Abstract
Context: The floral richness of the North-East Indian region cannot be neglected in context to its medicinal importance. Achyranthes aspera Linn. (Amaranthaceae; Prickly Chaff flower) is an indigenous plant species of this region. Although the local traditional healers have ethnomedical knowledge on the use of this plant, there is no scientific study on wound-healing activity of this plant.
Objective: The healing efficacy of methanol leaf extract of A. aspera (MEAA) in granulation tissue of burn wound and its antioxidant activity are investigated.
Materials and methods: Methanol extract of leaves of A. aspera was used for compounding 5% (w/w) ointment, which was applied topically twice daily in experimental burn wound in rats. Healing potential was assessed by rate of wound contraction, antioxidant and biochemical assay which was supported by gelatin zymography and histopathology.
Results: In the present study, 5% ointment of A. aspera showed significant (p < 0.05) wound healing, which was evident by wound contraction, elevation of various antioxidant enzymes viz. SOD, catalase, vitamin C and prohealing and biochemical parameters like hydroxyproline and protein content than the control animals. Up-regulated expression of matrix metalloproteinases (MMP-2 and 9) was also observed by gelatin zymography. Histopathological examination of the granulation tissues in the A. aspera-treated animals showed collagen deposition, fibroblast proliferation and formation of epidermis.
Discussion and conclusion: The methanol leaf extract of A. aspera showed excellent wound-healing activities which has great potential for development of plant-based product.
Introduction
Wound healing is a complex phenomenon that results in restoration of anatomic continuity and function, accomplished by several processes which involve different phases including inflammation, granulation, fibrogenesis, neovascularization, wound contraction and epithelialization (CitationClark, 1996). The basic principle of optimal wound healing is to minimize tissue damage and provide adequate tissue perfusion and oxygenation, proper nutrition and moist wound-healing environment to restore the anatomical continuity and function of the affected part (CitationPierce & Mustoe, 1995).
According to a WHO report, about 70%–80% of the world’s populations rely on nonconventional medicine mainly from herbal sources in their primary health care and in spite of phenomenal development of the synthetic drug industry and antibiotics, medicinal plants still constitute an important part of pharmacopoeias in both the developed and developing countries (CitationChan, 2003). The floral richness of the North-East Indian region cannot be neglected in context to its medicinal importance. However, climate change is affecting medicinal and aromatic plants around the world and could ultimately lead to losses of some key species. Global warming enhances the CO2 assimilation in plant and increases the plant temperature and plants need more water and due to lack of more water, the plants wilt up and get dried (CitationDhiman & Bhardwaj, 2010). Considering the rich biodiversity of this North-East Indian region, it is expected that screening and scientific evaluation of some traditionally used plants for their wound-healing activity may provide new drug molecule that can combat various side effects of the commercially available synthetic drugs and reduce the cost of medication.
Achyranthes aspera Linn. (Amaranthaceae; Prickly Chaff flower), locally known as Apang, is an annual, biennial, lower portion perennial erect undershrub or rather stiff herb growing up to 0.3 to 1.0 m in height. It grows in tropical and warmer regions. Yunani doctors and local kabiraj use the stem, leaves and fruits as a remedy for piles, renal dropsy, pneumonia, cough, kidney stone, skin eruptions, snake bite, gonorrhea, dysentery, and so on. The plant has antibacterial (CitationAziz et al., 2005), antitumor (CitationChakraborty et al., 2002), antiinflammatory (CitationVetrichelvan & Jegadeesan, 2003), abortifacient activity, increases pituitary and uterine wet weights in ovarectomized rats (CitationWorkineh et al., 2006) and also produces reproductive toxicity in male rats (CitationSandhyakumari et al., 2002). A. aspera extract also elevates thyroid hormone level and reported to have anticoagulant, antiarthritic, antitumor and antihepatocarcinogenic activity (CitationKartik et al., 2010). We have reported antidepressant (CitationBarua et al., 2009, Citation2010a) and analgesic (CitationBarua et al., 2010c) activity of this plant.
Although the local traditional healers have ethnomedical knowledge on the use of this plant, there is no scientific study on wound-healing activity of this plant. In our earlier study on the wound-healing activity of A. aspera using excision and incision wound model (in vivo) in normal Sprague Dawley rats and in Chorioallantoic membrane (CAM) model (in vitro) in 9-day-old embryonated chicken eggs, promising wound-healing and angiogenic activity of methanol extract of A. aspera was reported (CitationBarua et al., 2010b). The present study investigated the healing efficacy and antioxidant activity of A. aspera in impaired wound model viz. experimental full-thickness burn wound in rats and unravel its possible mechanism of action. The acute, subacute oral and dermal toxicity studies of the plant extract was also carried out to assess its toxicity, if any, in experimental animals.
Methods
Plant material
The leaves of the plants were collected from the medicinal garden of the Department of Pharmacology, College of Veterinary Science, Khanapara between February and June, 2010, identified by Taxonomist Dr. S. C. Nath of NEIST, Jorhat, Assam (Specimen No. AAU/CVSc/PHT/ 01) and a voucher specimen was deposited.
Preparation of methanol extract
Fresh leaves of the A. aspera were cleaned from extraneous materials, shade-dried, powdered mechanically, weighed, and stored in air tight container. About 250 g of powdered material was soaked in 1000 mL methanol for 72 h in beaker and mixture was stirred every 18 h using a sterile glass rod. Filtrate was obtained three times after passing through Whatman filter paper No. 1 and the solvent was removed by rotary evaporator under reduced pressure at <45°C temperature, leaving a dark brown residue, which was stored in air tight container at 4°C. Yield was 6.89% (w/w).
Ointment preparation
Ointment of three different concentrations were prepared by mixing 2.5, 5.0 and 7.5 g of MEAA with 97.5, 95 and 92.5 g of white soft petroleum jelly (S. D. Fine Chemicals, India) to prepare the desired concentration. Himax (Indradaru, Somvalk; Indian Herbs Research & Supply Co. Ltd. Darra Shivpuri, Saharanpur), a commonly used commercial polyherbal formulation against various types of wound in veterinary practice was used as standard positive control drug.
Experimental animals
Healthy adult albino rats of either sex, weighing between 150–180 g and Swiss mice (20–30 g) were used for the study. The animals were group housed in polypropylene cages under controlled conditions of temperature (21 ± 2°C), humidity (50 ± 5%), 12/12 h of light-dark cycle and standard pellet diet, water was provided ad libitum. All experiments were carried out during the light period (08.00–16.00 h). The study was conducted after obtaining the approval of the Institutional Animal Ethics Committee (No-770/03/ac/CPCSEA/FVSc, AAU/ IAEC/06/21) and conforms to the national guidelines on the care and use of laboratory animals, India.
Burn wound model
Rats were anesthetized by intra-peritoneal injection of thiopentone (25 mg/kg, i.p.), the dorsal surface of the rat was shaved and the underlying skin was cleaned with 70% ethanol. Full-thickness burn wound was created by using an aluminum metal rod (diameter 1.8 cm, area 250 mm2, melting point 660°C) heated to 85°C. The temperature of the metal rod was monitored with a fabricated digital computerized multimeter. The heated rod was exposed on the shaved area of the rat for 20 s, resting on its own weight of 30 g. No additional pressure was applied on the hand-leaded metal rod. Single burn wound was created on dorsal part of each rat. After 24 h, dead tissues were excised using sterile surgical blade (CitationPriya et al., 2002). Animals were allowed to recover from anesthesia and housed individually in sterile cages.
Experimental design
A preliminary study was conducted for selection of the most effective concentration of MEAA ointment by using 2.5, 5.0 and 7.5% (w/w) ointment for topical application in the burn wound. As 5% (w/w) ointment of MEAA showed optimum wound-healing activity, it was selected for further detail study. The experimental animals were randomly allocated into three groups of six animals each. Group I served as control and the rats received topical application of the vehicle, that is, soft white petroleum jelly. In Group II, 5% (w/w) ointment of MEAA was applied topically and the animals of Group III received topical application of positive control drug, that is, Himax ointment. They were applied twice daily for 7 days.
Wound-healing potential
The wound surface area was measured by tracing its contour using a transparent paper on the eighth day post wounding before wound excision to determine wound contraction. The area (mm2) within boundary was measured planimetrically (CitationUpadhyay et al., 2009). The percent wound contraction was calculated using the following formula:
The granulation tissue was excised to analyze pro-healing biochemical parameters viz. hydroxyproline (CitationWoessner, 1961) and total protein contents (CitationLowry et al., 1951). A 10% homogenate of granulation tissue was prepared in 0.15 M KCl containing 5 mM EDTA. After homogenization, samples were sonicated (10 bursts of 5 s each at 5 s intervals) and an aliquot was withdrawn for estimation of reduced glutathione (GSH; CitationBeutler et al., 1963). In the remaining homogenate, Triton X-100 was added at 0.1% (v/v). Then the samples were incubated at 4°C for 2.5 h and centrifuged at 4226 g. The supernatant was used for estimation of superoxide dismutase (SOD; CitationMarklund & Marklund, 1974), catalase (CAT; CitationAebi, 1984) and vitamin C content (CitationRae, 1984).
Gelatin zymography
Matrix metalloproteinases (MMPs) expression was studied in the granulation tissues by gelatin zymography assay. Granulation tissue was homogenized with Tris buffer (saline 0.9%, Tris 0.05 M, Triton X-100 0.25% and CaCl2 0.02 M) and centrifuged at 4226 g for 30 min. Tissue homogenate (50 µg) was subjected to 10% SDS-PAGE containing 0.1% SDS and 1 g/L gelatin under non-reducing conditions without prior boiling. After electrophoresis, gels were washed in 2.5% Triton X-100 for 30 min to remove SDS and allow protein to renature and gels were then subsequently immersed in activity buffer (50 mM Tris/HCl, 5 mM CaCl2, 0.2 M NaCl, 0.02% NaNO3) for 16 h at 37°C. The gels were next stained with 0.25% Coomassie brilliant blue (CBR-250) in methanol, acetic acid and water (4:1:5), then subsequently destained with methanol, acetic acid and water (4:1:5). Enzymatic activities were detected as clear bands of gelatin lysis against a blue background (CitationUpadhyay et al., 2009).
Histopathological study
For histological studies, granulation tissues collected on eighth day was fixed in 10% neutral formalin solution and dehydrated with a sequence of ethanol–xylene series of solution. The materials were processed by conventional paraffin embedding method. Microtome sections were prepared at 6 µ thicknesses, mounted on glass slides, stained with hematoxylin and eosin (CitationLee & Luna, 1968) and Van Gieson’s stain (CitationJocelyn & Bruce-Gregorios, 1974), followed by observation for histopathological changes under light microscope.
Safety evaluation of methanol leaf extract of A. aspera
Acute oral toxicity
Acute oral toxicity studies have been carried out to investigate the safety aspects of the MEAA. Healthy adult male albino mice were selected for the study and fasted overnight. MEAA was dissolved in 30% DMSO and administered to the mice at two different doses (2000 mg/kg and 4000 mg/kg body weight, p.o.) using a 20-gauge oral-feeding cannula. After 2 h of single administration of MEAA, the animals were provided with food and water. The animals were closely observed in their cages for general behavior, mortality or signs of severe toxic symptoms such as hypoactivity, piloerection, anorexia, salivation, diarrhea, syncope, muscle cramping, convulsions, if any, for 24 h and then daily for next 14 days. Control mice were administered orally with 2 mL/kg of 30% DMSO, once daily, for 14 days. They were observed for general behavior and mortality for 14 days.
On day 15, animals were fasted overnight and blood samples were collected from orbital sinus for clinical chemistry (CitationRiley, 1960) using capillary tubes (with and without heparin as per requirement) under mild ether anesthesia. Blood for hematological studies was collected into tubes containing ethylene-diamine-tetra-acetic acid (EDTA) as an anticoagulant. Hemoglobin and other hematological parameters were determined in control and MEAA-treated animals using standard operating protocol. The serum of the mice of test and control groups were separated and various biochemical parameters like SGOT (IFCC/Kinetic method using Enzokit, Diagnova, RFCL Limited, Haridware, Uttarkhand–249403), SGPT (IFCC/Kinetic method using Enzokit, Diagnova, RFCL Limited, Haridware, Uttarkhand–249403), total cholesterol (CHOD-PAP Diagnostic kits, Velon Pharmaceutical Pvt. Ltd., Mumbai, India) and total serum protein (CitationLowry et al., 1951) were estimated.
Subacute dermal toxicity study
MEAA (1000 mg/kg/d) was applied for 28 d on the skin of one flank of female Wistar rat using a gauze patch. The patch was held in place with a semiocclusive bandage for 4 h, after which the patch was removed and skin cleaned of residual MEAA. Adjacent areas of untreated skin from each animal served as controls. The body weight of the animals was recorded daily. During the experiment, food and water were freely available to the animals and they were closely observed in their cages for general behavior, any mortality and signs of toxic symptoms.
After termination of treatment schedule (28 d) animals were sacrificed, vital organs (kidney, brain, adrenal, ovary, spleen and liver) were carefully dissected out, cleaned of the adhering connective tissues, blotted and accurately weighed. Ratio of each organ to body weight of control and MEAA (1000 mg/kg/d)-treated animals were calculated. Blood samples for hematological studies were obtained from orbital sinus (CitationRiley, 1960) and hemoglobin, TLC, TEC and DLC were determined in control and MEAA-treated animals using standard operating protocol. The serum of the rats of the test and control groups were separated and serum activity of SGOT, SGPT, creatinine (CK-NAC kit), ALB (Albumin kit, BCG method, Merck Specialities Pvt. Ltd., Goa, India) and total serum protein (CitationLowry et al., 1951) of control and MEAA-treated animals were estimated.
Statistical analysis
Data were expressed as mean ± SE and statistical significance between experimental and control values were analyzed by one-way ANOVA followed by Dunnett’s test (Graph Pad Prism 2.01) and Duncan’s test (SPSS version 11.5). p < 0.05 was considered statistically significant.
Results
Wound-healing potential
Significant wound healing activity was observed with 5% (w/w) ointment of MEAA in the experimental burn wound model in rats (). On day 8, the rate of reduction of the wound area in the extract-treated rat was 89.93% which was significantly (p < 0.05) higher than the control (48.53%) animals and Himax (76.99%)-treated group ().
Table 1. Effect of methanol extract of A. aspera on wound area and percent wound contraction in experimental burn wounds in rats.
The protein, hydroxyproline and vitamin C content of the granulation tissues were significantly higher (p < 0.05) in the group treated with 5% (w/w) of MEAA than the control and Himax-treated group. The antioxidant parameters like CAT, SOD and GSH concentration in granulation tissue also increased significantly (p < 0.05) in comparison to the vehicle-treated control animals ().
Table 2. Effect of methanol extract of A. aspera (MEAA) on antioxidant and biochemical parameters in burn wounds on eighth day post wounding in rats.
Gelatin zymography
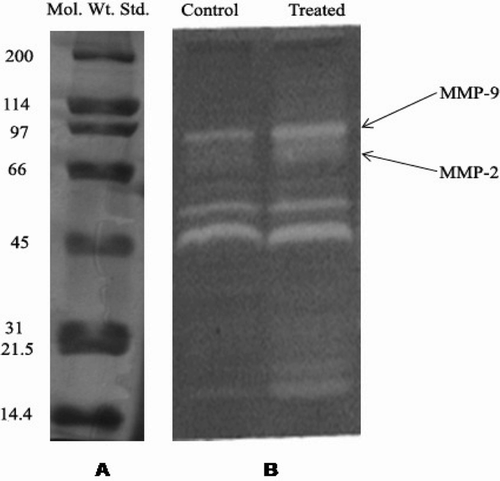
Gelatin zymography analysis of the granulation tissue after 7 days of treatment showed increased expression of both MMP-2 and 9 compared to the control wounds ( and ).
Figure 1. (A) Expression of standard marker proteins (Broad Range, BIO RAD, 10% SDS-PAGE) and (B) Matrix metalloproteinase expression by gelatin zymography (10% SDS-PAGE, 1 g/L gelatin) in methanol leaf extract of A. aspera (MEAA)-treated and untreated burn wound tissue of experimental rats after 7 days of treatment.
Histopathological examinations
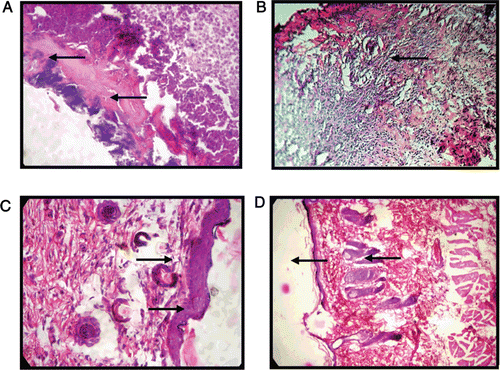
Histopathological changes of the granulation tissues of control, MEAA-treated and standard groups in experimental burns are shown in . The control group showed necrotic debris in the subcutaneous tissue and the wound surface (, whereas, granulation tissue of MEAA-treated group showed deposition of collagen fiber (, fibroblast proliferation and epidermis layer (, indicating wound-healing activity of MEAA. In contrast, Himax-treated standard group showed congestion, development of epidermis and keratin layer (. The histological studies showed an overall early recovery and regeneration in the MEAA-treated group when compared with control group. Furthermore, Vangeison’s staining showed uniform and regularly arranged collagen fibers in the wound tissue of MEAA-treated rats.
Figure 2. Photomicrograph showing histopathological changes of the granulation tissues in burn wound in rats, (A) Control group showing necrotic debris (H&E × 100), (B) MEAA-treated group showing collagen deposition (Vangiesons × 100), (C) MEAA-treated group showing collagen and fibroblast proliferation and formation of epidermis (H&E×100) and, (D) Himax-treated standard group showing congestion, development of epidermis and keratin layer (H&E × 100).
Acute oral-toxicity studies
In acute oral-toxicity studies, there was no increase or decrease in any of the parameters studied, in comparison to control animals. No significant change in any of the hematological variables, viz, hemoglobin, WBC and RBC count as well as biochemical parameters in animals treated with MEAA were observed ().
Table 3. Effect of methanol extract of A. aspera (MEAA) on hematological and biochemical in acute oral toxicity study.
Subacute dermal toxicity study
In the subacute dermal toxicity test in female rats, repeated dermal application of MEAA at limit test dose (1000 mg/kg/d) did not show any sign of toxicity in animals throughout the period of exposure and no morbidity or mortality were recorded. MEAA did not show any variation in body weight and organ body weight ratio of various vital organs as compared to control. There were no significant alteration of any of the hematological and biochemical parameters in the extract-treated group as compared to control group (), indicating A. aspera to be safe for topical application, as there was no induced dermal toxicity.
Table 4. Effect of methanol extract of A. aspera (MEAA) on body weight (g), organ weights relative to body weight (g %), hematological and serum biochemical parameters in subacute dermal toxicity study.
Discussion
The results of the present study clearly demonstrated that the methanol extract of A. aspera possessed a definite prohealing action in burn wound healing as observed by significant increase in the rate of wound contraction, augmented antioxidant levels and total protein contents in the granulation tissue, which was also supported by histopathological study. Triterpenes are known to promote wound-healing process mainly due to their astringent and antimicrobial property (CitationScortichini & Pia, 1991). As the methanol extract of A. aspera contains triterpenes (CitationBarua et al., 2010b), it might contribute towards wound contraction and increased rate of epithelialization in healing tissues.
Angiogenesis is a critical component of wound healing. Delayed or aberrant revascularization at the wound sites contributes to the etiology of chronic wounds (CitationGupta et al., 2008). In our previous study on wound-healing activity of methanol extract of leaves of A. aspera in normal wounds (excision and incision wound) as well as in vitro chick chorioallantoic membrane model, showed significantly higher rate of wound contraction, higher tensile strength and increased angiogenesis in the treated group (CitationBarua et al., 2010b). In the present study, histopathological studies in A. aspera-treated group promoted angiogenesis, thus corroborating the previous findings.
A major protein of extra-cellular matrix is collagen that ultimately contributes to wound strength (CitationSinger & Clark, 1991). The constituents present in A. aspera extract might be responsible for promoting the collagen formation at the proliferative stage of wound healing. Histopathological study showed better proliferation of collagen fibers in the extract-treated group compared to the control group. Measurement of the hydroxyproline could be used as an index for collagen turnover as breakdown of collagen liberates free hydroxyproline. In the present study, significant increase in the hydroxyproline content of the granulation tissue of the animals treated with A. aspera indicates enhanced collagen maturation by increased cross-linking.
Levels of different enzymatic and nonenzymatic antioxidants increased significantly after treatment with A. aspera. These findings suggest decreased oxidative injury in the wound tissue could be due to increased quenching or scavenging of oxygen free radicals by the elevated levels of antioxidants. Reactive Oxygen Species (ROS) are produced in response to cutaneous injury. They impede the healing process by causing damage to cellular membranes, DNA, proteins and lipids as well (CitationIba et al., 2004). Elimination of ROS could be an important strategy in healing of chronic wounds (CitationDissemond et al., 2002). Preventive antioxidants, such as super oxide dismutase (SOD), glutathione peroxidase and catalase (CAT) are the first line of defense against ROSs (CitationSinger & Clark, 1991). It seems reasonable to presume that their action is concerted, as SOD catalyses O2-dismutation producing H2O2, whereas CAT or peroxidases remove it (CitationMavelli et al., 1982). SOD-1 is a key enzyme in the dismutation of the potentially toxic superoxide radicals into hydrogen peroxide and dioxygen (CitationFridovich, 1978). A significant increase in the CAT activity observed in the extract-treated group suggests that H2O2 accumulated due to increased activity of SOD might be properly neutralized by CAT. Major endogenous thiol antioxidant in biological system is reduced glutathione (GSH), which serves as a coenzyme necessary for GPx to eliminate the lipid hydro peroxide (CitationSinger & Clark, 1991). In recent years, research has been increasingly focused on antioxidant activity of ascorbic acid, as “oxidative stress” may be a causal factor in the etiology of diverse and important disorders. The effect of ascorbic acid on collagen synthesis, antioxidant status and immunomodulation make it an appropriate supplement for wound-repair protocols (CitationMacKay et al., 2003). The increased ascorbic acid in the extract-treated group might be responsible for protecting the cells from oxidative stress leading to better wound healing.
Matrix metalloproteinases (MMPs) are key players in every phase of the healing process, that is, eliminate damaged protein, destroy provisional extracellular matrix, facilitate migration to the centre of the wound, remodel the granulation tissue, probably control angiogenesis and also regulate the activity of some growth factors (CitationInkinen et al., 2000). Increased expression of MMP-2 and 9 in MEAA-treated experimental rats suggested that the plant extract might be playing an important role in remodeling of the ECM.
The subacute dermal toxicity study of A. aspera (1000 mg/kg) in female rats did not show any change in body weight, organ body weight ratio, biochemical and hematological parameters. Although it was reported that feeding of 50% ethanol extract of A. aspera to male rats resulted in reduced sperm count, weight of epididymis, serum level of testosterone and testicular activity of 3-β-hydroxysteroid dehydrogenase suggesting reproductive toxicity of A. aspera in male rats (CitationSandhyakumari et al., 2002), but another study reported that A. aspera did not significantly influence the serum level of the ovarian hormones (CitationWorkineh et al., 2006). The present toxicity study with MEAA by both dermal and oral route did not show any organ toxicity, signifying that the plant extract might be safe at the given dose.
In conclusion, it can be interpreted that topical application of A. aspera exhibited significant wound-healing activity in experimental burn wound as evidenced by augmented endogenous antioxidants and increased angiogenesis. Since A. aspera is ubiquitous and abundantly grown, it could be a fairly economical therapeutic agent for wound management. Further pharmacodynamic investigations are required to identify the active fractions responsible for its wound-healing activity for formulation of a plant-based herbal product preserving the indigenous heritage with enormous beneficial value to the society.
Acknowledgments
The authors are grateful to Defence Research Development Organization (DRDO), Govt. of India, New Delhi for financial help, the Director of Research (Vety) and CIF, FVSc, Khanapara for providing necessary facilities. Thanks are also due to the Division of Pharmacology, DRDE, Gwalior, for conducting toxicity study.
Declaration of interest
The authors report no declaration of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aebi H. (1984). Catalase-Methods of Enzymatic Analysis. New York: Academic Press.
- Aziz A, Rahman M, Mondal AK, Muslim T, Rahman A, Quader A. (2005). 3-Acetoxy-6-benzoyloxyapagamide from Achyranthes aspera. Pharmaceutical J, 4, 1816–1820.
- Barua CC, Begum SA, Talukdar A, Barua AG, Borah P, Lahkar M. (2010a). Effect of Achyranthes aspera on modified forced swimming in rats. Pharmacologyonline, 1, 183–191.
- Barua CC, Begum SA, Talukdar A, Pathak DC, Sarma DK, Bora RS. (2010b). Wound healing activity of methanolic extract of leaves of Achyranthes aspera Linn using in vivo and in vitro model—a preliminary study. Indian J Anim Sci, 80, 969–972.
- Barua CC, Talukdar A, Begum SA, Buragohain B, Roy JD, Borah RS, Lahkar M. (2009). Antidepressant-like effects of the methanol extract of Achyranthes aspera Linn. in animal models of depression. Pharmacologyonline, 2, 587–594.
- Barua CC, Talukdar A, Begum SA, Lahon LC, Sarma DK, Pathak DC, Borah P. (2010c). Antinociceptive activity of methanolic extract of leaves of Achyranthes aspera Linn. (Amaranthaceae) in animal models of nociception. Indian J Exp Biol, 48, 817–821.
- Beutler E, Duron O, Kelly BM. (1963). Improved method for the determination of blood glutathione. J Lab Clin Med, 61, 882–888.
- Chakraborty A, Brantner A, Mukainaka T, Nobukuni Y, Kuchide M, Konoshima T, Tokuda H, Nishino H. (2002). Cancer chemopreventive activity of Achyranthes aspera leaves on Epstein-Barr virus activation and two-stage mouse skin carcinogenesis. Cancer Lett, 177, 1–5.
- Chan K. (2003). Some aspects of toxic contaminants in herbal medicines. Chemosphere, 52, 1361–1371.
- Clark RA. (1996). Wound Repair: An Overview and General Consideration-Molecular and Cellular Biology of Wound Repair. New York: Plenum Press.
- Dhiman SC, Bhardwaj P. (2010). Global warming in relation to the occurrence of medicinal plant, Ocimum basilicum Linn. and its tingid bug Monanthia globulifera Walker. J Env Bio Sci, 24, 175–178.
- Dissemond J, Goos M, Wagner SN. (2002). The role of oxidative stress in the pathogenesis and therapy of chronic wounds. Hautarzt, 53, 718–723.
- Fridovich I. (1978). The biology of oxygen radicals. Science, 201, 875–880.
- Gupta A, Upadhyay NK, Sawhney RC, Kumar R. (2008). A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen, 16, 784–790.
- Iba Y, Shibata A, Kato M, Masukawa T. (2004). Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int Immunopharmacol, 4, 1873–1880.
- Inkinen K, Turakainen H, Wolff H, Ravanti L, Kähäri VM, Ahonen J. (2000). Expression and activity of matrix metalloproteinase-2 and -9 in experimental granulation tissue. APMIS, 108, 318–328.
- Jocelyn H, Bruce-Gregorios MD. (1974). Histopathologic Techniques, Philippines: JMC Press Inc.
- Kartik R, Rao ChV, Trivedi SP, Pushpangadan P, Reddy GD. (2010). Amelioration effects against N-nitrosodiethylamine and CCl(4)-induced hepatocarcinogenesis in Swiss albino rats by whole plant extract of Achyranthes aspera. Indian J Pharmacol, 42, 370–375.
- Lee G, Luna HT. (1968). Manual of Histological Staining Methods of the Armed Forces. 3rd ed., New York: Institute of Pathology, American Registry of Pathology, Blakiston Division.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- MacKay D, Miller AL. (2003). Nutritional support for wound healing. Altern Med Rev, 8, 359–377.
- Marklund S, Marklund G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem, 47, 469–474.
- Mavelli I, Rigo A, Federico R, Ciriolo MR, Rotilio G. (1982). Superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Biochem J, 204, 535–540.
- Pierce GF, Mustoe TA. (1995). Pharmacologic enhancement of wound healing. Annu Rev Med, 46, 467–481.
- Priya KS, Gnanamani A, Radhakrishnan N, Babu M. (2002). Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharmacol, 83, 193–199.
- Rae JH. (1984). Chemical Determination of Ascorbic Acid, Dehydroascorbic Acid and Diketogluconic Acids—Methods of Biochemical Analysis. New York: Interscience Publishers.
- Riley V. (1960). Adaptation of orbital bleeding technic to rapid serial blood studies. Proc Soc Exp Biol Med, 104, 751–754.
- Sandhyakumary K, Boby RG, Indira M. (2002). Impact of feeding ethanolic extracts of Achyranthes aspera Linn. on reproductive functions in male rats. Indian J Exp Biol, 40, 1307–1309.
- Scortichini M, Pia RM. (1991). Preliminary in vitro evaluation of the antimicrobial activity of triterpenes and terpenoids towards Erwinia amylovora (Burrill). J Bacteriol, 71, 109–112.
- Singer AJ, Clark RA. (1999). Cutaneous wound healing. N Engl J Med, 341, 738–746.
- Upadhyay NK, Kumar R, Mandotra SK, Meena RN, Siddiqui MS, Sawhney RC, Gupta A. (2009). Safety and healing efficacy of Sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food Chem Toxicol, 47, 1146–1153.
- Vetrichelvan T, Jegadeesan M. (2003). Effect of alcohol extract of Achyranthes aspera Linn. on acute and subacute inflammation. Phytother Res, 17, 77–79.
- Woessner Jfr JF. (1961). The determination of hydroxyproline in tissue and protein sample containing small proportions of this amino acid. Arch Biochem Biophys, 93, 440–447.
- Workineh S, Eyasu M, Legesse Z, Asfaw D. (2006) Effect of Achyranthes aspera L. on fetal abortion, uterine and pituitary weights, serum lipids and hormones. African Health Sciences, 6, 108–112.
