Abstract
Context: Schistosomiasis is a major health problem worldwide. Thus, the search for new schistosomicidal agents from natural sources can provide prototypes for drug discovery.
Objective: The present study investigated the chemical composition of the EtOAc fractions of Styrax pohlii Pohl (Styracaceae) (EF-SP) aerial parts and S. camporum A. DC. leaves (EF-SC), as well as schistosomicidal activities against Schistosoma mansoni adult worms, which have not yet been studied.
Materials and methods: The crude ethanol extracts of S. camporum leaves and S. pohlii aerial parts (EE-SC and EE-SP) were partitioned with n-hexane, EtOAc, and n-BuOH. The EtOAc fractions were purified by preparative HPLC. The crude extracts, EtOAc fractions and pure compounds were tested against S. mansoni adult worms in vitro.
Results: The purification procedure resulted in the isolation of kaempferol-3-O-(2′′,4′′-di-O-(E)-p-coumaroyl)-β-d-glucopyranoside (1), kaempferol-3-O-(2′′,6′′-di-O-(E)-p-coumaroyl)-β-d-glucopyranoside (2), quercetin (3), and kaempferol (4). The bioassay results indicated that EE-SC, EF-SC, EF-SP, and compounds 2 and 4 are able to separate coupled S. mansoni adult worms. Additionally, EE-SC, EF-SP, and compound 4 killed the adult schistosomes in vitro at 100 µg/mL and 100 µM.
Discussion and conclusion: This is the first time that the presence of compounds 1–2 in S. pohlii and 3–4 in S. camporum has been reported. Additionally, biological results indicated that S. pohlii and S. camporum have great potential as a source of active compounds.
Introduction
Schistosomiasis is a major health problem worldwide. It is estimated that 200 million people are infected by trematode flatworms of the genus Schistosoma (CitationSteinmann et al., 2006). The treatment available for schistosomiasis includes praziquantel (PZQ) and oxamniquine. The emerging resistance to the available drugs, mainly in the case of PZQ, has led to the urgent need for new therapeutic agents against this disease (CitationCaffrey, 2007). Thus, the search for new schistosomicidal agents from natural sources can provide prototypes for drug discovery and treatment of neglected tropical diseases.
The Brazilian folk medicine contains references to the use of Styrax camporum Pohl and S. pohlii A. DC. (Styracaceae), use in the treatment of gastrointestinal diseases and fevers, respectively (CitationLorenzi, 1982; CitationRodrigues & de Carvalho, 2008). Styrax genus is also famous for production of a resinous material that is usually secreted when the bark and trunk are injured by sharp objects, and this material can be used as a substitute for the benzoin resin, which displays anti-inflammatory properties (CitationLorenzi, 1982).
Previous phytochemical studies on this genus have resulted in the isolation of lignans derivatives of 3,7-dioxabicyclo [3.3.0] octane, butanolide, and tetrahydrofuran; neolignans derivatives of dihydrobenzofuran; and benzofuran, phenylpropanoids, phenolic acids, pentacyclic saponins, and triterpenes (CitationPauletti et al., 2006).
As part of our ongoing biological and chemical studies on Styrax (CitationPauletti et al., 2000, Citation2002; CitationTeles et al., 2005) and of our investigations on the schistosomicidal activity of natural products (CitationBraguine et al., 2009; CitationMagalhães et al., 2009, Citation2010), we now report the isolation and structural identification of flavonoids from the EtOAc fractions of S. pohlii aerial parts and S. camporum leaves, as well as their schistosomicidal activities against Schistosoma mansoni adult worms, which have not yet been investigated.
Materials and methods
General
1H- and 13C-NMR spectra were recorded in DMSO-d6 on a Varian Unity 500 NMR spectrometer, using TMS as internal standard. Both analytical and preparative HPLC separation analyses were carried out on a Shimadzu LC-6AD system equipped with a degasser DGU-20A5, a UV-VIS detector SPD-20A series, a communication bus module CBM-20A, and a Reodyne manual injector. Separation of the micromolecules was carried out on a SHIMADZU Shim-pack ODS (particle diameter 5 μm, 250 × 4.60 mm, and 250 × 20 mm) column equipped with a pre-column of the same material. The MeOH used in the experiments was HPLC grade, J.T. Baker. Ultrapure water was obtained by passing redistilled water through a Direct-Q UV3 system, Millipore. Silica gel 60 (60–230 mesh, Sigma-Aldrich) and Sephadex LH-20 (Sigma-Aldrich, St. Louis, MO, USA) were employed for column chromatography, as well as silica on TLC Alu foils with fluorescent indicator 254 nm (Sigma-Aldrich). Silica gel 90 reverse phase ODS (Fluka, 230–400 mesh) was utilized for sample preparation prior to injection into the HPLC system.
Plant materials
The leaves of Styrax camporum and the aerial parts of Styrax pohlii were collected from the Brazilan Cerrado area located in the São Paulo State, in the city of Luis Antonio (21°33′–21°37′ S and 47°45′–47°57′ W), in October 2008. The materials were identified by Prof. V.M.M. Gimenez and Prof. M. Groppo. Vouchers specimens (SPFR12170 and SPFR12168, respectively) have been deposited in the Herbarium of the Department of Biology, Laboratory of Plant Systematics, Faculdade de Filosofia Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Brazil (Herbarium SPFR).
Extraction and isolation
The air-dried, powdered aerial parts (2.4 kg) of S. pohlii were extracted with EtOH. After filtration, the solvents were removed under reduced pressure to yield 87 g of the extract. The ethanol extract (EE, 40 g) was then dissolved in MeOH/H2O (2:8, v/v) and successively partitioned with n-hexane, EtOAc, and n-BuOH. After solvent removal using a rotary evaporator, each partition phase yielded 2.8, 8.9, 7.6, and 8.2 g, respectively. The EtOAc residue (3.0 g) was submitted to a solid phase extraction silica gel chromatographic column employing n-hexane/EtOAc/MeOH as eluents, which furnished five fractions. Fraction 4 (716 mg) was dissolved in MeOH/H2O (50:50, v/v) and chromatographed over reverse phase ODS using MeOH/H2O (50:50, v/v), (70:30, v/v) and 100% MeOH. The fraction eluted with MeOH/H2O (70:30, v/v, 100 mg) was subsequently submitted to preparative RP-HPLC purification using MeOH/H2O/AcOH (58:41.9:0.1, v/v/v), UV detection at 254 nm, and 9 mL/min. flow rate, which yielded eleven fractions. Fractions 6 and 7 gave compounds 1 (8.8 mg, Rt 51.7 min) and 2 (9.4 mg, Rt 51.3 min), respectively.
The air-dried leaves (0.77 kg) of S. camporum were powdered and exhaustively extracted by maceration with EtOH/H2O (70:30, v/v) at room temperature. After filtration, the solvent was removed under reduced pressure to yield 85 g. The extract (20 g) was then dissolved in MeOH/H2O (2:8, v/v) and successively partitioned with hexane, EtOAc, and n-BuOH. After solvent removal using a rotary evaporator, each partition phase yielded 0.2, 1.3, 4.6, and 2.8 g, respectively. The EtOAc residue (1.3 g) was dissolved in MeOH and submitted to a Sephadex LH-20 chromatographic column employing MeOH as eluent, which furnished eleven fractions. Fraction 7 (89 mg) was purified by RP-HPLC [MeOH/H2O/AcOH (30:69.9:0.1, v/v/v), UV detection at 254 nm; flow rate 9 mL/min], affording compounds 3 (8 mg, Rt 14.20 min.) and 4 (4 mg, Rt 21.47 min).
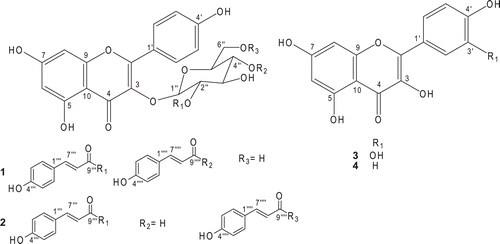
Kaempferol-3-O-(2′′,6′′-di-O-(E)-p-coumaroyl)-β-d-glucopyranoside (2)
1H-NMR (500 MHz, DMSO-d6) δ: 12.61 (s, 1H, 5-OH), 10.20 (s, 1H, OH), 8.02 (d, J = 8.9, 2H, H-2′ and H-6′), 7.58 (d, J = 16.0, 1H, H-7′′′), 7.53 (d, J = 8.4, 2H, H-2′′′ and H-6′′′), 7.35 (d, J = 8.4, 2H, H-2′′′′ and H-6′′′′), 7.34 (d, J = 16.0, 1H, H-7′′′′), 6.85 (d, J = 8.9, 2H, H-3′ and H-5′), 6.79 (d, J = 8.4, 4H, H-3′′′, H-5′′′, H-3′′′′ and H-5′′′′), 6.41 (d, J = 16.0, 1H, H-8′′′), 6.35 (d, J = 1.6, 1H, H-8), 6.12 (d, J = 16.0, 1H, H-8′′′′), 6.10 (d, J = 1.6, 1H, H-6), 5.72 (d, J = 7.6, 1H, H-1′′), 5.30 (t, J = 7.6, 1H, H-2′′), 4.40 (m, 1H, H-6′′), 4.20 (m, 1H, H-6′′), 3.95 (m, 1H, H-5′′), 3.90 (m, 2H, H-3′′ and H-4′′). 13C-NMR (125 MHz, DMSO-d6) δ: 177.0 (C-4), 167.0 (C-9′′′′), 167.0 (C-9′′′), 165.0 (C-7), 162.0 (C-5), 160.9 (C-4′), 161.0 (C-4′′′ and C-4′′′′), 157.0 (C-2), 157.0 (C-9), 145.6 (C-7′′′), 145.6 (C-7′′′′), 133.0 (C-3), 131.7 (C-2′ and C-6′), 131.0 (C-2′′′′ and C-6′′′′), 131.0 (C-2′′′ and C-6′′′), 126.0 (C-1′′′), 126.0 (C-1′′′′), 121.0 (C-1′), 116.0 (C-3′′′, C-5′′′, C-3′′′′ and C-5′′′′), 116.0 (C-3′ and C-5′), 115.0 (C-8′′′), 114.0 (C-8′′′′), 104.0 (C-10), 99.4 (C-6), 99.5 (C-1′′), 94.4 (C-8), 74.0 (C-5′′), 73.0 (C-2′′), 71.5 (C-4′′), 69.0 (C-3′′), 63.8 (C-6′′).
Kaempferol-3-O-(2′′,4′′-di-O-(E)-p-coumaroyl-β-d-glucopyranoside) (1)
1H-NMR (500 MHz, DMSO-d6) δ: 12.56 (s, 1H, 5-OH), 10.20 (s, 1H, OH), 7.96 (d, J = 8.9, 2H, H-2′ and H-6′), 7.61 (d, J = 16.0, 1H, H-7′′′), 7.55 (d, J = 8.9, 2H, H-2′′′ and H-6′′′), 7.38 (d, J = 8.9, 2H, H-2′′′′ and H-6′′′′), 7.35 (d, J = 15.8, 1H, H-7′′′′), 6.85 (d, J = 8.9, 2H, H-3′ and H-5′), 6.78 (d, J = 8.9, 4H, H-3′′′, H-5′′′, H-3′′′′ and H-5′′′′), 6.42 (d, J = 16.0, 1H, H-8′′′), 6.35 (d, J = 1.9, 1H, H-8), 6.13 (d, J = 15.8, 1H, H-8′′′′), 6.12 (d, J = 1.9, 1H, H-6), 5.72 (d, J = 8.0, 1H, H-1′′), 5.48 (t, J = 8.0, 1H, H-4′′), 4.90 (dd, J = 8.0 and 9.5, 1H, H-2′′), 4.28 (m, 1H, H-6′′), 4.05 (m, 1H, H-6′′), 3.55 (m, 1H, H-3′′), 3.40 (m, 1H, H-5′′). 13C-NMR (125 MHz, DMSO-d6) δ: 177.9 (C-4), 166.9 (C-9′′′′), 166.7 (C-9′′′), 165.0 (C-7), 162.0 (C-5), 161.0 (C-4′), 160.7 (C-4′′′ and C-4′′′′), 157.2 (C-2), 157.1 (C-9), 145.9 (C-7′′′), 145.5 (C-7′′′′), 133.4 (C-3), 131.7 (C-2′ and C-6′), 131.0 (C-2′′′′ and C-6′′′′), 131.1 (C-2′′′ and C-6′′′), 126.0 (C-1′′′), 125.8 (C-1′′′′), 121.5 (C-1′), 116.6 (C-3′′′, C-5′′′, C-3′′′′ and C-5′′′′), 116.0 (C-3′ and C-5′), 115.2 (C-8′′′), 114.5 (C-8′′′′), 104.7 (C-10), 99.6 (C-6), 99.1 (C-1′′), 94.5 (C-8), 75.2 (C-3′′), 74.7 (C-2′′ and C-4′′), 71.0 (C-5′′), 63.6 (C-6′′).
Quercetin (3)
1H-NMR (500 MHz, DMSO-d6) δ: 12.49 (s, 1H, 5-OH), 7.67 (d, J = 1.8, 1H, H-2′), 7.53 (dd, J = 8.4 and 1.8, 1H, H-6′), 6.87 (d, J = 8.4, 1H, H-5′), 6.39 (d, J = 1.8, 1H, H-8), 6.17 (d, J = 1.8, 1H, H-6). 13C-NMR (125 MHz, DMSO-d6) δ: 176.6 (C-4), 164.7 (C-7), 161.5 (C-5), 157.0 (C-9), 148.5 (C-4′), 145.9 (C-3′), 147.6 (C-2), 136.5 (C-3), 122.8 (C-1′), 120.8 (C-6′), 116.4 (C-5′), 115.9 (C-2′), 103.8 (C-10), 99.0 (C-6), 94.2 (C-8).
Kaempferol (4)
1H-NMR (500 MHz, DMSO-d6) δ: 12.48 (s, 1H, 5-OH), 8.03 (d, J = 8.8, 2H, H-2′ and H-6′), 6.92 (d, J = 8.8, 2H, H-3′ and H-5′), 6.42 (d, J = 1.8, 1H, H-8), 6.18 (d, J= 1.8, 1H, H-6). 13C NMR (125 MHz, DMSO-d6) δ: 176.7 (C-4), 164.8 (C-7), 161.5 (C-5), 157.0 (C-9), 160.0 (C-4′), 147.6 (C-2), 136.5 (C-3), 130.3 (C-2′ and C-6′), 122.5 (C-1′), 116.3 (C-3′ and C-5′), 103.8 (C-10), 99.1 (C-6), 94.3 (C-8).
In vitro schistosomicidal assays
The LE strain of S. mansoni was maintained by passage through Biomphalaria glabrata snails and Balb/c mice. After 8 weeks, S. mansoni adult worms were recovered under aseptic conditions from mice previously infected with 200 cercariae by perfusion of the livers and mesenteric veins (CitationManneck et al., 2010). The worms were washed in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen), kept at pH 7.5 with HEPES 20 mM, and supplemented with penicillin (100 UI/mL), streptomycin (100 µg/mL), and 10% bovine fetal serum (Gibco). After washing, one couple adult worms was transferred to each well of a 24-well culture plate containing 2 mL of the same medium and incubated at 37°C in a humid atmosphere containing 5% CO2 prior to use. At 24 h after incubation, extracts, fractions, and the isolated compounds (1–4) were dissolved in DMSO and added to RPMI 1640 medium, to give final concentrations of 100 µg/mL or µM. The parasites were kept for 5 days and monitored every 24 h, for evaluation of their general condition. The effects of EE-SC, EF-SC, EE-SP, EF-SP, and 1–4 on S. mansoni were assessed by observing the viability of the worms, as well as pairing, motor activity, and tegument alteration. The worms were considered dead when no movement was observed for at least 2 min of examination and no movement at the other observation time points was detected (CitationSmithers & Terry, 1965). The concentration of drug giving 50% separation of coupled worms (IC50), for compounds 2 and 4, and the lethal concentration that kill 50% of the parasites (LC50) for PZQ were calculated by non-linear regression analysis of percentage of separation and percentage of death versus concentration, respectively, ranging from 10–200 µM for 2 and 4 at 120 h and 0.05–10 µM at 24 h for PZQ. Quadruplicate measurements were accomplished for each employed concentration, and two independent experiments were performed. RPMI 1640 medium and RPMI 1640 with 1% DMSO (the highest concentration of drug solvent) were used as negative control groups. PZQ was used as positive control group. All experiments were authorized by the Ethics Committee for Animal Care of the University of Franca and University of São Paulo, and they were in accordance with the national and international accepted principles for laboratory animal use and care.
Results and discussion
The spectral data of all the isolated compounds () are in agreement with previously published data and allowed for identification of kaempferol-3-O-(2′′,6′′-di-O-(E)-p-coumaroyl)-β-d-glucopyranoside (2), quercetin (3), and kaempferol (4) (CitationYamashita et al. 1989; CitationAgrawal, 1989). kaempferol-3-O-(2′′,4′′-di-O-(E)-p-coumaroyl)-β-d-glucopyranoside (1) had been previously isolated from Quercus ilex L., although the structure was revised by the authors as being 2 (CitationRomussi et al., 1983, 1984, 1991). To the best of our knowledge, this is the first report of compounds 1–2 in S. pohlii and 3–4 in S. camporum.
Regarding the schistosomicidal assay (), pairs of coupled adult worms incubated with the ethanolic extract (EE-SC, 100 μg/mL) and EtOAc fraction (EF-SC, 100 μg/mL) of S. camporum promoted decreased motor activity and separation of the coupled worms. Only EE-SC (100 μg/mL) caused death (25%), and extensive tegumental alterations of the S. mansoni adult worms, thus EF-SC did not induce tegumental alteration and death. In addition, the ethanolic extract (EE-SP, 100 μg/mL) of S. pohlii did not cause death, separation, decreased motor activity, or extensive tegumental alterations in the case of the S. mansoni adult worms. However, the AcOEt fraction of S. pohlii (EF-SP, 100 μg/mL) led to separation of the coupled worms (100%) and caused death (100%) at 120 h as well as extensive tegumental alterations of the S. mansoni adult worms.
Table 1. In vitro effects of the crude extracts, EtOAc fractions, and compounds from S. camporum and S. pohlii against S. mansoni adult worms.
Considering the schistosomicidal activity of isolated the compounds (), worms incubated with compound 3 (100 μM) exhibited moderately reduced motor activity, without tegumental alterations. On the other hand, compounds 1, 2, and 4 (100 μM) did not display any tegumental alterations and motor activity reduction. Additionally, compounds 2 and 4 were able to separate adult worms into male and female (100%); separation occurred at 120 and 24 h for 2 and 4, respectively. Compounds 2 and 4 exhibit an IC50 values of 35.5 and 25.0 µM at 120 h regarding the paring of S. mansoni, respectively. Furthermore, only compound 4 was able to cause 25% death of the worms at 100 μM at 120 h. The appearance and motor activity of the worms in the 1% DMSO group were similar to those observed in the negative control group. In these groups, death, separation, decreased motor activity, or extensive tegumental alterations of the S. mansoni adult worms were not observed. PZQ (10 μM), used as positive control, caused death of the parasites and tegumental alterations without separation of worms. The LC50 for PZQ was determined to be 0.54 ± 0.02 µM.
Furthermore, it can be observed that compounds 1 and 2 are quite similar, differing mainly in the position of the (E)-p-coumaroyl groups in the sugar moiety of kaempferol. Therefore, considering the separation of adults worms, it is suggested that the positions at C-6″ of (E)-p-coumaroyl group in the sugar ring, as in the case of compound 2, may improve the activity of acylated flavonoid derivatives (1–2), since compound 2 was able to separate the male and female worms.
Compounds 3 and 4 are structurally analogous: both have a flavonol moiety and differ in terms of the presence of two hydroxyl group at C-3′ and C-4′ in 3. Thus, on the basis of the obtained results, it can be implied that the presence of the extra hydroxyl group culminates in only moderately decreased motor activity, with no activity regarding separation of worm couples and death of worms. As for compound 4, which contains only one hydroxyl group at C-4′, it was able to separate adult worms into male and female and to cause 25% death of the worms.
The mechanism by which flavonol derivatives, particularly 2 and 4, exert their in vitro schistosomicidal effect is not clear. However, quercetin (3) was identified as a selective inhibitor of the S. mansoni NAD+ catabolizing enzyme (SmNACE), which is localized in the outer surface (tegument) of the adult parasite. So 3 is presumably involved in parasite survival by manipulating the host’s immune regulatory pathways (CitationKuhn et al., 2010). Nevertheless, in our in vitro assay it was less effective and only moderately attenuated motor activity. Considering their antiparasitic activities, flavonols have been shown to possess antimalarial activity (CitationKaur et al., 2009). Moreover, compound 3 exhibits pronounced effect against chloroquine-sensitive (NF-54) Plasmodium falciparum strains and inhibits the P. falciparum enoyl-ACP-reductase (FabI) protein (CitationGupta et al., 2010).
In summary, chemical investigations of the EtOAc fractions from S. camporum and S. pohlii resulted in the isolation and identification of compounds 1-4, which had not yet been accomplished. Additionally, biological results indicated that EE-SC, EF-SC, EF-SP, and compounds 2 and 4 are able to separate coupled S. mansoni adult worms. Moreover, EE-SC, EF-SP, and compound 4 killed the adult schistosomes in vitro. Finally, since S. pohlii and S. camporum have great potential as a source of active compounds, further studies are in progress to disclose other important biological effects of these medicinal plants.
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenadoria de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for fellowships. FAPESP is also acknowledged for financial support (Grant # 2008/10.283-1).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agrawal PK. (1989). Carbon-13 NMR of Flavonoids. New York, USA: Elsevier.
- Braguine CG, Costa ES, Magalhães LG, Rodrigues V, da Silva Filho AA, Bastos JK, Silva ML, Cunha WR, Januário AH, Pauletti PM. (2009). Schistosomicidal evaluation of Zanthoxylum naranjillo and its isolated compounds against Schistosoma mansoni adult worms. Z Naturforsch, C, J Biosci, 64, 793–797.
- Caffrey CR. (2007). Chemotherapy of schistosomiasis: Present and future. Curr Opin Chem Biol, 11, 433–439.
- Gupta AK, Saxena S, Saxena M. (2010). Integrated ligand and structure based studies of flavonoids as fatty acid biosynthesis inhibitors of Plasmodium falciparum. Bioorg Med Chem Lett, 20, 4779–4781.
- Kaur K, Jain M, Kaur T, Jain R. (2009). Antimalarials from nature. Bioorg Med Chem, 17, 3229–3256.
- Kuhn I, Kellenberger E, Said-Hassane F, Villa P, Rognan D, Lobstein A, Haiech J, Hibert M, Schuber F, Muller-Steffner H. (2010). Identification by high-throughput screening of inhibitors of Schistosoma mansoni NAD(+) catabolizing enzyme. Bioorg Med Chem, 18, 7900–7910.
- Lorenzi H. (1982). Árvores Brasileiras − Manual de identificação e cultivo de plantas arbóreas nativas do Brasil, Vol. 1. São Paulo, Brazil: Instituto Plantarum de Estudos da Flora.
- Magalhães LG, Machado CB, Morais ER, Moreira EB, Soares CS, da Silva SH, Da Silva Filho AA, Rodrigues V. (2009). In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol Res, 104, 1197–1201.
- Magalhães LG, Kapadia GJ, da Silva Tonuci LR, Caixeta SC, Parreira NA, Rodrigues V, Da Silva Filho AA. (2010). In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitol Res, 106, 395–401.
- Manneck T, Haggenmüller Y, Keiser J. (2010). Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology, 137, 85–98.
- Pauletti PM, Araújo AR, Young MC, Giesbrecht AM, Bolzani VD. (2000). nor-Lignans from the leaves of Styrax ferrugineus (Styracaceae) with antibacterial and antifungal activity. Phytochemistry, 55, 597–601.
- Pauletti PM, Araújo AR, Young MCM, Bolzani VS. (2002). Triterpenos de Styrax camporum (Styracaceae). Quim Nova, 25, 349–352.
- Pauletti PM, Teles HL, Silva DHS, Araújo AR, Bolzani VS. (2006). The Styracaceae. Rev Bras Farmacogn, 16, 576–590.
- Rodrigues VEG, de Carvalho DA. (2008). Florística de plantas medicinais nativas de remanescentes de floresta estacional semidecidual na região do alto rio Grande, Minas Gerais. Cerne, 14, 93–112
- Romussi G, Cafaggi S, Ciarallo G. (1983). Ein neues acyliertes flavonoid-glycosid aus Quercus ilex L. Liebigs Ann Chem, 1983, 334–335.
- Romussi G, Sancassan F, Parodi B, Bignardi G. (1984). Constituents of Cupuliferae, 8. A novel, high acylated astragalin from Quercus ilex L. Liebigs Ann Chem, 1984, 1864–1866.
- Romussi G, Bignardi G, Pizza C, De Tommasi N. (1991). Constituents of Cupuliferae. XIII. New and revised structures of acylated flavonoids from Quercus suber L. Arch Pharm, 324, 519–524.
- Smithers SR, Terry RJ. (1965). The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology, 55, 695–700.
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. (2006). Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis, 6, 411–425.
- Teles HL, Hemerly JP, Paulettit PM, Pandolfi JR, Araujot AR, Valentini SR, Young MC, Bolzani VS, Silva DH. (2005). Cytotoxic lignans from the stems of Styrax camporum (Styracaceae). Nat Prod Res, 19, 319–323.
- Yamashita N, Etoh H, Sakata K, Yagi A, Ina H, Ina K. (1989). An acylated kaempferol glucoside isolated from Quercus dentata as a repellent against the blue mussell Mytilus edulis. Agric Biol Chem, 53, 1383–1385.