearing mice showed that gambogic acid (11) had higher distribution and longer retention time in tumor cells compared to the normal cells (CitationYang et al., 2007). Our group reported the selective cytotoxicity of isomorellin (3), isomorellinol (7), gambogic acid (11) and forbesione (39) against cholangiocarcinoma KKU-100 and KKU-M156 cells compared with normal peripheral blood mononuclear PBMCs (CitationHahnvajanawong et al., 2010). The selective inhibiting proliferation and inducing apoptosis in the cancer cell lines indicated that the prenylated caged xanthones might be an effective anticancer drug candidate with low toxicity to normal tissue. This report indicated the potential of prenylated caged xanthones for the treatment of cholangiocarcinoma, a form of cancer caused by liver fluke, which so far resistant all known chemotherapeutic agents.
The mechanism of action of a potential anticancer agent, gambogic acid (11) and its derivatives have been continuously reported by many research groups. Apoptosis is one of the main cytotoxicity mechanisms of prenylated caged xanthones studied in several cancer cells. The growth inhibition of gambogic acid on human gastric carcinoma MGC-803 (CitationZhao et al., 2004), BGC-823 (CitationLiu et al., 2005), human malignant melanoma A375 cells (CitationXu et al., 2009), KKU-100 and KKU-M156 cells (CitationHahnvajanawong et al., 2010) was related to the apoptosis induction of which the molecular mechanism is the regulation of gene Bcl-2, an apoptosis inhibitor and Bax, an apoptosis inducer. Gambogic acid could downregulate the expression of Bcl-2 mRNA and upregulate the expression of Bax mRNA. Our group also reported that the other prenylated caged xanthones, isomorellin (3), isomorellinol (7) and forbesione (39) also exhibited the potency in increasing the Bax/Bcl-2 protein expression ratio in KKU-100 and KKU-M156 cells (CitationHahnvajanawong et al., 2010).
Transferrin receptor (TfR), a membrane-bound protein involved in iron homeostasis, was discovered as another target for gambogic acid. TfR expression is increased in dividing cells and its overexpression has been reported in different types of cancers. Gambogic acid (11) could bind to TfR-1 which rapidly activated apoptosis in cells (CitationKasibhatla et al., 2005). Furthermore, gambogic acid (11) could enhance apoptosis induced by tumor necrosis factor (TNF) in human leukemia cancer cells by inhibiting NF-μB signaling pathway through its interaction with the transferrin receptor. Downregulation of TfR by RNA interference decreased sensitivity to gambogic acid-induced apoptosis and effects on NF-κB (CitationPandey et al., 2007).
Gambogic acid (11) was reported to be an effective telomerase inhibitor by repressing human telomerase reverse transcriptase hTERT promoter. Gambogic acid-treated human lung carcinoma (SCP-A1; CitationWu et al., 2004), human hepatoma (SMMC-7721; CitationGuo et al., 2004, Citation2006), and human gastric carcinoma (MGC-803, SGC-7901 and BGC-823; CitationYu et al., 2006, CitationZhao et al., 2008) cells experienced a profound downregulation of telomerase activity due to a suppressed expression of hTERT mRNA. Gambogic acid repressed hTERT transcriptional activity via downregulation of c-Myc expression in the cancer cell lines. Moreover, gambogic acid (11) could inhibit the posttranslational modification of hTERT by inhibiting the phosphorylation of Akt (CitationZhao et al., 2008).
The study on the apoptosis-inducing effect of gambogic acid on K562 cell line found that gambogic acid (11) could decrease the mitochondrial membrane potential and increase the activated caspase 3, caspase 8, and caspase 9 positive cell level (CitationZhang et al., 2009).
Moreover, gambogic acid-induced apoptosis is also mediated by the accumulation of reactive oxygen species (ROS). It was found that ROS accumulation induced by gambogic acid (11) resulted in the collapse of mitochondrial membrane potential in SMMC-7721, causing the release of cytochrome c and apoptosis-inducing factor from mitochondria to cytosol. Moreover, gambogic acid (11) elevated the phosphorylation of c-Jun-N-terminal protein kinase (JNK) and p38, which was the downstream effect of ROS accumulation. Therefore, accumulation of ROS played an important role in gambogic acid-induced mitochondrial signaling pathway (CitationNie et al., 2009).
Molecular mechanisms of cell-cycle arrest caused by gambogic acid (11) have been reported. Treatment of BGC-823 cells with gambogic acid (11) caused an irreversible arrest in the G2/M phase of the cell cycle associated with a significant decrease in CDC2/p34 expression by inhibition of cyclin-dependent kinase (CDK)-activating kinase (CDK7/cyclin H) activity (CitationYu et al., 2007). Moreover, it was found that gambogic acid (11) could depolymerize microtubules and increase the phosphorylation levels of JNK1 and p38, which caused G2/M cell cycle arrest and apoptosis in MCF-7 cells (CitationChen et al., 2008). Antiproliferative effect of gambogic acid in human cancer cells was reported that gambogic acid (11) could inhibit the catalytic activity of topoisomerase (Topo) IIα by directly binding to the ATPase domain and resulted in inhibiting DNA cleavage and ATP hydrolysis (CitationQin et al., 2007).
Gambogic acid (11) could inhibit the growth of osteosarcoma (MG63, HOS and U2OS) cells via the induction of cell cycle arrest and apoptosis in the cells. It was found that a decrease in phospho-GSK3-β (Ser9) and the expression of cyclin D1 by gambogic acid treatment mediated the G0/G1 phase arrest In U2OS cells. Gambogic acid (11) mediated G2/M cell cycle arrest in MG63 cells by decreasing in phospho-cdc2 (Thr 161) and cdc25B. Furthermore, gambogic acid (11) could elevate the Bax/Bcl-2 ratio which indicated the apoptosis induction in osteosarcoma cells (CitationZhao et al., 2011).
Gambogic acid (11) was reported as an inhibitor of heat shock protein 90 (Hsp90), a molecular chaperon involving the proliferation and survival of cancer cells. It was found that gambogic acid (11) could inhibit Hsp90 by binding to N-terminal ATP-binding domain and downregulates. In addition, gambogic acid (11) could inactivate of TNF-α/NF-κB and decrease X-linked inhibitor of apoptosis protein (XIAP) expression levels and the ratio of Bcl-2/Bax in HeLa cell (CitationDavenport et al., 2011; CitationZhang et al., 2010a, Citation2010b).
Gambogic acid (11) and gambogenic acid (46) exhibited inhibitory effects on hepatocellular carcinoma HCC cell proliferation via downregulation of the expression of stathmin 1 (STMN1), an important regulatory protein of microtubule dynamics. Moreover, it was found that overexpression of STMN1 in HCC cells decreased their sensitivity to the caged xanthones suggested that STMN1 might be a major target for gambogic acid (11) and gambogenic acid (46) (CitationWang et al., 2009b).
Gambogic acid (11) exhibits a potent proliferation inhibition by the G0/G1 phase cell cycle arrest and apoptosis induction in human lung adenocarcinoma A549 and human chronic myelogenous leukemia K562 cells, which might correlated with the downregulation of the expression of steroid receptor coactivator-3 (SRC-3), an important modulator of cell growth (CitationLi et al., 2009a, Citation2009b). Gambogic acid (11) also inhibited the activity of Akt kinase and influenced the expression of the apoptosis related gene Bcl-2 in K562 cells (CitationLi et al., 2009b).
Downregulations of nucleophosmin and nucleoporins by gambogic acid (11) correlated with the apoptosis induction in T-leukemia Jurkat cells. Moreover, most NUP88 and NUP214 nucleoporins were delocalized to the nuclear rim in the gambogic acid-treated cells. Therefore, nucleocytoplasmic transport of proteins may play important role for the antileukemic potency of gambogic acid (CitationShu et al., 2008).
Moreover, stabilization and activation of p53, a tumor suppressor protein were achieved through downregulating the expression of MDM2 by gambogic acid (11) treatment in variety of cancer cell lines. Gambogic acid could induce posttranslational modifications of p53 by phosphorylation at sites Ser15 and Ser20 causing DNA damage. It was found that gambogic acid-triggered DNA damage signaling induced p53/p21 (Waf1/CIP1) activation through the ATR-Chk1 pathway (CitationRong et al., 2010). Moreover, gambogic acid (11) could downregulate mutant p53 at posttranscription level without effect to the mRNA levels in human breast carcinoma MDA-MB-435 cells. The degradation of mutant p53 by gambogic acid was mediated by chaperones-assisted ubiquitin/proteasome degradation pathway in cancer cells (CitationWang et al., 2011).
Gambogic acid (11) was reported to be an effective angiogenesis inhibitor with low toxicity. Gambogic acid (11) significantly inhibited human umbilical vascular endothelial cell (HUVEC) proliferation, migration, invasion, tube formation, and microvessel growth. It was found that gambogic acid (11) exhibited antiangiogenic effect by inhibiting the activations of vascular endothelial growth factor receptor 2 and its downstream protein kinases, such as c-Src, focal adhesion kinase, and AKT (CitationYi et al., 2008). Furthermore, gambogic acid (11) could inhibit VEGF-induced tyrosine phosphorylation of KDR/Flk-1 and significantly decreased in VEGF-triggered phosphorylated forms of ERK, AKT, and p38 (CitationLu et al., 2007).
The potential activity in tumor metastasis of gambogic acid (11) was reported that gambogic acid (11) strongly inhibited the adhesion and migration of highly metastatic mouse melanoma B16-F10 cells in vitro and in vivo by downregulation of α4 integrin expression (CitationZhao et al., 2008).
Summary and future prospects
Prenylated caged xanthones have been demonstrated to be new class of bioactive compounds with great potential for the treatment of various diseases. These xanthones have travelled a long way through exciting scientific research, starting from the gamboges to pure compounds with proven biological activities. The chemical structure, biosynthesis, biology, and therapeutic potential of these compounds are truly astounding. The key structural feature that is crucial for biological activities of these compounds is the unique 4-oxo-tricyclo[4.3.1.03,7]dec-8-en-2-one scaffold. It can be said that the structure with the unusual scaffold is a truly “privileged structure” as evidence from their ability to interact with diverse drug targets. The ongoing exciting synthetic effort for this class of compounds is not covered in this review but has been adequately cover from a recent publication. SAR studies of this class of compounds are exciting areas of cutting edge research. The research on these compounds, concentrating mainly gambogic acid, and on their plant sources has merely scratched the surface. The future research on the chemistry and biology on this topic looks very bright, challenging, and with tremendous therapeutic applications.
Acknowledgments
The authors wish to thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC) and the Office of the Higher Education Commission and Mahidol University under the National Research Universities Initiative for financial support.
Declaration of interest
The authors report no declarations of interest.
References
- Adawadkar PD, Srinivasan R, Yemul SS. (1976). Coloring matters of Garcinia morella: Part VIII. Morellinol, dihydromorelloflavone and morelloflavone-7”-β-glucoside. Indian J Chem Sect B, 17, 19–21.
- Asano J, Chiba K, Tada M, Yoshii T. (1996). Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry, 41, 815–820.
- Batova A, Lam T, Wassscholowski V, Yu AL, Giannis A, Theodorakis EA. (2007). Synthesis and evaluation of caged Garcinia xanthones. Org Biomol Chem, 5, 494–500.
- Bhat HB, Nair PM, Venkataraman K. (1964). The colouring matters of Garcinia morella. Part V. Isolation of desoxymorellin and dihydroisomorellin. Ind J Chem, 2, 405–409.
- Callaghan R, Ford RC, Kerr ID. (2006). The translocation mechanism of P-glycoprotein. FEBS Lett, 580, 1056–1063.
- Cao SG, Wu XH, Sim KY, Tan BHK, Pereira JT, Wong WH, Hew NF, Goh SH. (1998a). Cytotoxic caged tetraprenylated xanthonoids from Garcinia gaudichaudii (Guttiferae). Tetrahedron Lett, 39, 3353–3356.
- Cao SG, Sng VHL, Wu XH, Sim KY, Tan BHK, Pereira JT, Goh SH. (1998b). Novel cytotoxic polyprenylated xanthonoids from Garcinia gaudichaudii (Guttiferae). Tetrahedron, 54, 10915–10924.
- Chantarasriwong O, Batova A, Chavasiri W, Theodorakis EA. (2010). Chemistry and biology of the caged Garcinia xanthones. Chem Eur J, 16, 9944–9962.
- Chen J, Gu HY, Lu N, Yang Y, Liu W, Qi Q, Rong JJ, Wang XT, You QD, Guo QL. (2008). Microtubule depolymerization and phosphorylation of c-Jun N-terminal kinase-1 and p38 were involved in gambogic acid induced cell cycle arrest and apoptosis in human breast carcinoma MCF-7 cells. Life Sci, 83, 103–109.
- Davenport J, Manjarrez JR, Peterson L, Krumm B, Blagg BS, Matts RL. (2011). Gambogic acid, a natural product inhibitor of Hsp90. J Nat Prod, 74, 1085–1092.
- El-Seedi HR, El-Barbary MA, El-Ghorab DMH, Bohlin L, Borg-Karlson AK, Göransson U, Verpoorte R. (2010). Recent insights into the biosynthesis and biological activities of natural xanthones. Curr Med Chem, 17, 854–901.
- Feng F, Liu WY, Chen YS, Guo QL, You QD. (2007). Five novel prenylated xanthones from Resina Garciniae. J Asian Nat Prod Res, 9, 735–741.
- Grieve M. (1931). Gamboge. A modern herbal [Online]. Available at: http://botanical.com/botanical/mgmh/g/gambog05.html. Accessed on 18 July 2011.
- Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. (2004). General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacol Sin, 25, 769–774.
- Guo QL, Lin SS, You QD, Gu HY, Yu J, Zhao L, Qi Q, Liang F, Tan Z, Wang X. (2006). Inhibition of human telomerase reverse transcriptase gene expression by gambogic acid in human hepatoma SMMC-7721 cells. Life Sci, 78, 1238–1245.
- Hahnvajanawong C, Boonyanugomol W, Nasomyon T, Loilome W, Namwat N, Anantachoke N, Tassaneeyakul W, Sripa B, Namwat W, Reutrakul V. (2010). Apoptotic activity of caged xanthones from Garcinia hanburyi in cholangiocarcinoma cell lines. World J Gastroenterol, 16, 2235–2243.
- Han QB, Wang YL, Yang L, Tso TF, Qiao CF, Song JZ, Xu LJ, Chen SL, Yang DJ, Xu HX. (2006a). Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. Chem Pharm Bull, 54, 265–267.
- Han Q, Yang L, Liu Y, Wang Y, Qiao C, Song J, Xu L, Yang D, Chen S, Xu H. (2006b). Gambogic acid and epigambogic acid, C-2 epimers with novel anticancer effects from Garcinia hanburyi. Planta Med, 72, 281–284.
- Han QB, Yang L, Wang YL, Qiao CF, Song JZ, Sun HD, Xu HX. (2006c). A pair of novel cytotoxic polyprenylated xanthone epimers from gamboges. Chem Biodivers, 3, 101–105.
- Han QB, Xu HX. (2009). Caged Garcinia xanthones: Development since 1937. Curr Med Chem, 16, 3775–3796.
- Jang SW, Okada M, Sayeed I, Xiao G, Stein D, Jin P, Ye K. (2007). Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death. Proc Natl Acad Sci USA, 104, 16329–16334.
- Karanjgaonkar CG, Madhavan Nair P, Venkataraman K. (1966). Morellic, isomorellic and gambogic acids. Tetrahedron Lett, 7, 687–691.
- Kartha G, Ramachandran GN, Bhat HB, Nair PM, Raghavan VKV, Venkataraman K. (1963). The constitution of morellin. Tetrahedron Lett, 4, 459–472.
- Kasibhatla S, Jessen KA, Maliartchouk S, Wang JY, English NM, Drewe J, Qiu L, Archer SP, Ponce AE, Sirisoma N, Jiang S, Zhang HZ, Gehlsen KR, Cai SX, Green DR, Tseng B. (2005). A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid. Proc Natl Acad Sci USA, 102, 12095–12100.
- Khalid RM, Jabit ML, Abas F, Stanslas J, Shaari K, Lajis NH. (2007). Cytotoxic xanthones from the leaves of Garcinia urophylla. Nat Prod Commun, 2, 271–6.
- Lee SB, Chen CM. (2006). Compounds isolated from gamboges resin having activity in inhibiting the growth of tumor/cancer cells and pharmaceutical compositions comprising the same. US Pat Appl Publ, 22 p.
- Leong Y, Harrison LJ, Bannett GJ, Tan HTW. (1996). Forbesione, a modified xanthone from Garcinia forbesii. J Chem Res, 392–393.
- Li R, Chen Y, Shu W, Zhao F, Liu Y, Wen L. (2009a). Effects of gambogic acid on regulation of steroid receptor coactivator-3 in lung adenocarcinoma A549 cells. Chin J Cancer Res, 21, 68–73.
- Li R, Chen Y, Zeng LL, Shu WX, Zhao F, Wen L, Liu Y. (2009b). Gambogic acid induces G0/G1 arrest and apoptosis involving inhibition of SRC-3 and inactivation of Akt pathway in K562 leukemia cells. Toxicology, 262, 98–105.
- Lin L, Lin L, Pezzuto JM, Cordell GA. (1993). Isogambogic acid and isomorellinol from Garcinia hanburyi. Mag Reson Chem, 31, 340–347.
- Liu W, Guo QL, You QD, Zhao L, Gu HY, Yuan ST. (2005). Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J Gastroenterol, 11, 3655–3659.
- Liu Y, Li W, Ye C, Lin Y, Cheang TY, Wang M, Zhang H, Wang S, Zhang L, Wang S. (2010a). Gambogic acid induces G0/G1 cell cycle arrest and cell migration inhibition via suppressing PDGF receptor ß tyrosine phosphorylation and Rac1 activity in rat aortic smooth muscle cells. J Atheroscler Thromb, 17, 901–913.
- Liu Y, Lin M, Li W, He Y, Shi Zeng, H, Wang S. (2010b). Gambogic acid inhibits cell proliferation via suppressing epithelial growth factor receptor tyrosine phosphorylation in rat aortic smooth muscle cell. Zhongguo Dongmai Yinghua Zazhi, 18, 527–531.
- Lu GB, Yang XX, Huang QS. (1984). Isolation and structure of neo-gambogic acid from Gamboge (Garcinia hanburryi). Yao Xue Xue Bao, 19, 636–639.
- Lu N, Yang Y, You QD, Ling Y, Gao Y, Gu HY, Zhao L, Wang XT, Guo QL. (2007). Gambogic acid inhibits angiogenesis through suppressing vascular endothelial growth factor-induced tyrosine phosphorylation of KDR/Flk-1. Cancer Lett, 258, 80–89.
- Mahabusarakam W, Nuangnaowarat W, Taylor WC. (2006). Xanthone derivatives from Cratoxylum cochinchinense roots. Phytochemistry, 67, 470–474.
- Na Z, Hu HB, Fan QF. (2010a). A novel caged-prenylxanthone from Garcinia bracteata. Chin Chem Lett, 21, 443–445.
- Na Z, Hu HB, Fan QF. (2010b). Three new caged prenylxanthones from Garcinia bracteata. Helv Chim Acta, 93, 958–963.
- Nair PM, Venkataraman K. (1964). The colouring matters of Garcinia morella. Part IV. Isomorellin. Ind J Chem, 2, 402–404.
- Nicolaou KC, Li J. (2001). “Biomimetic” Cascade reactions in organic synthesis: construction of 4-oxatricyclo[4.3.1.0]decan-2-one systems and total synthesis of 1-O-methylforbesione via tandem claisen rearrangement/Diels-Alder reactions. Angew Chem Int Ed, 40, 4264–4268.
- Nie F, Zhang X, Qi Q, Yang L, Yang Y, Liu W, Lu N, Wu Z, You Q, Guo Q. (2009). Reactive oxygen species accumulation contributes to gambogic acid-induced apoptosis in human hepatoma SMMC-7721 cells. Toxicology, 260, 60–67.
- Ollis WD, Ramsay MVJ, Sutherland IO, Mongkolsuk S. (1965). The constitution of gambogic acid. Tetrahedron, 21, 1453–1470.
- Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, Aggarwal BB. (2007). Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood, 110, 3517–3525.
- Qin Y, Meng L, Hu C, Duan W, Zuo Z, Lin L, Zhang X, Ding J. (2007). Gambogic acid inhibits the catalytic activity of human topoisomerase IIalpha by binding to its ATPase domain. Mol Cancer Ther, 6, 2429–2440.
- Quillinan AJ, Scheinmann F. (1971). Application of the Claisen rearrangement to the synthesis of heterocyclic bicyclo[2,2,2]octenones: an approach to the morellins based on new biogenetic suggestions. J Chem Soc, Chem Commun 966–967.
- Rao BS. (1937). Morellin, a constituent of the seeds of Garcinia morella. J Chem Soc, 853–855.
- Ren Y, Lantvit DD, Carcache de Blanco EJ, Kardono LB, Riswan S, Chai H, Cottrell CE, Farnsworth NR, Swanson SM, Ding Y, Li XC, Marais JP, Ferreira D, Kinghorn AD. (2010). Proteasome-inhibitory and cytotoxic constituents of Garcinia lateriflora: Absolute configuration of caged xanthones. Tetrahedron, 66, 5311–5320.
- Ren Y, Yuan C, Chai HB, Ding Y, Li XC, Ferreira D, Kinghorn AD. (2011a). Absolute configuration of (-)-gambogic acid, an antitumor agent. J Nat Prod, 74, 460–463.
- Ren Y, Matthew S, Lantvit DD, Ninh TN, Chai H, Fuchs JR, Soejarto DD, de Blanco EJ, Swanson SM, Kinghorn AD. (2011b). Cytotoxic and NF-κB inhibitory constituents of the stems of Cratoxylum cochinchinense and their semisynthetic analogues. J Nat Prod, 74, 1117–1125.
- Reutrakul V, Santisuk T, Noessner G, Schmidt J, Nickel B, Klenner T, Hose S. (2001). Novel xanthone compounds, their preparation and use medicament. Eur Pat Appl, EP 1065210 A1, 17 pp.
- Reutrakul V, Anantachoke N, Pohmakotr M, Jaipetch T, Sophasan S, Yoosook C, Kasisit J, Napaswat C, Santisuk T, Tuchinda P. (2007). Cytotoxic and anti-HIV-1 caged xanthones from the resin and fruits of Garcinia hanburyi. Planta Med, 73, 33–40.
- Rong JJ, Hu R, Song XM, Ha J, Lu N, Qi Q, Tao L, You QD, Guo QL. (2010). Gambogic acid triggers DNA damage signaling that induces p53/p21(Waf1/CIP1) activation through the ATR-Chk1 pathway. Cancer Lett, 296, 55–64.
- Rukachaisirikul V, Kaewnok W, Koysomboon S, Phongpaichit S, Taylor WC. (2000). Caged-tetraprenylated xanthones from Garcinia scortechinii. Tetrahedron, 56, 8539–8543.
- Rukachaisirikul V, Painuphong P, Sukpondma Y, Koysomboon S, Sawangchote P, Taylor WC. (2003). Caged-triprenylated and -tetraprenylated xanthones from the latex of Garcinia scortechinii. J Nat Prod, 66, 933–938.
- Rukachaisirikul V, Phainuphong P, Sukpondma Y, Phongpaichit S, Taylor WC. (2005). Antibacterial caged-tetraprenylated xanthones from the stem bark of Garcinia scortechinii. Planta Med, 71, 165–170.
- Shadid KA, Shaari K, Abas F, Israf DA, Hamzah AS, Syakroni N, Saha K, Lajis NH. (2007). Cytotoxic caged-polyprenylated xanthonoids and a xanthone from Garcinia cantleyana. Phytochemistry, 68, 2537–2544.
- Shu W, Chen Y, Li R, Wu Q, Cui G, Ke W, Chen Z. (2008). Involvement of regulations of nucleophosmin and nucleoporins in gambogic acid-induced apoptosis in Jurkat cells. Basic Clin Pharmacol Toxicol, 103, 530–537.
- Subba Rao GSR, Rathnamala S, Sivaramakrishnan R. (1978). Structure of moreollin, a pigment isolated from Garcinia morella Desser. Proc Indian Acad Sci, 87, 75–86.
- Sukpondma Y, Rukachaisirikul V, Phongpaichit S. (2005a). Antibacterial caged-tetraprenylated xanthones from the fruits of Garcinia hanburyi. Chem Pharm Bull, 53, 850–852.
- Sukpondma Y, Rukachaisirikul V, Phongpaichit S. (2005b). Xanthone and sesquiterpene derivatives from the fruits of Garcinia scortechinii. J Nat Prod, 68, 1010–1017.
- Tao SJ, Guan SH, Wang W, Lu ZQ, Chen GT, Sha N, Yue QX, Liu X, Guo DA. (2009). Cytotoxic polyprenylated xanthones from the resin of Garcinia hanburyi. J Nat Prod, 72, 117–124.
- Thoison O, Fahy J, Dumontet V, Chiaroni A, Riche C, Tri MV, Sévenet T. (2000). Cytotoxic prenylxanthones from Garcinia bracteata. J Nat Prod, 63, 441–446.
- Thoison O, Cuong DD, Gramain A, Chiaroni A, Hung NV, Sevenet T. (2005). Further rearranged prenylxanthones and benzoquinones from Garcinia bracteata. Tetrahedron, 61, 8529–8535.
- Tisdale EJ, Slobodov I, Theodorakis EA. (2004). Unified synthesis of caged Garcinia natural products based on a site-selective Claisen/Diels-Alder/Claisen rearrangement. Proc Natl Acad Sci USA, 101, 12030–12035.
- Velingkar VS, Dandekar VD. (2010). Modulation of P-glycoprotein mediated multidrug resistance (MDR) in cancer using chemosensitizers. Int J Pharm Sci Res, 1, 104–111.
- Wang LL, Li ZL, Xu YP, Liu XQ, Pei YH, Jing YK, Hua HM. (2008a). A new cytotoxic caged polyprenylated xanthone from the resin of Garcinia hanburyi. Chin Chem Lett, 19, 1221–1223.
- Wang T, Wei J, Qian X, Ding Y, Yu L, Liu B. (2008b). Gambogic acid, a potent inhibitor of survivin, reverses docetaxel resistance in gastric cancer cells. Cancer Lett, 262, 214–222.
- Wang J, Liu W, Zhao Q, Qi Q, Lu N, Yang Y, Nei FF, Rong JJ, You QD, Guo QL. (2009a). Synergistic effect of 5-fluorouracil with gambogic acid on BGC-823 human gastric carcinoma. Toxicology, 256, 135–140.
- Wang X, Chen Y, Han QB, Chan CY, Wang H, Liu Z, Cheng CH, Yew DT, Lin MC, He ML, Xu HX, Sung JJ, Kung HF. (2009b). Proteomic identification of molecular targets of gambogic acid: Role of stathmin in hepatocellular carcinoma. Proteomics, 9, 242–253.
- Wang J, Zhao Q, Qi Q, Gu HY, Rong JJ, Mu R, Zou MJ, Tao L, You QD, Guo QL. (2011). Gambogic acid-induced degradation of mutant p53 is mediated by proteasome and related to CHIP. J Cell Biochem, 112, 509–519.
- Wu J, Xu YJ, Cheng XF, Harrison LJ, Sim KY, Goh SH. (2001). A highly rearranged tetraprenyl xanthonoid from Garcinia gaudichaudii (Guttiferae). Tetrahedron Lett, 42, 727–729.
- Wu X, Cao S, Goh S, Hsu A, Tan BK. (2002). Mitochondrial destabilisation and caspase-3 activation are involved in the apoptosis of Jurkat cells induced by gaudichaudione A, a cytotoxic xanthone. Planta Med, 68, 198–203.
- Wu XH, Tan BKH, Cao SG, Sim KY, Goh SH. (2000). Two minor, cytotoxic caged xanthonoids from Garcinia gaudichaudii. Nat Prod Lett, 453–458.
- Wu ZQ, Guo QL, You QD, Zhao L, Gu HY. (2004). Gambogic acid inhibits proliferation of human lung carcinoma SPC-A1 cells in vivo and in vitro and represses telomerase activity and telomerase reverse transcriptase mRNA expression in the cells. Biol Pharm Bull, 27, 1769–1774.
- Xu X, Liu Y, Wang L, He J, Zhang H, Chen X, Li Y, Yang J, Tao J. (2009). Gambogic acid induces apoptosis by regulating the expression of Bax and Bcl-2 and enhancing caspase-3 activity in human malignant melanoma A375 cells. Int J Dermatol, 48, 186–192.
- Xu YJ, Yip SC, Kosela S, Fitri E, Hana M, Goh SH, Sim KY. (2000). Novel cytotoxic, polyprenylated heptacyclic xanthonoids from Indonesian Garcinia gaudichaudii (Guttiferae). Org Lett, 2, 3945–3948.
- Yang Y, Yang L, You QD, Nie FF, Gu HY, Zhao L, Wang XT, Guo QL. (2007). Differential apoptotic induction of gambogic acid, a novel anticancer natural product, on hepatoma cells and normal hepatocytes. Cancer Lett, 256, 259–266.
- Ye K. Gambogic amine, a selective TrkA agonist with neuroprotective activity. (2011). US Pat Appl: US 2011/0077293 A1, 15 pp.
- Yi T, Yi Z, Cho SG, Luo J, Pandey MK, Aggarwal BB, Liu M. (2008). Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res, 68, 1843–1850.
- Yu J, Guo QL, You QD, Lin SS, Li Z, Gu HY, Zhang HW, Tan Z, Wang X. (2006). Repression of telomerase reverse transcriptase mRNA and hTERT promoter by gambogic acid in human gastric carcinoma cells. Cancer Chemother Pharmacol, 58, 434–443.
- Yu J, Guo QL, You QD, Zhao L, Gu HY, Yang Y, Zhang HW, Tan Z, Wang X. (2007). Gambogic acid-induced G2/M phase cell-cycle arrest via disturbing CDK7-mediated phosphorylation of CDC2/p34 in human gastric carcinoma BGC-823 cells. Carcinogenesis, 28, 632–638.
- Zhang L, Yi Y, Chen J, Sun Y, Guo Q, Zheng Z, Song S. (2010a). Gambogic acid inhibits Hsp90 and deregulates TNF-a/NF-κB in HeLa cells. Biochem Biophys Res Commun, 403, 282–287.
- Zhang QG, Li CP, Chen JH, Ouyang J. (2009). Apoptosis-inducing effect of gambogic acid on K562 cells and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 17, 1443–1447.
- Zhang WH, Li C, Ding J, Frei B. (2010b). Gambogic acid inhibits adhesion molecule expression and NF-κB activation in human aortic endothelial cells. 15th World Congress on Heart Disease, International Academy of Cardiology, Annual Scientific Session 2010. Vancouver, BC, Canada, July 24–27 [Online]. Available at: www.cardiologyonline.com/wchd10/Abstracts/S25.../1361%20Zhang.doc.
- Zhao J, Qi Q, Yang Y, Gu HY, Lu N, Liu W, Wang W, Qiang L, Zhang LB, You QD, Guo QL. (2008). Inhibition of alpha(4) integrin mediated adhesion was involved in the reduction of B16-F10 melanoma cells lung colonization in C57BL/6 mice treated with gambogic acid. Eur J Pharmacol, 589, 127–131.
- Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. (2004). Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull, 27, 998–1003.
- Zhao Q, Yang Y, Yu J, You QD, Zeng S, Gu HY, Lu N, Qi Q, Liu W, Wang XT, Guo QL. (2008). Posttranscriptional regulation of the telomerase hTERT by gambogic acid in human gastric carcinoma 823 cells. Cancer Lett, 262, 223–231.
- Zhao W, Zhou SF, Zhang ZP, Xu GP, Li XB, Yan JL. (2011). Gambogic acid inhibits the growth of osteosarcoma cells in vitro by inducing apoptosis and cell cycle arrest. Oncol Rep, 25, 1289–1295.