Abstract
Context: Barringtonia acutangula (L.) Gaertn. (Lecythidaceae) has been used in folk medicine in the treatment of arthralgia, chest pain, dysmenorrhea, inflammation, hemorrhoids, diarrhea, and also in psychological disorders.
Objective: To investigate the antinociceptive, antidiarrheal, and neuropharmacological effect of the methanol extract of B. acutangula leaves and seeds in mice.
Materials and methods: The extracts (200 and 400 mg/kg; p.o.) were tested for antinociceptive activity by acetic acid-induced writhing, hot plate and tail immersion models; castor oil- and magnesium sulphate-induced diarrheal models were used to evaluate antidiarrheal activity whereas hole cross and open field models were employed for testing neuropharmacological activity.
Results: Both extracts exhibited significant antinociceptive effect (p < 0.001) in acetic acid and heat induced pain models in a dose-dependent manner. The extracts prolonged the latency period to the thermal stimuli in both hot plate and tail immersion test. The extracts also showed significant inhibition of defecation (p < 0.001, 0.01) in both diarrheal models. Again, the spontaneous motor activity was decreased (p < 0.001) by the extracts in both hole cross and open field test.
Discussion and conclusion: The results of this study suggest that the methanol extracts of B. acutangula leaves and seeds possess good antinociceptive, antidiarrheal, and central nervous system (CNS) depressant activities. This study validates the use of this plant in traditional medicine.
Introduction
Medicinal plants have been used for treating different human ailments from ancient times. This indigenous knowledge was passed down from generation to generation in various parts of the world and has significantly contributed to the development of different traditional systems of medicine (Jachak & Saklani, Citation2007). Modern allopathic medicine has its roots in the traditional system of medicine, and it is likely that many important new molecules with therapeutic potential will be discovered and used as lead compounds. Hence, medicinal potential of plants have been a major area of research in different countries with abundant flora. This traditional, use-based scientific exploration of medicinal plants resulted in discovery of drugs such as taxol, vincristine, vinblastine, d-tubocurarine, harpagoside, podophyllotoxin, lignans, and the like, and leads such as vasicin. Thirty percent or more of the present-day drugs are directly or indirectly derived from medicinal plants (Sharif & Banik, Citation2006). So, plant derived natural products have been recognized to be an ample source of bioactive compounds. However, only 1% of the approximately 500,000 plant species have been phytochemically investigated (CitationPalombo, 2006). This enormous potential has convinced the researchers to validate the traditional uses of medicinal plants and find out the rationale behind the bioactivity of plant constituents. The World Health Organization (WHO) also advocated all the countries to explore and identify the safe and effective natural remedies for microbial or nonmicrobial diseases (WHO, Citation1978).
Barringtonia acutangula (L.) Gaertn. (Lecythidaceae) is a 5–8 m tall, evergreen tree with obovate leaves, rough fissured grey bark, red flowers on pendulous racemes, and about 20 cm long four-sided fruits. The plant is also called Indian Oak and is locally known as Hijal in Bangladesh. It grows in the tropical areas of Southeast Asia, Australia, and Africa, frequently occurring on the bank of rivers, ponds, lakes, and low-lying areas (CitationKapoor, 1990; CitationYusuf et al., 1994). The plant is traditionally used in diarrhea, flatulence, hemorrhoids, inflammation, skin diseases, leprosy, arthralgia, dysmenorrhea, and as anthelmintic (CitationSatapathy & Brahmam, 1994; CitationSahoo et al., 2008). It is also used in different psychological disorders in Ayurvedic medicine (CitationKhare, 2007). The leaf juice is used in diarrhea and seeds are used in chest pain, cold, abdominal colic, and gonorrhea (CitationYusuf et al., 1994). Acutangulic acid, saponins, acutagenol A, acutagenol B, barringtogenols B, C, and D, stigmasterol, β-sitosterol, and β-amyrin have been isolated from leaf (CitationGhani, 2003). The leaf also contains a good amount of phenolic compounds (CitationDaduang et al., 2011). Seeds are reported to contain a triterpenoid glucoside (CitationPal et al., 1991). Pharmacological properties such as antibacterial activity of stem bark and twigs (CitationRahman et al., 2005; CitationSahoo et al., 2008), antioxidant and hepatoprotective activities of leaves (CitationRahman et al., 2010; CitationRashmi et al., 2011), hypolipidemic and antioxidant activities of root extract (CitationBabre et al., 2010a, Citation2010b), and anti-scorpion venom activity have been reported (CitationUawonggul et al., 2006). However, there are no experimental studies on antinociceptive, antidiarrheal, and neuropharmacological activities of this plant. The objective of this study is therefore to investigate the antinociceptive, antidiarrheal, and neuropharmacological activities of methanol extract of B. acutangula leaves and seeds.
Materials and methods
Plant collection
The leaves and seeds of B. acutangula were collected from a natural population of Brammonkhali, Rupgonj, Narayangonj, Bangladesh in August, 2010. The collected samples were then identified by Sarder Nasir Uddin, Senior Scientific Officer, Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh. A voucher specimen (DACB: 35245) has been deposited in the Herbarium for further reference.
Preparation of extract
The leaves and seeds were washed with water, dried for 7 days under a shade at the room temperature, and then woven dried at 40°C for 48 h and were powdered. The powder of leaves (75 g) and seeds (100 g) were extracted separately with 300 mL of methanol by a Soxhlet apparatus for 2 days at 50°C. The extracts were then filtered using a Buchner funnel and a sterilized cotton filter. The solvent was completely removed by rotary evaporator and 15.95 g leaf extract (yield 21.27%) and 5.70 g seed extract (yield 5.7%) were obtained. These crude extracts were used for the investigations.
Chemicals
Acetic acid and magnesium sulphate were product of Merck, Germany, and castor oil of Well’s Health Care, Spain. Diclofenac sodium, nalbuphine, and diazepam were purchased from local manufacturers. All other chemicals used were of analytical grade.
Phytochemical screening
The crude methanol extracts of B. acutangula leaves (BALE) and seeds (BASE) were qualitatively tested for detection of carbohydrates, saponins, flavonoids, tannins, alkaloids, glycosides, glucosides, reducing sugars, proteins, and steroids following standard phytochemical procedures (CitationGhani, 2003).
Animals
Swiss albino mice (25–30 g) were collected from the Animal Resources Branch of International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR,B). The animals were kept in standard laboratory conditions (relative humidity 55–60%; room temperature 25 ± 2°C; 12 h light–dark cycle) and were provided with standard diet (ICDDR,B formulated) and clean water ad libitum. The animals were acclimatized to the environment for a period of 14 days prior to performing the experiments. All the experimental animals were treated following the Ethical Principles and Guidelines for Scientific Experiments on Animals (1995) formulated by The Swiss Academy of Medical Sciences and the Swiss Academy of Sciences.
Drugs and treatments
BALE and BASE were dissolved in distilled water and administered to the mice at 200 and 400 mg/kg per orally by gavage. The vehicle at the same volume (10 mL/kg) was administered by gavage to the control group. All drugs, used as standard, were dissolved in 0.9% saline and administered intraperitoneally (i.p.) except loperamide. Loperamide (3 mg/kg) was suspended in vehicle for oral administration. Diclofenac sodium (10 mg/kg, i.p.) and nalbuphine (10 mg/kg, i.p.) were used as standard peripheral and central analgesic agents, respectively. Diazepam (1 mg/kg, i.p.) was used as reference CNS depressant drug.
Acute toxicity test
Mice were divided into control and test groups (n = 6). The test groups received BALE or BASE per orally at the doses of 500, 1000, 1500, and 2000 mg/kg. Then the animals were kept in separate cages and were allowed to food and water ad libitum. The control group received the vehicle. The animals were observed for possible behavioral changes, allergic reactions, and mortality for the next 72 h (CitationWalker et al., 2008).
Antinociceptive activity
Acetic acid-induced writhing test
The peripheral antinociceptive activity of the extracts was studied using acetic acid-induced writhing test. The animals were divided into control, reference drug group and test groups (n = 5). Acetic acid (0.7%) was intraperitoneally injected to each mouse after 30 min of oral administration of the extract or vehicle. The reference drug diclofenac sodium was administered 15 min before injection of acetic acid. After an interval of 5 min, the mice were observed for writhing (abdominal contraction, elongation of the body, and extension of the hind limb) for the next 10 min (CitationVogel, 2007).
Hot plate test
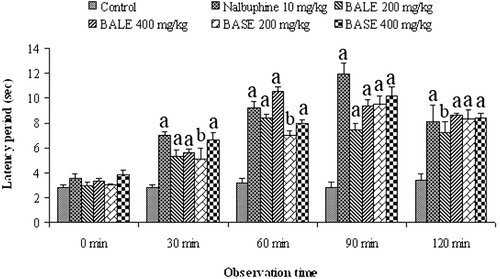
The hot plate method is a thermal pain model to test antinociceptive activity based on the procedure described by CitationEddy and Leimbach (1953). The mice which showed forepaw licking, withdrawal of the paws or jumping response within 15 s on a hot plate were selected for this study. Mice were fasted overnight with water given ad libitum. The mice were treated with vehicle, extracts or the reference drug nalbuphine. Then the animals were placed on Eddy’s hot plate kept at a temperature of 55 ± 1°C and the latency period was recorded. The response in the form of forepaw licking, withdrawal of the paws or jumping were recorded at 0, 30, 60, 90, and 120 min following the treatments (n = 5). A cut off period of 20 s was maintained to avoid damage to the paw.
Tail immersion test
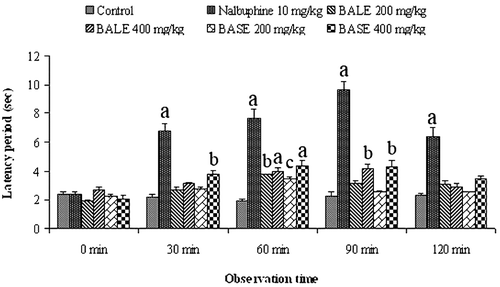
This test is based on the observation that morphine like drugs selectively prolongs the reaction time of the typical tail-withdrawal reflex in mice (CitationToma et al., 2003). The mice were treated with vehicle, extract or nalbuphine and then 1–2 cm of the tail of the mice was immersed in warm water kept constant at 52 ± 1°C. The tail withdrawal or deflection time was recorded to get the latency period. The latency period of the tail-withdrawal response was taken as the index of antinociception and was determined at 0, 30, 60, 90, and 120 min after the treatments. A cutoff period of 20 s was maintained to avoid injury to the tail of mice.
Antidiarrheal activity
Antidiarrheal activity test in castor oil-induced diarrhea
The antidiarrheal activity was studied in castor oil-induced diarrhea in mice according to the method described by CitationShoba and Thomas (2001). The mice were screened for the experiment observing the diarrhea induced after giving 0.5 mL of castor oil to each mouse (p.o.). Mice fasted for 24 h were treated with vehicle, extracts, or standard drug per orally. After 1 h, 0.5 mL of castor oil was administered orally to each mouse. Then each animal was placed in a separate cage with blotting paper lined floor. The mice were observed for the next 4 h to record the characteristic diarrheal droppings. The percent (%) inhibition of defecation was calculated using the formula: % Inhibition of defecation = [(A − B) / A] × 100; where, A = mean number of defecation caused by castor oil and B = mean number of defecation caused by drug or extract.
Antidiarrheal activity test in magnesium sulphate-induced diarrhea
The experiment was carried out according to the method described by CitationDoherty (1981). Diarrhea was induced by oral administration of magnesium sulphate at the dose of 2 g/kg. The mice were screened and divided into different groups. Mice were treated with magnesium sulphate (2 g/kg, p.o.) after 30 min of the administration of the vehicle, drug or extracts. After observing the diarrheal droppings for the next 4 h, the percent (%) inhibition of defecation was calculated (CitationUddin et al., 2005).
Neuropharmacological activity
Hole cross test
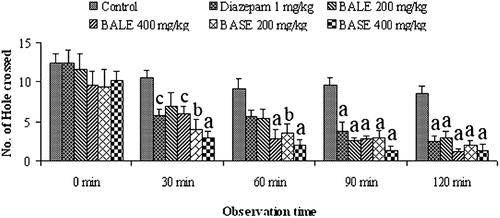
The method was carried out as described by CitationTakagi et al. (1971). A cage (30 × 20 × 14 cm) with a fixed partition in the middle, having a hole of 3 cm diameter was used. Mice were treated with vehicle, extract, or diazepam and were place in one side of the cage. Then the number of passage of a mouse through the hole from one chamber to other was counted for a period of 3 min at 0, 30, 60, 90, and 120 min after the treatments.
Open field test
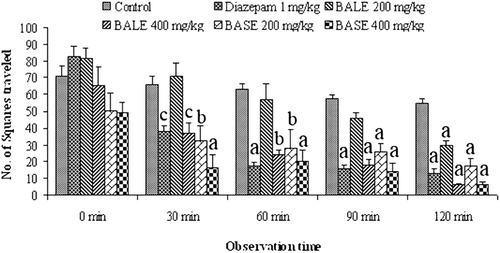
Open field behavioral test is routinely used to evaluate both locomotor activity and emotionality in rodents. The open field apparatus consisted of a wooden field of half square meter, with a series of squares alternatively painted in black and white. It had a 50 cm high wall and was placed in a dimly lit room. Mice were treated with vehicle, extracts or diazepam and were place in the middle of the open field. Then the number of squares visited by the mice was counted for 3 min at 0, 30, 60, 90, and 120 min after the treatments (CitationGupta et al., 1971).
Statistical analysis
The statistical analyses of the results were carried out by one way analysis of variance (ANOVA) followed by Dunnett’s post hoc test using SPSS 17.0. p < 0.05–0.001 were considered as statistically significant.
Results
Phytochemical screening
Preliminary phytochemical screening of B. acutangula revealed the presence of carbohydrate, alkaloid, glycoside, glucoside, saponin, steroids, and tannin in BALE, and glycoside, glucoside, and saponin in BASE.
Acute toxicity
Oral administration of BALE and BASE at the doses of 500–2000 mg/kg did not produce any mortality or noticeable behavioral changes in mice within 72 h observation period. Therefore, it can be suggested that BALE and BASE have low toxicity profile with LD50 greater than 2000 mg/kg.
Antinociceptive activity
Acetic acid-induced writhing test
The antinociceptive effect of BALE and BASE at 200 and 400 mg/kg doses were found significant (p < 0.001) in acetic acid-induced writhing model. The extracts showed a dose-dependent decrease in the number of writhing induced by acetic acid (). At 400 mg/kg dose BALE and BASE showed 68.60 and 83.33% inhibition of writhing respectively in comparison to control. The standard drug diclofenac sodium showed 62.02% inhibition of writhing at 10 mg/kg dose in the observation period.
Table 1. Antinociceptive effect of methanol extract of B. acutangula leaf and seed on acetic acid-induced writhing in mice.
Hot plate test
Hot plate test results are shown in . Oral administration of BALE and BASE significantly (p < 0.01, 0.001) prolonged the latency period (seconds) at both 200 and 400 mg/kg doses when compared to the control group. Both extracts increased the latency time in a dose-dependent manner. Nalbuphine (10 mg/kg), the standard drug, showed highest prolongation of latency period. Maximum effect of the extracts was observed at 60 and 90 min.
Tail immersion test
The tail-withdrawal reflex time of the mice to the hot water-induced pain significantly increased after administration of BALE and BASE (). The maximal effect of the extracts was recorded at 60 min. The effect was found significant (p < 0.05–0.001) in comparison to control. However, the increase in latency was less significant than that were observed in the hot plate test.
Figure 2. The antinociceptive effect of B. acutangula extracts and nalbuphine in tail immersion test. Each value is presented as the mean ± SEM (n = 5). ap < 0.001, bp < 0.01, cp < 0.05, Dunnett’s test compared with control group. BALE = B. acutangula leaf extract; BASE = B. acutangula seed extract.
Antidiarrheal activity
Castor oil-induced diarrhea
In castor oil-induced diarrhea, both extracts showed dose-dependent reduction in fecal dropping (). Significant (p < 0.001) level of reduction in fecal dropping was found with both BALE and BASE at 200 and 400 mg/kg doses. At 400 mg/kg dose, BALE and BASE showed 75 and 74.14% inhibition of defecation, respectively.
Table 2. Antidiarrheal effect of methanol extract of B. acutangula leaf and seed on mice in castor oil and MgSO4-induced diarrhea.
Magnesium sulphate-induced diarrhea
In magnesium sulphate-induced diarrhea, both extracts showed a dose-dependent reduction in fecal dropping in a similar manner like in castor oil-induced diarrhea (). Significant (p < 0.001) reduction in fecal dropping was shown by BALE at 400 mg/kg (72.92%) and BASE at 400 mg/kg (60.42%) doses. BALE and BASE at 200 mg/kg doses also showed 47.92 and 35.42% inhibition of diarrheal droppings, respectively (p < 0.05).
Neuropharmacological activity
Hole cross test
In hole cross test, both BALE and BASE showed marked decrease in the locomotor activity which represent the CNS depressant effect of the extracts (). The effect was noticeable from the 2nd observation (30 min) to 5th observation at both 200 and 400 mg/kg doses. Maximum depressant effect was observed from 3rd (60 min) to 5th (120 min) observation period. The effect was dose-dependent and statistically significant (p < 0.001) compared to control.
Open field test
The decrease in locomotion was also evident from the results of open field test. Both extracts showed dose-dependent and statistically significant (p < 0.001) CNS depressant effect (). The maximum effect was observed from 4th observation (90 min) to 5th observation (120 min). The depressant effect of the extracts increased with time.
Discussion
The present study demonstrates that BALE and BASE possess marked antinociceptive activities in chemical and heat-induced pain and antidiarrheal activity in chemical-induced diarrheal models. The extracts also possess CNS depressant activity. No acute toxicity was observed after oral administration of BALE and BASE even at the dose of 2000 mg/kg in mice.
Acetic acid-induced writhing test is a visceral pain model. The pain is induced at the peritoneal receptors by the increasing amount of endogenous mediators of pain, such as prostaglandin E2, prostaglandin E2α, kinins, and the like (CitationDeraedt et al., 1980; CitationBentley et al., 1983). The stimulation of the nociceptive neurons, which is sensitive to NSAIDs and narcotics, by the endogenous mediators also contribute in the pain induction (CitationAdzu et al., 2003). Acetic acid-induced abdominal writhing is widely used to screen peripherally acting analgesics (CitationSánchez-Mateo et al., 2006). The findings of the present study indicate that the extracts of B. acutangula possess a significant peripheral antinociceptive activity.
The hot plate test is used to evaluate the centrally acting analgesics (CitationWong et al., 1994). The paw-licking or jumping responses in hot plate are complex supraspinally organized behavior of mice (CitationChapman et al., 1985). So, a decrease in licking or increase in latency indicates the centrally acting analgesic properties of the treatment. The results of the hot plate test showed that B. acutangula extracts produced antinociceptive effect against heat-induced pain. The effect was evident from the elongation of the latency time till the 5th observation (120 min).
Tail immersion model is an acute pain model. The tail-withdrawal response is predominantly considered to be selective for centrally acting analgesics, whereas the peripherally acting drugs are known to be inactive on heat-induced pain (CitationSrinivasan et al., 2003). The significant increase (p < 0.05) in tail-withdrawal time by the extracts suggests centrally acting analgesic activity of BALE and BASE.
Both tail immersion and hot plate test measure the latency time of mice to thermal stimuli. Tail immersion monitors a spinal reflex involving μ2- and δ-opioid receptors, whereas the hot plate demonstrates supraspinal reflex mediated by μ1- and μ2-opioid receptors (CitationJinsmaa et al., 2004, Citation2005; CitationArslan & Bektas, 2010). Therefore, the results of the present study indicate that the central antinociceptive effect of B. acutangula may be prominent on μ-opioid receptors. Influx of calcium ions at the terminal of the axon of the afferent nerve by different compounds in the extracts may also decrease the activity of adenylyl cyclase. This may lead to decreased cAMP level, potassium ion efflux and subsequent hyperpolarization of the nerves and give the antinociceptive effect (CitationYaksh, 1999). The antinociceptive effect of the extracts in these three models implies that the extracts contain pharmacologically active phytoconstituents that may act both centrally and peripherally.
The inhibition of diarrhea in mice, induced by castor oil and magnesium sulphate, is used to determine the antidiarrheal activity of plant extracts. Castor oil stimulates the release of prostaglandin E in the colon (CitationBeubler & Jaun, 1979), decreases Na+,K+ ATPase activity (CitationGaginella & Phillips, 1975), and alters the intestinal histology and permeability (CitationMascolo et al., 1993). These reduce or reverse absorption of water and electrolytes from the intestinal lumen and colon and cause secretory diarrhea in mice. Magnesium sulphate causes increased loss of intestinal content due to the reduction in reabsorption of water and cholecystokinin release from the duodenal mucosa. These increase the secretion and motility of small intestine, prevent the reabsorption of sodium chloride and water, and induce diarrhea (CitationGálvez et al., 1993; CitationUddin et al., 2005). In this study, the extracts showed a dose-dependent reduction in fecal droppings in both castor oil- and magnesium sulphate-induced diarrhea (). Phytochemical screening detected the presence of alkaloid, glycoside, saponin, steroids, and tannin in the extracts. These phytoconstituents may be attributed to the antidiarrheal effects in the animal model. Tannins may impart antidiarrheal effect possibly by inhibiting the intestinal motility (CitationGálvez et al., 1991) and reducing the intestinal secretion (CitationMukherjee et al., 1995). It may be possible that the antidiarrheal activity is due to the tannins (CitationAgunu et al., 2005), alkaloids (CitationMandal et al., 2010), glycosides (CitationAbere et al., 2010), and other phytoconstituents.
Rearing of mice is considered as a function of the excitability level of the CNS (CitationMasur et al., 1971). The decrease in rearing and locomotion in hole cross and open field tests therefore confirms the CNS depressant activity of BALE and BASE. The depressant activity may be due to the presence of alkaloids in the extracts (CitationMorais et al., 1998). This general depressant and sedative effect of the extracts may be due to the action of alkaloids on the cerebral mechanism involved in the regulation of sleep (CitationN’Gouemo et al., 1994). Tannins have also been reported to show nonspecific CNS depression in mice (CitationTakahashi et al., 1986). So the reported central depressant effect of the extracts of B. acutangula may be due to the presence of tannin-like constituents in the plant.
Conclusion
The findings of the present study provide convincing evidence that BALE and BASE possess remarkable antinociceptive, antidiarrheal, and CNS depressant activities. These effects are rapid, long lasting, and statistically significant at both 200 and 400 mg/kg doses. The mechanisms of peripheral and central antinociceptive effects of BALE and BASE are not completely understood and need further studies with different antagonists (such as opioid, adrenergic, serotonergic, etc.). Determination of antidiarrheal effect in other models as well as the effect on gut motility may give a clear idea about the mechanism(s) of antidiarrheal activity. Animal models for testing sedative and anxiolytic activities may also be used to determine specific type of effect on nervous system. However, the results of this study affirm the traditional uses of the plant in the treatment of painful conditions such as arthralgia, chest pain, dysmenorrhea, inflammation, hemorrhoids, in diarrhea and psychological disorders. Chemical and pharmacological studies are required to isolate the bioactive compounds and elucidate the precise mechanisms responsible for the pharmacological activities of the plant. It seems quite possible that B. acutangula contains chemical constituents with analgesic, antidiarrheal, and CNS depressant properties which may be used as lead compound for new drug development.
Acknowledgments
The authors are grateful to Professor Dr. Abdul Ghani, Chairman, Department of Pharmacy, Stamford University Bangladesh, for his permission to use the facilities of the Pharmacology Laboratory.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abere TA, Okoto PE, Agoreyo FO. (2010). Antidiarrhoea and toxicological evaluation of the leaf extract of Dissotis rotundifolia Triana (Melastomataceae). BMC Complement Altern Med, 10, 71.
- Adzu B, Amos S, Kapu SD, Gamaniel KS. (2003). Anti-inflammatory and anti-nociceptive effects of Sphaeranthus senegalensis. J Ethnopharmacol, 84, 169–173.
- Agunu A, Yusuf S, Andrew GO, Zezi AU, Abdurahman EM. (2005). Evaluation of five medicinal plants used in diarrhoea treatment in Nigeria. J Ethnopharmacol, 101, 27–30.
- Arslan R, Bektas N. (2010). Antinociceptive effect of methanol extract of Capparis ovata in mice. Pharm Biol, 48, 1185–1190.
- Babre NP, Debnath S, Manjunath YS, Deshmaukh G, Hariprasath K, Sharon K. (2010a). Hypolipidemic effect of hydro-alcoholic extract of Barringtonia acutangula Linn root extract on streptozotocin-induced diabetic rats. J Pharm Sci Tech, 2, 368–371.
- Babre NP, Debnath S, Manjunath YS, Parameshwar P, Wankhede SV, Hariprasath K. (2010b). Antioxidant potential of hydroalcoholic extract of Barringtonia acutangula Linn roots on streptozotocin-induced diabetic rats. Int J Pharm Pharm Sci, 4, 201–203.
- Bentley GA, Newton SH, Starr J. (1983). Studies on the antinociceptive action of alpha-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol, 79, 125–134.
- Beubler E, Juan H. (1979). Effect of ricinoleic acid and other laxatives on net water flux and prostaglandin E release by the rat colon. J Pharm Pharmacol, 31, 681–685.
- Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. (1985). Pain measurement: An overview. Pain, 22, 1–31.
- Daduang J, Vichitphan S, Daduang S, Hongsprabhas P, Boonsiri P. (2011). High phenolics and antioxidants of some tropical vegetables related to antibacterial and anticancer activities. Afr J Pharm Pharmacol, 5, 608–615.
- Deraedt R, Jouquey S, Delevallée F, Flahaut M. (1980). Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol, 61, 17–24.
- Doherty NS. (1981). Inhibition of arachidonic acid release as the mechanism by which glucocorticoids inhibit endotoxin-induced diarrhoea. Br J Pharmacol, 73, 549–554.
- Eddy NB, Leimbach D. (1953). Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther, 107, 385–393.
- Gaginella TS, Phillips SF. (1975). Ricinoleic acid: Current view of an ancient oil. Am J Dig Dis, 20, 1171–1177.
- Gálvez J, Crespo ME, Jiménez J, Suárez A, Zarzuelo A. (1993). Antidiarrhoeic activity of quercitrin in mice and rats. J Pharm Pharmacol, 45, 157–159.
- Gálvez J, Zarzuelo A, Crespo ME, Utrilla MP, Jiménez J, Spiessens C, de Witte P. (1991). Antidiarrhoeic activity of Sclerocarya birrea bark extract and its active tannin constituent in rats. Phytother Res, 5, 276–278.
- Ghani A. (2003). Medicinal Plants of Bangladesh: Chemical Constituents and Uses. 2nd ed. Dhaka, Bangladesh: The Asiatic Society of Bangladesh.
- Gupta BD, Dandiya PC, Gupta ML. (1971). A psycho-pharmacological analysis of behaviour in rats. Jpn J Pharmacol, 21, 293–298.
- Jachak SM, Saklani A. 2007 Challenges and opportunities in drug discovery from plants. Curr Sci, 92, 1251–1257.
- Jinsmaa Y, Fujita Y, Shiotani K, Miyazaki A, Li T, Tsuda Y, Okada Y, Ambo A, Sasaki Y, Bryant SD, Lazarus LH. (2005). Differentiation of opioid receptor preference by [Dmt1]endomorphin-2-mediated antinociception in the mouse. Eur J Pharmacol, 509, 37–42.
- Jinsmaa Y, Okada Y, Tsuda Y, Shiotani K, Sasaki Y, Ambo A, Bryant SD, Lazarus LH. (2004). Novel 2′,6′-dimethyl-L-tyrosine-containing pyrazinone opioid mimetic mu-agonists with potent antinociceptive activity in mice. J Pharmacol Exp Ther, 309, 432–438.
- Kapoor LD. (1990). Handbook of Ayuvedic Medicinal Plants. Boca Raton: CRC Press.
- Khare CP. (Ed.). (2007). Indian Medicinal Plants. New York: Springer Science+Business Media.
- Mandal S, Nayak A, Kar M, Banerjee SK, Das A, Upadhyay SN, Singh RK, Banerji A, Banerji J. (2010). Antidiarrhoeal activity of carbazole alkaloids from Murraya koenigii Spreng (Rutaceae) seeds. Fitoterapia, 81, 72–74.
- Mascolo N, Izzo AA, Barbato F, Capasso F. (1993). Inhibitors of nitric oxide synthetase prevent castor-oil-induced diarrhoea in the rat. Br J Pharmacol, 108, 861–864.
- Masur J, Märtz RM, Carlini EA. (1971). Effects of acute and chronic administration of Cannabis sativa and (-) delta9-trans-tetrahydrocannabinol on the behavior of rats in an open-field arena. Psychopharmacologia, 19, 388–397.
- Morais LC, Barbosa-Filho JM, Almeida RN. (1998). Central depressant effects of reticuline extracted from Ocotea duckei in rats and mice. J Ethnopharmacol, 62, 57–61.
- Mukherjee PK, Das J, Balasubramanian R, Saha K, Pal M, Saha BP. (1995). Antidiarrhoeal evaluation of Nelumbo nucifera rhizome extract. Indian J Pharmacol, 27, 262–264.
- N’Gouemo P, Nguemby-Bina C, Baldy-Moulinier M. (1994). Some neuropharmacological effects of an ethanolic extract of Maprounea africana in rodents. J Ethnopharmacol, 43, 161–166.
- Pal BC, Achari B, Price KR. (1991). A triterpenoid glucoside from Barringtonia acutangula. Phytochem, 30, 4177–4179.
- Palombo EA. (2006). Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: Modes of action and effects on intestinal function. Phytother Res, 20, 717–724.
- Rahman MA, Sikder MAA, Kaiser MA, Rahman MS, Hasan CM, Rashid MA. (2010). In vitro antioxidant activity of Barringtonia acutangula (L.). Bangladesh Pharm J, 13, 38–41.
- Rahman MM, Polfreman D, MacGeachan J, Gray AI. (2005). Antimicrobial activities of Barringtonia acutangula. Phytother Res, 19, 543–545.
- Rashmi K, Shenoy KB, Hegde K, Shabaraya AR. (2011). Hepatoprotective effect of Barringtonia acutangula (L.) Gaertn leaf extracts against CCl4 induced hepatic damage. J Pharm Res, 4, 540–542.
- Sahoo S, Panda PK, Mishra SR, Parida RK, Ellaiah P, Dash SK. (2008). Antibacterial Activity of Barringtonia acutangula against selected urinary tract pathogens. Indian J Pharm Sci, 70, 677–679.
- Sánchez-Mateo CC, Bonkanka CX, Hernández-Pérez M, Rabanal RM. (2006). Evaluation of the analgesic and topical anti-inflammatory effects of Hypericum reflexum L. fil. J Ethnopharmacol, 107, 1–6.
- Satapathy KB, Brahmam M. (1994). Ethnobotanical survey on tribal area plant Barringtonia acutangula. Fourth Int Cong Ethnobiol. Lucknow: NBRO.
- Sharif MM, Banik GR. 2006. Status and utilization of medicinal plants in Rangamati of Bangladesh. Res J Agr Biol Sci, 2, 268–273.
- Shoba FG, Thomas M. (2001). Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol, 76, 73–76.
- Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Raviprakash V, Kumar D. (2003). Antinociceptive and antipyretic activities of Pongamia pinnata leaves. Phytother Res, 17, 259–264.
- Takagi K, Watanabe M, Saito H. (1971). Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. Jpn J Pharmacol, 21, 797–810.
- Takahashi RN, de Lima TC, Morato GS. (1986). Pharmacological actions of tannic acid; II. Evaluation of CNS activity in animals. Planta Med, 52, 272–275.
- Toma W, Gracioso JS, Hiruma-Lima CA, Andrade FD, Vilegas W, Souza Brito AR. (2003). Evaluation of the analgesic and antiedematogenic activities of Quassia amara bark extract. J Ethnopharmacol, 85, 19–23.
- Uawonggul N, Chaveerach A, Thammasirirak S, Arkaravichien T, Chuachan C, Daduang S. (2006). Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J Ethnopharmacol, 103, 201–207.
- Uddin SJ, Shilpi JA, Alam SM, Alamgir M, Rahman MT, Sarker SD. (2005). Antidiarrhoeal activity of the methanol extract of the barks of Xylocarpus moluccensis in castor oil- and magnesium sulphate-induced diarrhoea models in mice. J Ethnopharmacol, 101, 139–143.
- Vogel HG. (2007). Drug discovery and evaluation: Pharmacological Assays. Berlin: Springer.
- Walker CI, Trevisan G, Rossato MF, Franciscato C, Pereira ME, Ferreira J, Manfron MP. (2008). Antinociceptive activity of Mirabilis jalapa in mice. J Ethnopharmacol, 120, 169–175.
- Wong CH, Dey P, Yarmush J, Wu WH, Zbuzek VK. (1994). Nifedipine-induced analgesia after epidural injection in rats. Anesth Analg, 79, 303–306.
- World Health Organization. 1978. The promotion and development of traditional medicine. Technical report series, 622.
- Yaksh TL. (1999). Spinal systems and pain processing: development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol Sci, 20, 329–337.
- Yusuf M, Chowdhury JU, Wahab MA, Begum J. (1994). Medicinal Plants of Bangladesh. Chittagong, Bangladesh: BCSIR Laboratories.