Abstract
Context: The incidence and mortality of thrombotic disorders are rapidly increasing throughout the world. Therefore, attempts have been made to develop new anticoagulant and antithrombotic drugs. Our previous studies showed that a novel protein, named Fu-P, had fibrinogenolytic activity and much higher fibrinolytic activity on the fibrin plate than urokinase in vitro.
Objective: The antithrombotic activities of Fu-P in vivo are investigated here for the first time.
Materials and methods: Antithrombotic activity of Fu-P was studied in a rat model of artery-vein bypass thrombosis. The anticoagulant activity of Fu-P was measured by clotting assay of activated partial thrombinplastin time and prothrombin time (PT). The effects of Fu-P on the factor Xa and thrombin were assayed using the chromogenic substrate S-2765 and S-2238.
Results: Intravenous injection of Fu-P produced a 58.4% inhibition ratio of thrombus formation at 0.1 mg/kg body weight, while heparin produced 42.5% inhibition ratio of thrombus formation at 0.6 mg/kg body weight. Fu-P significantly prolonged fibrinogen clotting time, activated partial thrombinplastin time and thrombin time, which also prolonged PT. The inhibition assay of the coagulant factors using chromogenic substrates S-2238 and S-2765 showed that Fu-P was not the inhibitor of the thrombin and Xa.
Discussion and conclusion: These findings demonstrated that the novel fibrinolytic enzyme (Fu-P) might also be used as a natural agent for thrombolytic therapy or thrombosis prevention.
Introduction
Fibrin is the major protein component of blood clots, which is formed from fibrinogen by thrombin (CitationVoet et al., 1990). Accumulation of fibrin in the blood vessels usually increases thrombosis, leading to myocardial infarction and other cardiovascular diseases (CitationButenas et al., 2002). Anticoagulant therapy plays an important role in the treatment of cardiovascular diseases. Based on their different working mechanisms, the coagulation system is modulated by four major inhibitory pathways: The protein C anticoagulant pathway, thrombin and factor Xa inhibitors, tissue factor pathway inhibitor, and fibrinogenolytic mechanism (CitationRosenberg &, Rosenberg, 1984; CitationHirsh, 2003; CitationWeitz, 2001). Classic anticoagulant drugs such as heparin and warfarin are very effective, save lives and have been used for more than 50 years. Nevertheless, both heparin and warfarin are nonspecific drugs which have several drawbacks. Heparin is not effective in clot-bound thrombin and may induce immunologically-mediated thrombocytopenia (CitationBates et al., 2006). Warfarin has a small therapeutic window, and requires careful laboratory monitoring. In addition, there is always the burden of drug and food interactions (CitationNutescu, 2003). Therefore, attempts have been made to develop new anticoagulant and antithrombotic drugs.
Our previous studies showed that a novel protein named Fu-P from Fusarium sp. CPCC 480097 was a 28 kDa single protein. The first 15 amino acids of the N-terminal sequence of Fu-P were Q-A-S-S-G-T-P-A-T-I-R-V-L-V-V and have no homology with that of other known fibrinolytic enzymes. In vitro, Fu-P showed fibrinogenolytic activity and much higher fibrinolytic activity on the fibrin plate than urokinase (Wu et al., 2009). This study was performed to investigate antithrombotic effect of Fu-P in vivo.
Materials and methods
Materials
Fu-P was purified from the fermentation broth of Fusarium sp. CPCC 480097 which was kept by Shanghai Health Creation Center of Biopharmaceutical R & D (Shanghai, China). Sprague–Dawley male rats were purchased from Shanghai Slaccas Experimental Animal Co., Ltd (Shanghai, China). The rats had free access to a commercial pellet diet and drinking water before the experiment. Human fibrinogen was purchased from Green Cross China Biological Product Co., Ltd (Huainan, China). Activated partial thrombinplastin time (APTT), prothrombin time (PT) and thrombin time (TT) assay kits were purchased from Dede Behring Marburg Gmbh Inc (Germany). Factor Xa, S-2238 and S-2275 were obtained from Sigma (USA). Heparin was obtained from Shanghai Chemical Reagent Company of China Pharmaceutical Group Corporation (Shanghai, China).
Determination of protein concentration
Protein concentration was determined by Bradford method (CitationBradford, 1976), using bovine serum albumin as standard.
In vivo antithrombotic activity assay
Antithrombotic activity of Fu-P was studied in a rat model of artery-vein bypass thrombosis (CitationMaksimenko et al., 1999). Fifty male SD rats weighting 190–230 g were used. We followed the institutional guidelines for animal care and experimentation. In brief, different doses of Fu-P were administered with an intravenous injection. Control rats were given an equal volume of normal saline (as negative control) or 0.6 mg/kg body weight of heparin (as positive control). After 5 min stabilization period, rats were anaesthetized with an intravenous injection of 30 mg/kg body weight of pentobarbital sodium. Then, the left carotid artery and the right jugular vein were isolated and dissected. A catheter with a 7.5 cm long segment of silk suture in it was inserted into the carotid artery and the jugular vein to form a bypass. After 30 min circulation, the thrombus formed on the silk suture was isolated and weighted. The inhibition ratio of thrombosis was calculated as follow equation:
In which Weight0 was the thrombotic weight when injected normal saline. Weight1 was the thrombotic weight when injected Fu-P and heparin.
In vitro fibrinogen clotting time assay
Fibrinogen clotting time (FCT) was determined according to the method of Zingali with minor modification (CitationZingali et al., 1993). (1) Fu-P (30 µL, 0.3 mg/mL) was incubated with 50 µL of 0.5% human fibrinogen (20 mM Tris-HCl, pH 7.4) for 3 min at 37°C. Then, 30 µL of 5 U/mL human thrombin was added, and the clotting time was determined. (2) Fu-P (30 µL, 0.3 mg/mL) was incubated with 30 µL of 5 U/mL human thrombin for 3 min at 37°C. Then 50 µL of 0.5% human fibrinogen (same buffer) was added, and then the clotting time was determined. (3) Fu-P (30 µL, 0.3 mg/mL) was incubated with 50 µL of 0.5% human fibrinogen for 3 min at 37°C. Then, 60 µL of 5 U/mL human thrombin was added, and the clotting time was determined. (4) Fu-P (30 µL, 0.3 mg/mL) was incubated with 100 µL of 0.5% human fibrinogen for 3 min at 37°C. Then, 30 µL of 5 U/mL human thrombin was added, and the clotting time was determined.
Anticoagulant assay
The anticoagulant activity of Fu-P was measured by clotting assay of APTT, PT and TT. In brief, different doses of Fu-P, normal saline (as negative control) and heparin (same concentration, as positive control) were injected intravenously as described before. After administering 30 min, the fresh rat blood was collected. Nine parts of the fresh rat blood were drawn into one part of 3.8% trisodium citrate. The platelet-poor-plasma was obtained by centrifuging for 20 min at 2500g. Measurements of plasmin APTT, PT and TT were performed by using Sysmex CA1500 and an assay kit as described (CitationKim & Lee, 2006; CitationMotoyashiki, 2003; CitationGuglielmone et al., 2002).
Concentration of fibrinogen assay
After administering, the concentration of fibrinogen (FBG) in the platelet-poor-plasma was measured by the method of CitationVon Clauss (1957) with minor modification.
Amidolytic activity of the enzyme
The assays were adapted to 96-well microtiter plates. The factor Xa assay used the chromogenic substrate S-2765 (CitationChu et al., 2003). 60 µL Tris-HCl buffer (50 mM Tris, 100 mM NaCl, pH 8), 20 µL distilled water (as control) or 20 µL Fu-P solution (30, 50, and 100 µg/mL) and 20 µL factor Xa solution (1.5 U/mL) were pipetted into a well. After 10 min incubation at room temperature, 20 µL of S-2765 (2 mM) was added to each well. This mixture was incubated for 15 min at room temperature. The hydrolysis of the substrate was stopped by adding 80 µL of acetic acid. The absorbance change was measured at 405 nm with Bio-RAD model 405 microplate spectrophotometer.
The effect of Fu-P on the hydrolysis rate of thrombin was preformed similarly as described in the factor Xa assay (CitationPhilomene et al., 1998). Instead of factor Xa and S-2765, thrombin (5 U/mL) and S-2238 (2 mM) were used.
Statistical analysis
The experimental results were presented as the mean ± SEM. The data were analysed using ANOVA-test. The differences were considered as statistically significant at p < 0.05.
Results
Effect of Fu-P on thrombosis in vivo
The effect of Fu-P on thrombosis model in vivo was investigated using an experimental artery-vein bypass thrombosis model in rats. An injection of Fu-P caused a dose-dependent inhibition of thrombus formation (). Fu-P was more effective to inhibition thrombosis than heparin. With a dose of 0.1 mg/kg body weight, a 58.4% inhibition of thrombosis was observed. However, a 42.5% inhibition of thrombosis was observed at the dose of 0.6 mg/kg body weight.
Table 1. Effects of Fu-P on artery-vein bypass thrombosis (n = 10).
Effect of Fu-P on FCT
As shown in , when Fu-P was first incubated with fibrinogen, no coagulant was observed in 3 min with Fu-P at 0.3 mg/mL. But, if Fu-P was first incubated with thrombin, Fu-P prolonged the clotting time and reached 50.2 s at same concentration, then the clot disappeared at 150 s. Augmentation of thrombin showed no effect on FCT. However, augmentation of fibrinogen showed a notable effect on prolongation of FCT, the clotting time of which reached 126.5 s.
Table 2. Effects of Fu-P on FCT.
Effect of Fu-P on the anticoagulant assays
To investigate the interactions of Fu-P with coagulant factors, the effects of Fu-P on coagulant time were evaluated by APTT, PT and TT and summarized in . APTT and TT were strongly prolonged and the inhibition of clot formation was also Fu-P dose-dependent. The APTT of Fu-P in the experiment was 21.2 s at 0.01 mg/kg body weight, 25.7 s at 0.03 mg/kg body weight and 151.52 s at 0.1 mg/kg body weight. Fu-P prolonged the TT and reached 49.7 s at 0.01 mg/kg body weight, 55.1 s at 0.03 mg/kg body weight and 103.3 s at 0.1 mg/kg body weight. A prolonged effect on PT of Fu-P was observed at 0.1 mg/kg body weight. Heparin was used as a positive control. Respectively, Fu-P showed higher anticoagulant activity than that of heparin, which showed APTT as 134.4 s and TT as 92.4 s at 0.6 mg/kg body weight.
Table 3. Effects of Fu-P on rat plasma coagulant time (n = 10).
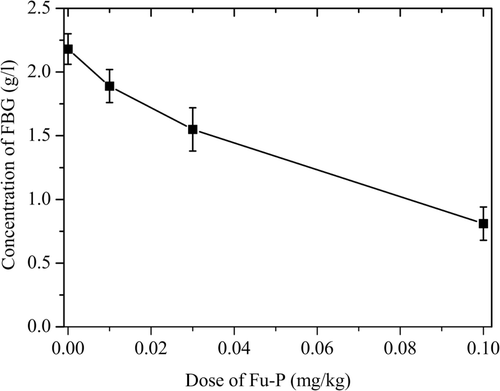
Effect of Fu-P on the concentration of fibrinogen in vivo
In order to find the effect of Fu-P on fibrinogen in vivo, we detected the concentration of fibrinogen in the platelet-poor-plasma after administering. As shown in , the effect of Fu-P on the concentration of fibrinogen in vivo was dose-dependent. Heparin, as positive control, did not show any effect on FBG (data no shown).
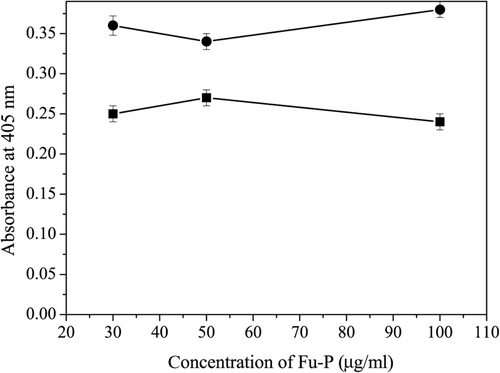
Chromogenic substrate assay
The chromogenic substrate S-2765 was used to test whether Fu-P had any effect on factor Xa. In our experiment, Xa activity could not be inhibited by Fu-P, showing that Fu-P could not inhibit Xa activity (). The chromogenic substrate S-2238 is a specific substrate for thrombin. The hydrolysis of S-2238 by thrombin was not affected by the addition of Fu-P, indicating that Fu-P was unable to inhibit thrombin activity ().
Discussion
It has been previously demonstrated that Fu-P from Endophytic fungus Fusarium sp. CPCC 480097 was a novel protease and showed fibrinogenolytic activity and fibrinolytic activity in vitro. In this study, the present work was aimed at determining the potent antithrombotic activity in vivo.
The model of artery-vein bypass thrombosis was widely used in evaluating the properties of antithrombotic preparations. In our experimental conditions, Fu-P showed the significant in vivo inhibition of thrombus formation. The blood coagulant mechanism was an extremely complicated scheme. Prolongation of ATPP showed inhibition of the intrinsic and or common system. The result of TT tested suggested two possibilities for the site of action of Fu-P. They were: (1) fibrinogen itself was affected; (2) thrombin itself was affected. As shown by the FCT assay, the results implied that the possible target of Fu-P was fibrinogen. Upon further study, the effect of Fu-P on the concentration of FBG in vivo was dose-dependent. The amidolytic assays were a reliable method in testing the effect of antithrombotic preparations on coagulation factors. As shown by the chromogenic assay, Fu-P showed no the inhibition to thrombin and Xa activity. From this researche, it was clear that the mechanism of Fu-P affecting antithrombotic activity should be due to inhibition of fibrin polymerization by hydrolyzing FBG.
Fibrinogen, the final molecule in coagulant cascade before the deposition of fibrin, is involved in primary haemostasis, platelet aggregation and a major determinant of plasma viscosity (CitationDahlbäck, 2000; CitationDrouet, 1996; CitationHoward et al., 2008). Elevation of fibrinogen levels is associated with increased cardiovascular risk in the general population, in patients with coronary artery disease and hypertension (CitationKannel, 1997). Fibrinogen levels also predict recurrent events in patients with coronary heart disease and prior cerebrovascular disease (CitationTanne et al., 2001; CitationRothwell et al., 2004). Defibrase and defibrin could significantly reduce the levels of fibrinogen and produce a good therapeutic effect in the treatment of acute cerebral infarction in clinical research (CitationPeng & Huang, 2004; CitationFrizzell, 2005). Furthermore, practice has verified that an appropriate decrease of fibrinogen levels plays an important role in reducing the formation of new thrombosis (CitationVlachopoulos et al., 2007).
In summary, Fu-P, a novel protein, showed significant antithrombotic activity and fibrinogenolytic activity. Therefore, this enzyme may be used to prevent the formation of venous blood clots.
Declaration of interest
This work was partly supported by the National Infrastructure of National resources for Science and Technology (Grant no: 2005DKA21203).
References
- Bates SM, Weitz JI. (2006). The status of new anticoagulants. Br J Haematol, 134, 3–19.
- Bin Wu, Licheng, Wu, Daijie Chen, Zhijun, Yang, Minyu Luo. (2009). Purification and characterization of a novel fibrinolytic protease from Fusarium sp. CPCC 480097. J Ind Microbiol Biot, 36, 451–459.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248–254.
- Butenas S, Mann KG. (2002). Blood coagulant. Biochemistry, 67, 5–15.
- Chu AJ, Beydoun S, Mathews ST, Hoang JL. (2003). Novel anticoagulant polyethylenimine: Inhibition of thrombin-catalyzed fibrin formation. Arch Biochem Biophys, 415, 101–108.
- Dahlbäck B. (2000). Blood coagulation. Lancet, 355, 1627–1632.
- Drouet L. (1996). Fibrinogen: A treatable risk factor? Cerebrovasc Dis, 6, 2–6.
- Frizzell JP. (2005). Acute stroke: Pathophysiology, diagnosis, and treatment. AACN Clin Issues, 16, 421–40.
- Guglielmone HA, Agnese AM, Núñez Montoya SC, Cabrera JL. (2002). Anticoagulant effect and action mechanism of sulphated flavonoids from Flaveria bidentis. Thromb Res, 105, 183–188.
- Hirsh J. (2003). Current anticoagulant therapy–unmet clinical needs. Thromb Res, 109, 1–8.
- Howard SC, Algra A, Rothwell PM; (2008) Cerebrovascular cohort studies collaboration. Effect of age and glycaemic control on the association between fibrinogen and risk of acute coronary events after transient ischaemic attack or stroke. Cerebrovasc Dis, 25, 136–143.
- Kannel WB. (1997). Influence of fibrinogen on cardiovascular disease. Drugs, 54, 32–40.
- Kim MS, Lee KA. (2006). Antithrombotic activity of methanolic extract of Umbilicaria esculenta. J Ethnopharmacol, 105, 342–345.
- Maksimenko AV, Tischenko EG, Golubykh VL. (1999). Antithrombotic activity of the superoxide dismutase-chondroitin sulfate complexes in a rat model of arterial injury. Cardiovasc Drugs Ther, 13, 479–484.
- Motoyashiki T, Tu AT, Azimov DA, Ibragim K. (2003). Isolation of anticoagulant from the venom of tick, Boophilus calcaratus, from Uzbekistan. Thromb Res, 110, 235–241.
- Nutescu EA. (2003). The future of anticoagulation clinics. J Thromb Thrombolysis, 16, 61–63.
- Peng X, Huang WY. (2004). Clinical analysis of defibrase injection in treatment of acute cerebral infarction. China J Mod Med, 14, 36–38.
- Philomene WK, Masao Y, Katsushito A, Chinzei Y. (1998). Purification and characterization of a thrombin inhibitor from the salivary glands of a malarial vector mosquito, Anopheles stephensi. Biochim Biophys Acta, 1381, 227–233.
- Rosenberg RD, Rosenberg JS. (1984). Natural anticoagulant mechanisms. J Clin Invest, 74, 1–6.
- Rothwell PM, Howard SC, Power DA. (2004). The cerebrovascular cohort studies collaboration: Fibrinogen concentration and risk of ischaemic stroke and acute coronary events in 5113 patients with transient ischaemic attack and minor ischaemic stroke. Stroke, 35, 2300–2305.
- Tanne D, Benderly M, Goldbourt U. (2001). The bezafibrate infarction prevention study group: A prospective study of plasma fibrinogen levels and the risk of stroke among participants in the bezafibrate infarction prevention study. Am J Med, 111, 457–463.
- Vlachopoulos C, Pietri P, Aznaouridis K, Vyssoulis G, Vasiliadou C, Bratsas A, Tousoulis D, Xaplanteris P, Stefanadi E, Stefanadis C. (2007). Relationship of fibrinogen with arterial stiffness and wave reflections. J Hypertens, 25, 2110–2116.
- Voet D, Voet JG. (1990). Biochemistry. New York; Wiley; pp 87–1095.
- Von Clauss A. (1957). Rapid physiological coagulant method in determination of fibrinogen. Acta Haematol, 17, 237–244.
- Weitz JI. (2001). New anticoagulant drugs. J Thromb Thrombolysis, 12, 7–17.
- Zingali RB, Jandrot-Perrus M, Guillin MC, Bon C. (1993). Bothrojaracin, a new thrombin inhibitor isolated from Bothrops jararaca venom: Characterization and mechanism of thrombin inhibition. Biochemistry, 32, 10794–10802.