Abstract
Context: Phyllanthus (Euphorbiaceae) species are traditionally well-known for their medicinal properties including hepatoprotective activity.
Objective: The study assessed the hepatoprotective and antioxidant activities of 11 Phyllanthus species, P. amarus Schumach., P. urinaria L., P. debilis Klein ex Willd, P. tenellus Roxb., P. virgatus G. Forst., P. maderaspatensis L., P. reticulatus Poir., P. polyphyllus Willd., P. emblica L., P. indofischerii Bennet. and P. acidus (L.) Skeels.
Materials and methods: The dried leaves and stems of each plant species were extracted in methanol and successively in water. The extracts were screened for hepatoprotective activity at a concentration of 50 µg/mL against tert-butyl hydroperoxide (t-BH) induced toxicity in HepG2 cells. Seven extracts from five species that showed hepatoprotective activity were assessed for their 50% effective concentration (EC50) values and their antioxidant activity using a DPPH assay. Phyllanthin and hypophyllanthin contents were also determined in these Phyllanthus species.
Results: The methanol extracts of P. polyphyllus, P. emblica and P. indofischeri showed high levels of hepatoprotective activity with EC50 values of 12, 19 and 28 µg/mL and IC50 of 3.77, 3.38 and 5.8 µg/mL for DPPH scavenging activity respectively against an IC50 of 3.69 µg/mL for ascorbic acid. None of these activities could be attributed to phyllanthin and hypophyllanthin.
Discussion and conclusion: The hepatoprotective and antioxidant activities of P. indofischeri are demonstrated for the first time in literature. The study also confirms the hepatoprotective and antioxidant activities of leaves of P. emblica and P. polyphyllus. The molecule(s) responsible for the activities is being investigated.
Introduction
The genus Phyllanthus (Euphorbiaceae) constitutes one of the most important groups of plants with high medicinal value (CitationVed & Goraya, 2008). In India, Phyllanthus species traded as raw drugs (CitationSrirama et al., 2010) have been traditionally used for treating liver disorders, intestinal infections and diabetes (CitationSharma et al., 2000; CitationMahishi et al., 2005; CitationRajakumar & Shivanna, 2009). Recently, CitationShivanna and Rajkumar (2011) reported that P. amarus is extensively recommended by local communities in Hosanagara taluk of Shimoga district in Karnataka, India for treating jaundice in humans. These plants are also used as an astringent, diuretic, antipyretic, laxative, tonic, antibacterial, antioxidative, immunomodulatory, antiviral, antiatherosclerotic and antineoplasic as well as to treat dropsy (CitationThyagarajan & Jayaram, 1992; CitationPrakash et al., 1995; CitationCalixto et al., 1998).
Fifty-three species of Phyllanthus are distributed in India of which 23 are endemic (CitationBalakrishnan & Chakrabarty, 2007). Only a few of these Phyllanthus species have been investigated for their medicinal value. For example, P. amarus, P. urinaria, P. emblica, P. acidus, P. tenellus, P. polyphyllus, P. fraternus, P. simplex, P. maderaspatensis, and P. reticulatus have been reported for a variety of medicinal activities including antioxidant, anticancer, nephro-protective, hepatoprotective, antibacterial, anti-inflammatory, antitumor, anti-HIV, anti-herpes Simplex Virus activities (CitationRavikanth et al., 2011; CitationEldeen et al., 2011; CitationFaremi et al., 2008; CitationLin et al., 2008; CitationLuo et al., 2009, Citation2011; CitationSharma et al., 2009; CitationSultana et al., 2008; CitationKundu et al., 2009; CitationRao et al., 2006; CitationAsha et al., 2007; CitationShabeer et al., 2009). Although a number of molecules have been isolated and chemically characterized from Phyllanthus species, the bioactivity of only few have been determined (CitationCalixto et al., 1998; CitationKinjo et al., 2003; CitationRao et al., 2006). With particular reference to their hepatoprotective activity, only few species (P. amarus, P. urinaria) have been screened in HepG2 cells against tert-butyl hydroperoxide.
The present study aims to investigate the hepatoprotective activity of 11 Phyllanthus species against liver damage mediated by toxin (t-BH) in HepG2 cells and determine its antioxidant activity.
Materials and methods
Plant material
Phyllanthus species (Euphorbiaceae) – P. amarus Schumach., P. urinaria L., P. debilis Klein ex Willd, P. tenellus Roxb., P. virgatus G.Forst., P. maderaspatensis L., P. reticulatus Poir., P. polyphyllus Willd., P. emblica L., P. indofischerii Bennet. and P. acidus (L.) Skeels were selected for the study. Individual plant specimens of the above species were collected in 2009–2010 from southern India and Andaman and Nicobar Islands. The plant species were identified and authenticated by the Botanical Survey of India, Kolkata. The specimens of these plant species were deposited at the School of Ecology and Conservation, University of Agricultural Sciences, Bangalore. The details of voucher and place of deposition are given in . These species comprised of 6 herbs, 2 shrubs and 3 trees.
Table 1. List of Phyllanthus species with the voucher number and location details.
Preparation of the plant extract
The leaves and stems of each plant species were dried and ground into a fine powder. The tissue powder (10 g) was extracted with methanol under reflux three-times for 30–40 min each and filtered using a Whatman qualitative filter paper (Grade 1–11 µm particle size) to obtain methanol extract. The remaining residue after methanol extraction was successively refluxed in water three-times for 30–40 min and the pooled aqueous extract was filtered using a Whatman Qualitative filter paper (Grade 1–11 µm particle size). The solvents were then dried in a rotary evaporator under reduced pressure at a temperature of 60°C to obtain a dark brown to black paste in case of methanol and a dark brown powdered form in case of aqueous extracts. The methanol and successive water extract yield of all species was about 10% except P. maderaspatensis and P. virgatus which yielded about 6–8%.
Determination of phyllanthin and hypophyllanthin
Phyllanthin and hypophyllanthin are the two major lignans of the Phyllanthus genus. These two compounds present in P. amarus have been shown to have hepatoprotective effect against carbon tetrachloride and galactosamine and ethanol induced hepatotoxicity in primary cultured rat hepatocytes and HepG2 cells (Syamsunder et al., 1985; Krithika et al., 2009; Chirdchupunseree & Pramyothin, 2010). Here, we attempted to examine if these lignans were also responsible in the hepatoprotective activity exhibited by other Phyllanthus species.
Estimation of phyllanthin and hypophyllanthin was done based on CitationSharma et al. (1993). The methanol extracts (100 mg) and 300 mg of the aqueous extracts of Phyllanthus species were dissolved in 10 mL of HPLC grade methanol. The samples were sonicated for 3 min. These samples were kept in boiling water bath for 5 min and then allowed to stand until they reached the room temperature. The samples were made up to 10 mL with HPLC grade methanol. They were sonicated again for 6 min and filtered with 0.22 µ filters (Rankem). The filtrate was used for estimation of phyllanthin and hypophyllanthin using HPLC. The standard phyllanthin and hypophyllanthin were dissolved separately at a concentration of 0.16 mg/mL in methanol were used as the standards.
The phyllanthin and hypophyllanthin was detected using a C18 column at 230 nm in HPLC (Agilent). The mobile phase consisted of acetonitrile and phosphate buffer in the ratio of 83:17. The phyllanthin and hypophyllanthin standards (Natural Remedies Private Limited, Bangalore) were used for obtaining the standard curves. The flow rate was adjusted to 1.9 mL/min. The presence of phyllanthin and hypophyllanthin in the extracts were determined depending on the retention time, and the concentrations corresponding to their peak area was estimated using the standard curves obtained from reference phyllanthin and hypophyllanthin.
Assessment of hepatoprotective activity of extracts against t–BH induced cytotoxicity in HepG2 cells
The hepatoprotective activity of the extracts of Phyllanthus species were assessed on HepG2 cells treated in the presence or absence of tert-butyl hydroperoxide toxin. HepG2 (ATCC No. HB-8065 HepG2) is a cultured immortalized hepatoma cell line from humans widely used for cytotoxicity, xenobiotic metabolism and carcinogenesis studies (CitationThabrew et al., 1997). The HepG2 cells have many of the morphological and biochemical characteristics of normal hepatocytes including the synthesis and secretion of plasma proteins (CitationKnowles et al., 1980; CitationThabrew et al., 1997; CitationSohn et al., 2005).
The methanol extracts were prepared in plain DMSO at a stock concentration of 20 mg/mL whereas the successive water extract was prepared in Dulbecco’s phosphate buffer saline (DPBS) at a stock concentration of 2 mg/mL, filtered though 0.22 µm sterilized filter and further diluted in Dulbecco’s phosphate buffer saline (DPBS).
The HepG2 cells were seeded at 5 × 104 cells/well in a 96 well plate containing Earle’s minimum essential media (EMEM) amended with 10% fetal bovine serum (FBS) and incubated overnight at 37°C in a humidified atmosphere containing 5% CO2. The used media was discarded and the wells were washed with (DPBS). Fresh EMEM with 10% FBS media along with different concentrations (3.125, 6.25, 12.5, 25, 50, 100 and 200 µg/mL) of the extracts and silymarin as the positive control were added to the plate and incubated for 2 h.
The plate with the HepG2 cells and the extracts was divided into two halves. The t-BH toxin (1 mM) was added to one half of the plate and the other half was devoid of t-BH to assess the cytotoxicity of the extracts. The plate was incubated at 37°C for 2 h. The used media with the extracts and with silymarin in the positive control was discarded, washed with DPBS twice and fresh EMEM with 10% FBS media and [3-(4,5-d-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT)] (500 µg/mL) were added and incubated for 1 h. MTT, a yellow formazole is reduced to a purple formazon in living cells as these indicate the viability of cells. Post incubation, the supernatant was removed, and 100% DMSO was added to dissolve the formazan crystals. The absorbance of each well was measured at 570 nm using a VERSAmax tunable Micro plate reader (Molecular Devices) (CitationChandrasekaran et al., 2010). From the assay, we arrived at the non-cytotoxic concentration of the extract to further assess their hepatoprotective activity.
The absorbance data was converted to number of cells following the standard relation between cell number and optical density. For each concentration of the extract, the mean ± standard deviation (SD) of cell number was computed based on the five replicates.
These results were compared using one-way ANOVA and Dunnett’s multiple comparison tests. The differences between the groups were considered statistically significant at p < 0.05 and p < 0.01. The results are expressed in percentage hepatoprotection. The percentage protection was calculated according to CitationKinjo et al. (2003).
where, sample value = viable cell number treated with the concentrations of extracts and t-BH
t-BH control = cell number treated with the toxin t-BH only
Cell control = cell number of the negative control
The EC50 was calculated by using median effective plot. All the statistical analysis was performed using GraphPad Prism program (GraphPad Software, Inc., San Diego, CA, U.S.A.).
DPPH assay
The antioxidant activity of the Phyllanthus species was assessed based on the free radical scavenging activity against 2, 2-diphenyl-1-picrylhydrazil (DPPH) free radical. The assay was performed according to CitationSharma and Bhat (2009) with slight modification. A total reaction mixture of 200 µl was prepared in a 96 micro well plate. The reaction mixture consisted of 20 µl of the methanol and aqueous extracts of Phyllanthus species or positive control (ascorbic acid) dissolved in methanol or negative control (methanol), 80 µl of 50 mM Tris HCl buffer and 100 µl of 50 µM DPPH solutions. The extracts and positive control were diluted to a final concentration of 100 µg/mL (3.125, 6.25, 12.5, 25, 50, 100 µg/mL) and 10 µg/mL (0.3125, 0.625, 1.25, 2.5,5 and 10 µg/mL), respectively. The reaction mixture was incubated at 37°C for 30 min. The decrease in the absorbance value was measured at 517 nm using ELISA reader (TECAN). The radical scavenging activity was computed and 50% Inhibitory concentration (IC50) was calculated for the antioxidant activity by linear regression.
Results and discussion
Among the species of Phyllanthus studied, the methanol extracts of P. debilis, P. virgatus, P. polyphyllus, P. emblica and P. indofischeri and successive water extracts of P. virgatus and P. polyphyllus showed hepatoprotective activity at 50 µg/mL against t-BH induced cytotoxicity in HepG2 cells (). The EC50 of the positive control silymarin was 32 µg/mL, while that of the methanol extracts of P. polyphyllus, P. emblica and P. indofischeri were 12, 19 and 28 µg/mL, respectively (). The antioxidant activity of the methanol extracts of these three species was determined by the DPPH assay. The IC50 for P. polyphyllus, P. emblica and P. indofischeri were 3.77, 3.38 and 5.8 µg/mL respectively compared to an IC50 of 3.69 µg/mL from the positive control ascorbic acid ().
Table 2. Hepatoprotective and antioxidant activities along with phyllanthin and hypophyllanthin content of eleven Phyllanthus species.
Figure 1. Hepatoprotective activity of methanol extracts of (A) P. polyphyllus, (B) P. emblica and (C) P.indofischeri against t-BH induced cell damage in HepG2 cells. *p < 0.001 as compared to t-BH (1 mM). Error bars indicate standard deviation.
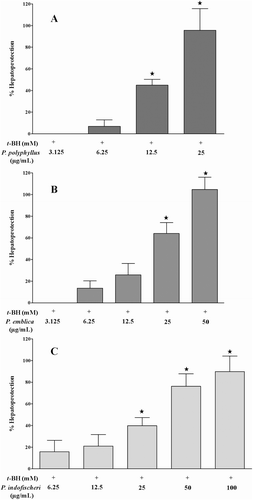
The results of the study indicated a significant hepatoprotective activity of P. polyphyllus, P. emblica, and P. indofischeri. The former two species have been studied previously for their hepatoprotective and antioxidant activities (CitationPramyothin et al., 2006; CitationLiu et al., 2008; CitationRajkapoor et al., 2008; CitationLuo et al., 2009). CitationRajkapoor et al. (2008) reported the hepatoprotective and antioxidant activity of P. polyphyllus in acetaminophen-induced hepatotoxicity in rat model. The hepatoprotective activity of P. polyphyllus was attributed to the inhibition of lipid peroxidation, enhancement of the antioxidant enzyme levels and by free radical scavenging (CitationRajkapoor et al., 2008). In addition, preliminary phytochemical studies suggest that the flavonoids present in the plant could be responsible for the hepatoprotective activity (CitationRajkapoor et al., 2008). However, a strong hepatoprotective activity and antioxidant activity of P. indofischeri is reported for the first time in literature. P. indofischeri is an endemic tree found only in the Deccan plateau of India and shares morphological similarities with P. emblica (amla, Indian gooseberry) (CitationGanesan, 2003; CitationBalakrishnan & Chakrabarty, 2007). The fruits of P. indofischeri are sold in the trade name of Amla (CitationGanesan, 2003; CitationRavikanth et al., 2011) but this species has not been studied for its bioactivity. The results of the experiment also indicated that the traditionally known hepatoprotective activity of admixtures of Phyllanthus raw drug could be due to the invariable presence of P. emblica, P. indofischeri and P. polyphyllus, all possessing hepatoprotective activity. The presence of P. amarus in the admixture might also contribute to the above activity (Khatoon et al., 2006).
Traditionally, P. amarus has been predominantly used in treating liver disorders (CitationSharma et al., 2000). Two phytochemicals namely phyllanthin and hypophyllanthin that are present in P. amarus have been argued to be responsible for the hepatoprotective activity. However, none of the species examined other than P. amarus was found to have phyllanthin and hypophyllanthin in the extracts studied. Clearly the hepatoprotective activity of the species reported here could be due to the activity of other chemical constituents.
Conclusion
In conclusion, the methanol extracts of P. polyphyllus, P. emblica and P. indofischeri show promising hepatoprotective activity. Characterization of the active principles, their mode of action and other in vivo experiments are needed to evaluate their therapeutic value as natural hepatoprotective agents. The biomolecule(s) with activity is currently being investigated.
Acknowledgements
The work was supported by grants from the Department of Biotechnology, Government of India. We acknowledge the cooperation of the Forest Departments of Karnataka, Kerala and Andaman and Nicobar Islands for providing the necessary permission to visit the various forest divisions in the respective regions.
Declaration of interest
The work was supported by grants from the Department of Biotechnology, Government of India (BT/PR 8359/NDB/51/145/2006).
References
- Asha VV, Sheeba MS, Suresh V, Wills PJ. (2007). Hepatoprotection of Phyllanthus maderaspatensis against experimentally induced liver injury in rats. Fitoterapia, 78, 134–141.
- Balakrishnan NP, Chakrabarty T. (2007). The Family Euphorbiaceae in India - A Synopsis of its Profile, Taxonomy and Bibliography. Bishen Singh Mehendra Pal Singh, Dehra Dun, India.
- Calixto JB, Santos AR, Cechinel Filho V, Yunes RA. (1998). A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology, and therapeutic potential. Med Res Rev, 18, 225–258.
- Chandrasekaran CV, Sundarajan K, David K, Agarwal A. (2010). In vitro efficacy and safety of poly-herbal formulations. Toxicol In Vitro, 24, 885–897.
- Eldeen IMS, Seow EM, Abdullah R, Sulaiman SF. (2011). In vitro antibacterial, antioxidant, total phenolic contents and anti-HIV-1 reverse transcriptase activities of extracts of seven Phyllanthus sp. S Afr J Bot, 77, 75–79.
- Faremi TY, Suru SM, Fafunso MA, Obioha UE. (2008). Hepatoprotective potentials of Phyllanthus amarus against ethanol-induced oxidative stress in rats. Food Chem Toxicol, 46, 2658–2664.
- Ganesan R. (2003). Identification, distribution and conservation of Phyllanthus indofischerii, another source of Indian gooseberry. Curr Sci India 84, 1515–1518.
- Kinjo J, Hirakawa T, Tsuchihashi R, Nagao T, Okawa M, Nohara T, Okabe H. (2003). Hepatoprotective constituents in plants. 14. Effects of soyasapogenol B, sophoradiol, and their glucuronides on the cytotoxicity of tert-butyl hydroperoxide to HepG2 cells. Biol Pharm Bull, 26, 1357–1360.
- Knowles BB, Howe CC, Aden DP. (1980). Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science, 209, 497–499.
- Kundu S, Nandy D, Bishayi B. (2009). Immunomodulatory effects of Phyllanthus acidus and Parkia javanica whole plant extracts on murine splenic macrophages. Int J Infect Dis, 13, 58
- Lin SY, Wang CC, Lu YL, Wu WC, Hou WC. (2008). Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem Toxicol, 46, 2485–2492.
- Liu X, Zhao M, Wang J, Yang B, Jiang Y. (2008). Antioxidant activity of methanolic methanol extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. J Food Compos Anal, 21, 219–228.
- Luo W, Zhao M, Yang B, Ren J, Shen G, Rao G. (2011). Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem, 126, 277–282.
- Luo W, Zhao M, Yang B, Shen G, Guohua Rao G. (2009). Identification of bioactive compounds in Phyllanthus emblica L. fruit and their free radical scavenging activities. Food Chem, 114, 499–504.
- Mahishi P, Srinivasa BH, Shivanna MB. (2005). Medicinal plant wealth of local communities in some villages in Shimoga District of Karnataka, India. J Ethnopharmacol, 98, 307–312.
- Prakash A, Satyan KS, Wahi SP, Singh RP. (1995). Comparative hepatoprotective activity of three Phyllanthus species, P. urinaria, P. amarus and P. simplex on carbon tetrachloride induced liver injury in the rats. Phytother Res, 9, 594–596.
- Pramyothin P, Samosorn P, Poungshompoo S, Chaichantipyuth C. (2006). The protective effects of Phyllanthus emblica Linn. extract on ethanol induced rat hepatic injury. J Ethnopharmacol, 107, 361–364.
- Rajkapoor B, Venugopal Y, Anbu J, Harikrishnan N, Gobinath M, Ravichandran V. (2008). Protective effect of Phyllanthus polyphyllus on acetaminophen induced hepatotoxicity in rats. Pak J Pharm Sci, 21, 57–62.
- Rajakumar N, Shivanna MB. (2009). Ethno-medicinal application of plants in the eastern region of Shimoga District, Karnataka, India. J Ethnopharmacol, 126, 64–73.
- Rao YK, Fang SH, Tzeng YM. (2006). Anti-inflammatory activities of constituents isolated from Phyllanthus polyphyllus. J Ethnopharmacol, 103, 181–186.
- Ravikanth G, Srirama R, Senthilkumar U, Ganeshaiah KN, Uma Shaanker R. (2011). Genetic resources of Phyllanthus in Southern India: Identification of geographic and genetic hot-spots and its implications for conservation. In: Kuttan R and Hari Kumar HB ed Phyllanthus Species: Scientific Evaluation and Medicinal Applications. Florida: C.R.C Press: Taylor & Francis Group, 97–118.
- Shabeer J, Srivastava RS, Singh SK. (2009). Antidiabetic and antioxidant effect of various fractions of Phyllanthus simplex in alloxan diabetic rats. J Ethnopharmacol, 124, 34–38.
- Sharma A, Sharma MK, Kumar M. (2009). Modulatory role of Emblica officinalis fruit extract against arsenic induced oxidative stress in Swiss albino mice. Chem Biol Interact, 180, 20–30.
- Sharma A, Singh RT, Handa SS. (1993). Estimation of phyllanthin and hypophyllanthin by high performance liquid chromatography in Phyllanthus amarus. Phytochem Analysis, 4, 226–229.
- Sharma OP, Bhat TK. (2009). DPPH antioxidant assay revisited. Food Chem, 113, 1202–1205.
- Sharma PC, Yelne MB, Dennis TJ. (2000). Database on medicinal plants used in Ayurveda. In: Central Council for Research in Ayurveda & Siddha, Janakpur, New Delhi, 3: 512–536.
- Shivanna MB, Rajkumar N. (2011). Traditional medico-botanical knowledge of local communities in Hosenagara Taluk of Shimoga dt. in Karnataka, India. J Herbs Spices Med Plants, 17, 291–317.
- Sohn JH, Han KL, Lee SH, Hwang JK. (2005). Protective effects of panduratin A against oxidative damage of tert-butylhydroperoxide in human HepG2 cells. Biol Pharm Bull, 28, 1083–1086.
- Srirama R, Senthilkumar U, Sreejayan N, Ravikanth G, Gurumurthy BR, Shivanna MB, Sanjappa M, Ganeshaiah KN, Shaanker RU. (2010). Assessing species admixtures in raw drug trade of Phyllanthus, a hepato-protective plant using molecular tools. J Ethnopharmacol, 130, 208–215.
- Sultana S, Ahmed S, Jahangir T. (2008). Emblica officinalis and hepatocarcinogenesis: A chemopreventive study in Wistar rats. J Ethnopharmacol, 118, 1–6.
- Thabrew MI, Hughes RD, McFarlane IG. (1997). Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J Pharm Pharmacol, 49, 1132–1135.
- Thyagarajan SP, Jayaram P. (1992). Natural history of Phyllanthus amarus in the treatment of hepatitis B. Indian J Med Microbiol, 10, 64–80.
- Ved DK, Goraya GS. (2008). Demand and Supply of Medicinal Plants in India. FRLHT.