Abstract
Context: Andrographis paniculata Nees. (Acanthaceae) is an annual herbaceous plant widely cultivated in southern Asia, China, and Europe. It is used in the treatment of skin infections in India, China, and Malaysia by folk medicine practitioners.
Objective: Antifungal activity of the whole plant extracts and isolation of active principles from A. paniculata were investigated.
Materials and methods: Dichloromethane (DCM) and methanol (MEOH) extracts of A. paniculata whole plant were screened for their antifungal potential using broth microdilution method in vitro against seven pathogenic fungal species responsible for skin infections. Active principles were detected through bioguided assays and isolated using chromatography techniques. Structures of compounds were elucidated through spectroscopy techniques and comparisons were made with previously reported data for similar compounds.
Results: DCM extract revealed lowest minimum inhibitory concentration (MIC) value (100 μg/mL) against Microsporum canis, Candida albicans, and Candida tropicalis, whereas MEOH extract revealed lowest MIC (150 µg/mL) against C. tropicalis and Aspergillus niger. DCM extract showed lowest minimum fungicidal concentration (MFC) value (250 µg/mL) against M. canis, C. albicans, C. tropicalis and A. niger, whereas MEOH extract showed lowest MFC (250 µg/mL) against Trichophyton mentagrophytes, Trichophyton rubrum, M. canis, C. albicans, C. tropicalis and A. niger. Bioassay guided isolation from DCM and MEOH extract afforded 3-O-β-d-glucosyl-14-deoxyandrographiside, 14-deoxyandrographolide, and 14-deoxy-11,12-didehydroandrographolide as antifungal compounds. The lowest MIC (50 µg/mL) and MFC (50 µg/mL) was exerted by 14-deoxyandrographolide on M. canis.
Discussion and conclusion: This is first report on the isolation of antifungal substances through bioassay-guided assay from A. paniculata. Our finding justifies the use of A. paniculata in folk medicines for the treatment of fungal skin infections.
Introduction
Infectious diseases are one of the main causes of morbidity and mortality worldwide (CitationMeena et al., 2010). During the past several years, there has been an increasing incidence of fungal infections due to an increase in immune-compromised population such as organ transplant recipients, cancer, and HIV/AIDS patients. Fungal infections though not as frequent as bacterial or viral infections, have nonetheless been increasing in incidence in the human population over the last 20 years. This fact coupled with the resistance to antibiotics and with the toxicity during prolonged treatment with several antifungal drugs, is the reason for an extended search for new drugs to treat opportunistic fungal infections (CitationFostel & Lartey, 2000). Hence, effective antifungal therapy could prove a significant role in health care; the screening of traditional medicinal plants in search of novel antifungal agents is now more frequently performed. The search for novel antifungal agents relies greatly on ethnobotanical information and ethno-pharmacological exploration (CitationMotsei et al., 2003; CitationFortes et al., 2008).
Andrographis paniculata (Burm.f.) Wall. ex Nees., (Acanthaceae) (King of Bitters, Hempedu Bumi in Malay) is an annual herbaceous plant widely cultivated in southern Asia, China, and some parts of Europe. The whole plant and roots have traditionally been used over the centuries in Asia and Europe as a folk medicine for a wide variety of ailments or as herbal supplements for health promotion (CitationChopra et al., 1982; CitationKhory & Katrak, 1984; CitationChang & But, 1987). In traditional Chinese medicine, it is widely used to get rid of body heat, as in fevers, and to dispel toxins from the body (CitationTang & Eisenbrand, 1992). In European countries, it is frequently used to prevent the common cold (CitationCaceras et al., 1997). A. paniculata has been reported to have a wide range of pharmacological activities – antidiabetic (CitationSyahrin et al., 2006), antiviral (CitationWiart et al., 2005), antibacterial (CitationSingha et al., 2003; CitationSule et al., 2011a, Citation2011b), anticancer (CitationTan et al., 2005; CitationGeethangili et al., 2008), antiinflammatory (CitationShen et al., 2002), hepatoprotective (CitationTrivedi & Rawal, 2005), immunestimulatory/immunomodulatory (CitationIruretagoyena et al., 2005; CitationWang et al., 2010), and antisnakebite activity (CitationSamy et al., 2008).
Evidence of its wide use by the traditional clerics in India, Malaysia, and China for treating some skin infections (CitationTapsell et al., 2006) and its promising in vitro antibacterial activity (CitationSingha et al., 2003; CitationSule et al., 2011a,Citationb) prompted us to choose this plant for further evaluation in order to ascertain its antifungal potential and responsible agents to treat skin infections that are caused by pathogenic fungi. This area of the pharmacological activity of this plant has not yet been thoroughly investigated.
Materials and methods
Instruments
Melting points of all compounds were determined using Buchi B-545 instrument (Buchi, Switzerland). UV spectra were determined on SECOMAM UV-Vis Spectrophotometer (Jena Analytic, Germany). IR spectra were recorded on Perkin Elmer Spectrum RXI FT-IR Spectrometer using KBr disks. 1H- and 13C-NMR spectra were recorded on a Bruker instrument at 600 MHz and 150 MHz, respectively.
Collection of plant material
Fresh whole plant of A. paniculata was procured from the botanical garden of Forest Research Institute of Malaysia (FRIM), Kuala Lumpur, Malaysia, during the month of April, 2009. The plant was identified by Dr. Richard Chung Cheng Kong (Taxonomist), FRIM, Malaysia. The voucher specimen (NMPC-Q25) has been deposited in the Herbarium, Faculty of Pharmacy, IIUM, Kuantan, Pahang DM, Malaysia for future references.
Preparation of dichloromethane (DCM) and methanol (MEOH) extracts
The fresh A. paniculata whole plant (5 kg) was dried in a PROTECH laboratory air dryer (FDD-720-Malaysia) at 40°C for 7 days and pulverized [600 g (12%)] using Fritsch Universal Cutting Mill-PULVERISETTE 19-Germany. It was then stored in a desiccator at 2°C until further use. All solvents were double distilled before use. Dry powdered A. paniculata whole plant was successively extracted with DCM and MEOH using Soxhlet apparatus for 6 h separately. The extracts were filtered and concentrated using a rotary evaporator (Buchi Rotary Evaporator, R-210, Switzerland). Final concentrated extracts upon freeze drying [DCM extract 10.10 g (4.04%) and MEOH extract 19.25 g (7.07%)] were stored at 2°C in labeled sterile bottles and kept as aliquots until further antifungal evaluation. All chemicals used in this study were of analytical grade and double distilled.
Test microorganisms
Seven reference fungal strains notable for skin pathogenesis were chosen for antifungal investigation: three dermatophytes [Trichophyton mentagrophytes (IMR T-44), Trichophyton rubrum (ATCC-28188), Microsporum canis (ATCC-36299)], one non-dermatophyte [Aspergillus niger (IMR A-102)], and three yeasts [Candida albicans (ATCC-90028), Candida krusei (IMR C-368), Candida tropicalis (IMR C-353)]. All fungal strains were purchased either from the Institute for Medical Research (IMR), Malaysia or from American Type Culture Collection (ATCC), USA, respectively. All fungal stock cultures were maintained on Potato dextrose agar (PDA) (for non-dermatophyte and yeasts) and Sabouraud dextrose agar (SDA) (for dermatophytes) slants (OXOID Ltd., England) at 4°C and pH 5.6.
Screening for antifungal activity
Determination of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
Broth microdilution method recommended by Clinical and Laboratory Standards Institute, USA (CitationCLSI, 2008a,b) was used for the determination of MIC and MFC values for each plant extract and bioguided isolated compounds. A two-fold dilution series of plant extracts (DCM and MEOH) and compounds dissolved in 10% DMSO (which had no inhibitory activity against test microorganisms) was prepared: 400, 350, 300, 250, 200, 150, 100, 50 and 01 µg/mL, this was then added to the broth media in a 96-wells microtiter plates. Subsequently, 100 µL of inoculum (adjusted to 107 CFU/mL) was added to each well. Fungal suspensions with 10% DMSO was used as negative control, whereas broth containing standard drugs, nystatin (NY) and griseofulvin (GR) (30 µg/mL) were used as positive controls. Each extract was assayed in duplicate and each time two sets of microtiter plates were prepared, one was kept for incubation while another set was kept at 4°C for comparing the turbidity in the wells of microtitre plate. MIC values were determined as the lowest concentration of the extracts/compounds that showed no turbidity after incubation. The turbidity of the wells in the microtiter plate was interpreted as visible growth of microorganisms. The MFC was determined by subculturing 50 µL from each well showing no apparent growth onto freshly prepared SDA plates incubated at 32°C for 48 h (dermatophytes) and PDA plates incubated at 28°C for 48 h (yeast and non-dermatophyte). Lowest concentration of extract/compounds showing no visible growth on subculturing was taken as MFC.
Bioautography
MEOH and DCM (10 µL each) extracts were applied as small spots separately on sterilized 10 × 10 cm TLC plates and developed in hexane: acetone (2:1). Broth cultures of A. niger, C. tropicalis and M. canis (adjusted to 107 CFU/mL) were mixed with 30 mL molten SDA separately. Mixture of agar and microbial suspensions were spread aseptically onto the TLC plates in square Petri dishes, allowed for 25 min to solidify, and then the plates were incubated at 32°C for 48 h. At the end of incubation time, 0.5% p-iodonitrotetrazolium violet (INT) was uniformly sprayed on the TLC plates and the active antifungal compounds in the plant extracts were detected as whitish TLC spots on a deep pink background after incubation in the moist atmosphere for 2–3 days at 25°C (CitationRahalison et al., 2007).
Bioguided isolation of active principles
The air dried and powdered whole plant (5 kg) of A. paniculata was extracted by macerating in methanol (20.0 L) at room temperature for 24 h, filtered, and evaporated under reduced pressure. The whole process was repeated three times to ensure maximum yield of methanol soluble compounds from the plant powder. Each time, filtrate was evaporated under reduced pressure and combined. The dark blackish green residue was further extracted with DCM and MEOH, respectively, in order to get DCM and MEOH soluble compounds separately. The DCM soluble portion upon evaporation under reduced pressure yielded 120 g DCM extract and MEOH soluble portion yielded 217 g MEOH extract. DCM and MEOH extracts were chromatographed separately on a silica gel column using different polarity to obtain several fractions. These fractions were further chromatographed on a silica gel column to afford three antimicrobial compounds viz., AB-1 (22 mg), AB-2 (25 mg), and AB-3 (13 mg). The structures of isolated antifungal constituents were unambiguously elucidated based on chemical evidences, spectral analysis and comparison with already reported data.
Identification and isolation of antifungal compounds (AB-1 and AB-2) from MEOH extract
MEOH extract (100 g) was loaded onto column (10 × 50 cm) packed with silica gel 60 particle size 0.063–0.2 mm (70–230 mesh) (Fluka Chemika). The column was eluted with pure hexane (100%) through hexane:ethyl acetate with increasing polarity (90:10, 80:20, 70:30, 50:50, 30:70, 20:80, 10:90, 0:100) and finally with ethyl acetate:methanol with increasing polarity (95:5, 90:10, 80:20, 70:30, 50:50, 30:70, 20:80, 0:100); 50 mL were collected in individual test tubes, 214 fractions were obtained and eluents from test tubes that exhibited similar R.f. values as indicated by TLC analysis in different solvent systems were pooled together and a total of 20 (AM–TM) similar fractions were eventually obtained. Antifungal active fractions IM–LM and NM–OM afforded crystallized products of two compounds which were further purified using preparative column chromatography on silica gel 60 and eluted with hexane:ethyl acetate to produce various fractions. Eluents in test tubes 78–85, 88–97, 98–107 and 108–122 upon crystallization with absolute ethyl alcohol afforded pure compound AB-1 (white crystals) (22 mg); M.P. 242–244°C; R.f. 0.78 (chloroform:methanol:ethylacetate (CME) (16: 0.8: 1.2)); UV λmax MeOH nm: 202; IR (cm−1) ν: 3351, 1732, 165, 899 and AB-2 (colorless crystals) (25 mg); M.P. 172–174°C, R.f. 0.66 (CME); UV λmax MeOH nm: 223 and IR (cm−1) ν: 3367, 1736, 1646, 896. 1H-NMR and 13C-NMR spectral data of AB-1 and AB-2 are given in .
Table 1. 1H- and 13C-NMR chemical shifts of AB-1 (3-O-β-d-glucosyl-14-deoxyandrographiside), AB-2 (14-deoxyandrographolide) and AB-3 (14-deoxy-11,12-dihydrondrographolide), (chemical shifts in δ ppm), 600 and 150 MHz in CDCl3, “o” denotes overlapping signals, J values are given in Hertz (Hz).
Identification and isolation of antifungal compound (AB-3) from DCM extracts
DCM extract (100 g) was loaded onto column (10 × 50 cm) packed with silica gel 60 particle size 0.063–0.2 mm (70–230 mesh) (Fluka Chemika). The column was eluted with 750 mL at a gradient system beginning with pure hexane (100%) through hexane:acetone with increasing polarity (90:10, 80:20, 70:30, 50:50, 30:70, 20:80, 10:90, 0:100) and finally with acetone:methanol with increasing polarity (95:5, 90:10, 80:20, 70:30, 50:50, 30:70, 20:80, 0:100). 50 mL were collected in test tubes, 145 fractions were obtained and eluents from test tubes that exhibited similar R.f. values pooled together and a total of 13 (AD–MD) similar fractions were eventually obtained. These were put in 25 mL conical flask in ethanol for crystallization and recrystallization to obtain pure compounds. Antifungal active fractions FD–ID and JD–MD were pooled together based on TLC profile and kept for recrytallization to afford the mixture of three crystallized substances which were further purified by using preparative column chromatography on silica gel 60 and eluted with hexane:ethylacetate to produce various fractions. Eluents in test tubes 32–36, 62, 101–106, 107–112 and 113–145 upon recrystallization afforded compound AB-3 (whitish crystals) (13 mg); M.P. 208–209°C; R.f. 0.45 (CME); UV λmax MeOH nm: 209 and IR (cm−1) ν: 3373, 1741, 1642, 898. 1H-NMR and 13C-NMR spectral data of AB-3 are given in .
Results
Results of our study showed that the whole plant extracts have good inhibitory effects against most of the pathogenic fungal species taken into consideration. MIC and MFC of active extracts were determined by broth microdilution assay. Highest MIC value was exerted by DCM extract at 250 µg/mL on C. krusei, A. niger, and the lowest was exerted at 100 µg/mL on M. canis, C. albicans, C. tropicalis, whereas MEOH extract revealed highest MIC (200 µg/mL) against T. mentagrophytes, T. rubrum, M. canis, C. albicans, C. krusei and showed lowest MIC (150 µg/mL) against C. tropicalis and A. niger. DCM extract showed highest MFC (300 µg/mL) against T. mentagrophytes, T. rubrum, C. krusei, and lowest MFC (250 µg/mL) against M. canis, C. albicans, C. tropicalis and A. niger. MEOH extract showed highest MFC (350 µg/mL) on C. krusei and the lowest MFC (250 µg/mL) T. mentagrophytes, T. rubrum, M. canis, C. albicans, C. tropicalis and A. niger ().
Table 2. Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of DCM and MEOH extracts of A. paniculata whole plant.
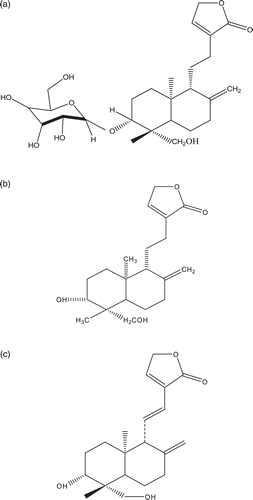
Bioguided isolation from DCM and MEOH extracts of the whole plant of A. paniculata afforded three antifungal compounds viz., 3-O-β-d-glucosyl-14-deoxyandrographiside (AB-1), 14-deoxyandrographolide (AB-2) and 14-deoxy-11,12-dihydrondrographolide (AB-3). Their structures were unambiguously characterized through the analysis of UV, IR, 1H-NMR and 13C-NMR spectral data and direct comparison with the previously reported spectral data of similar compounds (CitationZhou et al., 2008; CitationPoonam et al., 2010).
Compound AB-1 obtained from MEOH extract showed positive Legal and Kedde test, suggests the presence of an α, β-unsaturated lactone in the compound. The 1H- and 13C-NMR spectra of AB-1 revealed signals due to a β-glucopyranosyl group [δH 4.24 (d, J = 7.2 Hz, 1H)] and δC 103.10, 71.72, 72.73, 74.03, 76.23 and 70.72 and the characteristic signals for the double bond containing one hydrogen at carbon 14 in γ-lactone ring were observed at δ 7.10 (t, 1H) in 1H-NMR as well as in 13C-NMR spectra at δ 143.84, respectively (), which corresponds to the 3-O-β-d-glucosyl-14-deoxyandrographiside previously isolated from A. paniculata and reported by CitationZhou et al. (2008) (). Compound AB-2 was also obtained from MEOH extract exhibited positive test for the Legal and Kedde reactions, suggests the presence of an α, β-unsaturated lactone in the molecule. The characteristic NMR spectral data indicated that compound AB-2 was a labdane-type diterpene with α, β-unsaturated γ-lactone. In 1H-NMR spectrum of AB-2, two methyl singlets were observed at δ 0.71 and 1.59, respectively. The characteristic exocyclic methylene protons for AB-2 diterpenoids were observed at δ 4.59 (brs, 1H) and 4.45 (brs, 1H) in 1H-NMR as well as at δ 108.86 in 13C-NMR spectra respectively (). The 1H- and 13C-NMR (in CDCl3) spectra of AB-2 suggested a diterpenoid compound with a structure similar to that of 14-deoxyandrographolide previously isolated from A. paniculata by CitationPoonam et al. (2010) (). However, compound AB-3 was isolated from the DCM extract and its characteristic NMR spectral data indicated to be a labdane-type diterpene with an α, β-unsaturated γ-lactone. AB-3 was also found to be positive for the Legal and Kedde reactions, suggests the presence of an α, β-unsaturated lactone in the same molecule. In 1H-NMR spectrum, two methyl singlets were observed at δ 0.82 and 1.27, respectively. The characteristic exocyclic methylene protons for diterpenoids were observed at δ 4.89 (d, J = 1.8, 1H) and 4.60 (brs, 1H) in 1H-NMR as well as at δ 109.2 in 13C-NMR spectra respectively and the signals for the presence of one hydrogen each residing at C-11 and C-12 were observed in the form of doublet of doublets and single doublet at δ 6.90 (dd, J = 15.6, 9.6 Hz, 1H) and 6.13 (d, J = 15.6 Hz, 1H) as well as at δ 134.71 and 121.12 in 13C-NMR, respectively. The characteristic signals for the double bond containing one hydrogen at carbon 14 for diterpenoids were observed at δ 7.10 (t, 1H) in 1H-NMR as well as in 13C-NMR at δ 129.30, respectively. Two signals in the form of doublet of doublets and doublet for didehydro at carbon 11 and carbon 12 in the 1H-NMR spectrum were observed at δ 6.90 (dd, J = 15.6, 9.6 Hz, 1H) and 6.13 (d, J = 15.6 Hz, 1H), respectively which were also correlated to the signals at δ 134.71 and 121.12 in 13C-NMR spectrum (). The 1H- and 13C-NMR (in CDCl3) spectra of AB-3 explicitly suggested a diterpenoid compound with a structure similar to that of 14-deoxy-11,12-didehydrondrographolide previously isolated from A. paniculata and reported by CitationPoonam et al. (2010) ().
Figure 1. (a) Structure of AB-1 (3-O-β-d-glucosyl-14-deoxyandrographiside) obtained from MEOH extract of A. paniculata whole plant.(b) Structure of AB-2 (14-deoxyandrographolide) obtained from MEOH extract of A. paniculata whole plant. (c) Structure of AB-3 (14-deoxy-11,12-didehydrondrographolide) obtained from DCM extract of A. paniculata whole plant.
All three compounds were further investigated for their thorough antifungal activity by broth microdilution method against three fungal strains (M. canis, A. niger, and C. albicans) on which the plant extracts revealed the most potent antifungal activity. MIC values for all antifungal compounds ranged from 50–150 µg/mL and MFC values ranged from 50–200 µg/mL. The highest MIC (150 µg/mL) was exerted by compound AB-2 and AB-3 against C. tropicalis and M. canis, whereas AB-2 revealed the lowest MIC (50 µg/mL) indicates the most potent antifungal activity against M. canis. The highest MFC (200 µg/mL) was exerted by compound AB-2 on C. tropicalis and the lowest MFC (50 µg/mL) was exerted by compound AB-2 on M. canis. Compound AB-3 (14-deoxy-11,12-didehydrondrographolide) showed broad spectrum antifungal activity as it was found to exhibit inhibitory activity against all fungal strains taken into consideration. In contrast, positive control, nystatin showed the lowest MIC (5 µg/mL) and MFC (10 µg/mL) on M. canis, and griseofulvin revealed the lowest MIC (10 µg/mL) and MFC (20 µg/mL) on A. niger (). The results clearly revealed that all compounds including standard antibiotics were selective in their mode of action on the tested strains. As observed from the results, the antifungal activities of the compounds were more prominent on dermatophytes followed by non-dermatophyte and yeast strains tested. The isolated compounds demonstrated their antifungal effect (high MIC and MFC values) though to a lesser extent as compared to the positive controls i.e., standard antibiotics.
Table 3. Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of active principles and positive controls against selected fungal strains.
Discussion
Various phytochemical compounds which are naturally present in plants as secondary metabolites have been implicated in the conferment of antifungal activities (CitationHostettmann & Marston, 1994; CitationGrayer & Harborne, 1994; CitationOsbourn, 1996; CitationAl-Barwani & Eltayeb, 2004; CitationAthikomkulchal et al., 2006; CitationShanker et al., 2007, CitationFabri et al., 2011). The presence of some of such secondary metabolites in a significant amount in the investigated part of A. paniculata may have conferred the strong antifungal activity on the whole plant extracts. In this regard, higher concentration of these substances may have been responsible for a higher degree of inhibition on the tested strains. Previous studies have indicated that active principles exhibit antimicrobial activity at a relatively lower concentration in comparison to plant extracts; however, plants which display antimicrobial activity at lower concentrations are considered potent antimicrobial plants for further studies (Rios & Recio, 2005). Hence, the inhibitory effects at low concentrations on the microorganisms may in part be mediated through the chemical constituents of the plant.
Results obtained from our study further confirm the traditional use of A. paniculata against pathogenic fungi. A. paniculata whole plant extracts displayed prominent antifungal activity on the dermatophytes, non-dermatophytes and yeasts strains tested. The results also indicate that crude extract of the whole plant A. paniculata exhibited prominent antifungal activity. This behavior could be associated with a possible synergistic action of the compounds isolated from this plant which is in accordance with previous studies of antimicrobial activity of isolated compounds which displayed stronger antimicrobial activity than the plant extracts (CitationMbaveng et al., 2008; CitationSopa et al., 2008). The use of different plant extracts in the treatment of infections caused by various bacteria, viruses, and fungi have already been reported and recognized (CitationKosalec et al., 2005). Natural products, either as pure compounds or as standardized plant extracts, provide unlimited opportunities for new drug leads because of the unmatched availability of chemical diversity (CitationCos et al., 2006).
Conclusion
The TLC bioautography-guided strategy was effectively used to separate the antifungal compounds from the DCM and MEOH extracts of the whole plant of A. paniculata. Three antifungal compounds were successfully isolated. The isolated compounds, 3-O-β-d-glucosyl-14-deoxyandrographiside, 14-deoxyandrographolide and 14-deoxy-11,12-didehydrondrographolide, demonstrated potent antifungal activities against the selected microbial strains. Further investigation of the activities of these compounds and their potential use in the treatment of fungal diseases are still warranted. To our knowledge, this is first report on the isolation of antifungal substances through bioassay-guided assay from A. paniculata.
Acknowledgement
Authors are grateful to the Research Management Center, IIUM for financial assistance through endowment grant # EDW B 0904-267 to accomplish this work. Authors are also grateful to Centre for Research and Instrumentation Management, University Kebangsaan Malaysia (CRIM, UKM), for conducting NMR analysis of all compounds.
Declaration of interest
The authors declare no conflicts of interest.
References
- Al-Barwani FM, Eltayeb EA. (2004). Antifungal compounds from induced Conium maculatum L. plants. Biochem Syst & Ecol, 32, 1097–1108.
- Athikomkulchai S, Prawat H, Thasana N, Ruangrungsi N, Ruchirawat S. (2006). COX-1, COX-2 inhibitors and antifungal agents from Croton hutchinsonianus. Chem Pharm Bull, 54, 262–264.
- Caceras DD, Hancke JL, Burgos RLA, Wickman JK. (1997). Prevention of common cold with Andrographis paniculata dried extract. A pilot double blind trial. Phytomedicine 4, 101–104.
- Chang HM, But PPH. (1987). Pharmacology and applications of Chinese Materia Medica. English translation by Shem Chang-Shing, Yeung, Sih, Cheng-Yao and Lai-Ling, Wang (Chinese Medicinal Material Research Centre, The Chinese University of Hong Kong), Singapore: World Scientific Publishing Co. Pte. Ltd; 2, 918–928.
- Clinical and Laboratory Standards Institute (CLSI). (2008a). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, 3rd ed, CLSI document M27-A3, Clinical and Laboratory Standards Institute, Wayne, PA, USA.
- Clinical and Laboratory Standards Institute (CLSI). (2008b). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd Edition, CLSI document M38-A2. Wayne, PA, USA: Clinical and Laboratory Standards Institute.
- Chopra RN, Chopra IC, Handa KL, Kapur LD. (1982). Indigenous Drugs of India. 2nd Edition. Calcutta, India: Academic Publishers. p 238.
- Cos P, Vlietinck AJ, Berghe DV, Maes L. (2006). Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J Ethnopharmacol, 106, 290–302.
- Fabri RL, Nogueira MS, Moreira Jdos R, Bouzada ML, Scio E. (2011). Identification of antioxidant and antimicrobial compounds of Lippia species by bioautography. J Med Food, 14, 840–846.
- Fortes TO, Alviano DS, Tupinambá G, Padrón TS, Antoniolli AR, Alviano CS, Seldin L. (2008). Production of an antimicrobial substance against Cryptococcus neoformans by Paenibacillus brasilensis Sa3 isolated from the rhizosphere of Kalanchoe brasiliensis. Microbiol Res, 163, 200–207.
- Fostel JM, Lartey PA. (2000). Emerging novel antifungal agents. Drug Discov Today, 5, 25–32.
- Hostettmann K, Marston A. (1994). Search for new antifungal compounds from higher plants. Pure & Appl Chem, 66, 2231–2234.
- Geethangili M, Rao YK, Fang SH, Tzeng YM. (2008). Cytotoxic constituents from Andrographis paniculata induce cell cycle arrest in jurkat cells. Phytother Res, 22, 1336–1341.
- Grayer RJ, Harborne JB. (1994). A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry, 37, 19–42.
- Iruretagoyena MI, Tobar JA, González PA, Sepúlveda SE, Figueroa CA, Burgos RA, Hancke JL, Kalergis AM. (2005). Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J Pharmacol Exp Ther, 312, 366–372.
- Khory RN, Katrak NN. (1984). Materia Medica of India and Their Therapeutics. Delhi, India: Neeraj Publishing House. p 64.
- Kosalec I, Pepeljnjak S, Kustrak D. (2005). Antifungal activity of fluid extract and essential oil from anise fruits (Pimpinella anisum L., Apiaceae). Acta Pharm, 55, 377–385.
- Mbaveng AT, Kuete K, Nguemeving JR, Beng VP, Nkengfack AE, Meyer JJM, Lall N, Krohn K. (2008). Antimicrobial activity of the extracts and compounds obtained from Vismia guineensis (Guttiferae). Asian J Trad Med, 3, 211–223.
- Meena AK, Kaur R, Singh B, Yadav AK, Singh U, Sachan A, Pal B, Rao MM. (2010). Review on antifungal activities of Ayurvedic medicinal plants. Drug Invention Today, 2, 146–148.
- Motsei ML, Lindsey KL, van Staden J, Jäger AK. (2003). Screening of traditionally used South African plants for antifungal activity against Candida albicans. J Ethnopharmacol, 86, 235–241.
- Osbourn AE. (1996). Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell, 8, 1821–1831.
- Poonam K, Tiwari UK, Shukla A, Gaur AK. (2010). Constituents isolated from Andrographis paniculata. Indian J Chem, 49, 356–359.
- Rahalison L, Hamburger M, Hostettmann K. (2007). A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phytochemical Anal, 2, 199–203.
- Ríos JL, Recio MC. (2005). Medicinal plants and antimicrobial activity. J Ethnopharmacol, 100, 80–84.
- Samy RP, Thwin MM, Gopalakrishnakone P, Ignacimuthu S. (2008). Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J Ethnopharmacol, 115, 302–312.
- Shanker KS, Kanjilal S, Rao BV, Kishore KH, Misra S, Prasad RB. (2007). Isolation and antimicrobial evaluation of isomeric hydroxy ketones in leaf cuticular waxes of Annona squamosa. Phytochem Anal, 18, 7–12.
- Shen YC, Chen CF, Chiou WF. (2002). Andrographolide prevents oxygen radical production by human neutrophils: Possible mechanism(s) involved in its anti-inflammatory effect. Br J Pharmacol, 135, 399–406.
- Singha PK, Roy S, Dey S. (2003). Antimicrobial activity of Andrographis paniculata. Fitoterapia, 74, 692–694.
- Sopa K, Supinya T, Sanan S. (2008). Antimicrobial activity of the ethanol extract and compounds from the rhizomes of Kaempferia parviflora. Songklanakarin J Science & Tech, 30, 463–466.
- Sule A, Ahmed QU, Samah OA, Omar, MN. (2011a). Bacteriostatic and bacteriocidal activity of the polar and non-polar extracts of Andrographis paniculata against skin disease causing pathogenic bacteria. J Med Plants Res, 5, 7–14.
- Sule A, Ahmed QU, Samah OA, Omar MN, Hassan NM, Kamal LZM, Yarmo, MA. (2011b). Bioassay guided isolation of antibacterial compounds from Andrographis paniculata (Burm.f.) Wall. ex Nees (Hempedeu bumi). American J Applied Sci, 8, 525–534.
- Syahrin AS, Amrah SS, Chan KL, Lim BY, Hasenan N, Hasnan J, Mohsin SSJ. (2006). Effect of spray dried ethanolic extract of Andrographis paniculata (Burms F.) Nees on streptozotocin induced diabetic rats. Int J Diabeties in Developing Countries 26, 163–168.
- Tan ML, Kuroyanagi M, Sulaiman SF, Najimudin N, Tengku’ Muhammad TS. (2005). Cytotoxic activities of major diterpenoid constituents of Andrographis paniculata in a panel of human tumor cell lines. Pharm Biol, 43, 501–508.
- Tapsell LC, Hemphill I, Cobiac L, Sullivan DR, Fenech M, Patch CS, Roodenrys S, Keogh JB, Clifton PM, Williams PG, Fazio VA, Inge KE. (2006). Health benefits of herbs and spices: The past, the present and the future. Med J Aust, 185, S1–S24.
- Tang W, Eisenbrand G. (1992). Chinese drugs of plant origin, chemistry, pharmacology and use in traditional and modern medicine. Springer Verlag Berlin, 97–103.
- Trivedi N, Rawal UM. (2005). Hepatoprotective and toxicological evaluation of Andrographis paniculata on severe liver damage. Indian J Pharmacol, 32, 288–293.
- Wang W, Wang J, Dong SF, Liu CH, Italiani P, Sun SH, Xu J, Boraschi D, Ma SP, Qu D. (2010). Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol Sin, 31, 191–201.
- Wiart C, Kumar K, Yusof MY, Hamimah H, Fauzi ZM, Sulaiman M. (2005). Antiviral properties of ent-labdene diterpenes of Andrographis paniculata nees, inhibitors of herpes simplex virus type 1. Phytother Res, 19, 1069–1070.
- Zhou KL, Chen LX, Zhuang YL, Wang NL, Yao XS, Qiu F. (2008). Two new ent-labdane diterpenoid glycosides from the aerial parts of Andrographis paniculata. J Asian Nat Prod Res, 10, 939–943.