Abstract
Context: Prunella vulgaris L. (Labiatae) is a perennial plant common in China and Europe and is rich in rosmarinic acid (RA), ursolic acid (UA), and oleanolic acid (OA). The dried spica of P. vulgaris has been used as traditional medicine in China for over a hundred years. To our best knowledge, no study has been conducted to determine the influence of harvesting time on concentrations of bioactive compounds of P. vulgaris.
Objective: In the current study, changes in the bioactive compounds present in spicas were investigated at five harvest times over 2 months.
Materials and methods: Plant material were collected at five fixed dates: 5th May, 20th May, 7th June, 15th June, and 25th June and assayed for chemical contents by high-performance liquid chromatography (HPLC).
Results: Among the different harvest times, the highest levels of RA (56.81 mg·g−1), UA (2.77 mg·g−1), and OA (0.91 mg·g−1) were found on 5th May, whereas the lowest levels of RA (1.66 mg·g−1), UA (2.27 mg·g−1), and OA (0.43 mg·g−1) were observed on 25th June.
Discussion and conclusion: As each medicinal product has its own content requirement for different bioactive components, the optimum harvest time might be determined according to the accumulation dynamics of target compound in dried spicas of P. vulgaris. These results may be useful for determining the optimal harvest time when bioactive components are at the maximum level, which is in early May.
Introduction
Prunella vulgaris L. (Labiatae), also known as the “self-heal”, is a perennial plant common in China and Europe. Prunellae Spica, the dried spica of P. vulgaris is a standard medicinal material in the Chinese Pharmacopoeia (CitationBoard of Pharmacopoeia of P. R. China, 2010) that has been used in China for over a hundred years (Chen et al., 2010). Because of its good performance and few side effects, as confirmed by a long-term clinical application (CitationPinkas et al., 1994; CitationCheung & Zhang, 2008), the dried spica has been widely used either alone or with other herbal ingredients to treat sore throats, fevers, goiters, hypertension, tuberculosis, and mammary gland hyperplasia (CitationBoard of Pharmacopoeia of P. R. China, 2010). In addition to its pharmaceutical uses, the spicas of P. vulgaris are manufactured as a refrigerated beverage, and the fresh leaves are consumed as a vegetable dish in southeast China (Chen et al., 2010).
Previous phytochemical studies on the biologically active components of P. vulgaris have been isolated and reported to mainly contain triterpenoids, phenolics, flavonoids, tannins, caffeic acids, and anionic polysaccharide prunelline (CitationRyu et al., 2000; CitationWang et al., 2000; CitationJiang et al., 2008). Rosmarinic acid (RA), one of the major phenolics, suppresses lipoperoxidation (CitationLaranjinha et al., 1994), scavenges superoxide radicals (CitationOsakabe et al., 2002), and exhibits anti-inflammatory (CitationOsakabe et al., 2004) and antioxidant (CitationPsotova et al., 2003) bioactivity. RA has also been used as the standard for quality control of Prunellae Spica by the Chinese Pharmacopoeia (CitationBoard of Pharmacopoeia of P. R. China, 2010). Of the triterpenes, ursolic acid (UA; ) and oleanolic acid (OA; ) have beneficial and notable effects on hepatoprotective, antifungal, anti-inflammation, antitumor, and antihyperlipidemia activities (CitationLiu, 1995). There is increasing evidence that UA is the main active substance in antidiabetic medicines due to its ability to lower blood sugar, urine sugar, water intake, and urine volume, whereas OA is the main compound that protects the liver (CitationLiu et al., 2003).
Because of its pharmacological and industrial importance, demand for P. vulgaris has increased steadily in recent years. The wild population of P. vulgaris cannot meet this growing need, and therefore, it has been proposed since the 1990s that P. vulgaris be cultivated in China (CitationChen et al., 2011). At present, approximately 10 million kg of P. vulgaris is required for prescriptions or export from China each year (CitationGuo et al., 2010). Hence, production protocols for cultivating uniform and high-quality P. vulgaris are needed to meet the market demand.
Figure 1. Chemical structures of the ursolic acid (UA), oleanolic acid (OA), and rosmarinic acid (RA).
An important aspect of P. vulgaris production is the timing of harvest to maximize yields and content of the desirable compounds. The spica maturity stage (mid-June) traditionally has been harvest time (CitationLiu et al., 2010; CitationWang et al., 2011) because it provides the highest spica yields and best marketing attributes (CitationXu, 2001); however, large variations in the amounts of the major bioactive components at this stage result in different optimal harvest times (CitationWang et al., 1993; CitationZhang et al., 2007). In previous studies, variations due to cultivation patterns, such as planting date and growth years, have received little interest (CitationJiang et al., 2009). Furthermore, only the yield (i.e., spica dry matter) or content of single active components was considered (CitationWang et al., 1993, Citation2011), and different harvesting dates were used in these studies. Our previous studies have shown that early May was considered an optimal harvest time of P. vulgaris in ancient China (Chen et al., 2010). In addition, P. vulgaris used in some studies was wild material from different regions, and growth conditions and cultivation methods were not clearly recorded, producing inconsistent results (CitationLuo et al., 2005).
Therefore, information about the content variations of the major bioactive compounds in spicas of P. vulgaris in relation to harvest time is needed. It is also important to determine the optimal harvest time of desirable compounds for growers. In our study, we determined RA, UA, and OA content at five harvest times over 2 months.
Materials and methods
Plant materials
Seeds of P. vulgaris were collected from Queshan County in the Henan Province of North China on June 10, 2008 and authenticated by Professor Qiaosheng Guo of the Institute of Chinese Medicinal Materials, Nanjing Agricultural University, Jiangsu Province, P.R. China. Twenty seeds of approximately the same size were sown in separate pots on October 7, 2008 and germinated in the greenhouse. After 1 month, the seedlings were transplanted to the experimental field located in a flat land site (117°23′E, 31°24′N, 48.64 altitude) of Lujiang County, Anhui Province, P.R. China. The spacing was 30 cm between rows and 25 cm between plants within a row. The field was cultivated using conventional commercial methods from October 2008 to June 2009. The material represented 20 plants were randomly collected at five fixed dates: 5th May, 20th May, 7th June, 15th June, and 25th June. After collected, the whole plants were dissected into spica and other tissues. Spicas were oven dried to a constant weight at 60°C, weighed, ground, and passed through a 0.3 mm sieve. RA, UA, and OA were analyzed by high-performance liquid chromatography (HPLC).
Chemicals and reagents
HPLC-grade methanol was purchased from Fisher Scientific Co. (Fair Lawn, NJ). Deionized water was purified by the Milli-Q system (Millipore, Bedford, MA, USA). Analytical-grade phosphoric acid and ethanol were from Nanjing Liudu Fine Chemicals Co. Ltd. (Nanjing, China). Three authentic standards of RA, UA, and OA were purchased from the National Institute for Control of Biological and Pharmaceutical Products (Beijing, China).
Apparatus and analytical conditions
HPLC was performed with a chromatographic pump (LC-20AT), manual sample injector, and a diode array detector (SPD-M20A, Shimadzu, Kyoto, Japan). The HPLC fingerprint was performed on a C18 column (Shimadzu, ODS-C18, 4.6 mm × 250 mm, 5 µm) at 25°C with a sample injection volume of 10 µL. The data were collected with the model N2000 chromatography workstation (Zhejiang University, Hangzhou, China).
The chromatographic conditions used to detect the UA and OA components were as follows: The detection wavelength was 210 nm for the analysis. The mobile phase, consisting of methanol and 0.5% ammonium acetate (90:10, v/v), was at a flow rate of 0.6 mL·min−1, and the column temperature was maintained at 25°C. The chromatographic conditions for the RA component were as follows: the mobile phase consisting of methanol and 0.01% aqueous phosphoric acid (52:48, v/v), the detection wavelength was 330 nm, the flow rate was 1.0 mL·min−1, and the column temperature was maintained at 25°C.
Making the calibration curves of active constituents
Stock solutions of RA, UA, and OA were prepared in deionized water and diluted with 75% methanol to six different concentrations to construct the calibration plots. The linearity of each standard curve was confirmed by plotting the peak area (y) against the corresponding concentration (x, µg·mL−1) of the analytes. The regression equations are listed in .
Table 1. Calibration curves of RA, UA, and OA standard chemicals.
Preparation of sample solutions
The samples for HPLC were prepared using modifications of the methods by CitationFang et al. (2010). Powdered spica samples (0.10 g) were mixed with 20 mL of 75% methyl alcohol for 30 min, extracted through a 30 min ultrasonic treatment at 20°C and centrifuged at 10,000 rpm for 10 min. The upper solution was filtered through a 0.45 µm organic membrane before injection into the HPLC system.
Statistical analysis
All data were analyzed using SPSS 11.5 for Windows. The data were initially compared by analysis of variance (ANOVA), and the differences between means were detected using the Duncan test. p values ≤ 0.05 were considered significant.
Results and discussion
Changes in RA, UA, and OA in the spicas of P. vulgaris at different harvest times
The HPLC chromatograms fingerprint of the RA, UA and OA from P. vulgaris spicas and its reference standards are shown in and . The amounts of RA, UA, and OA in the spicas of P. vulgaris collected at five harvest times are presented in . The results indicate that the amounts of these major bioactive compounds varied with harvest times.
Table 2. Changes in RA, UA, and OA contents (mg·g−1) in the spicas of P. vulgaris at different harvest times.
Figure 2. HPLC chromatogram fingerprint of the OA and UA from P. vulgaris spicas and RS (reference standards). (1) OA; (2) UA.
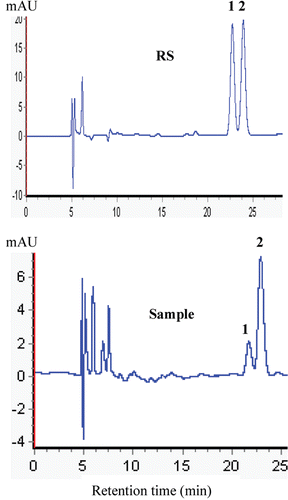
Figure 3. HPLC chromatogram fingerprint of the RA from P. vulgaris spicas and RS (reference standards). (3) RA.
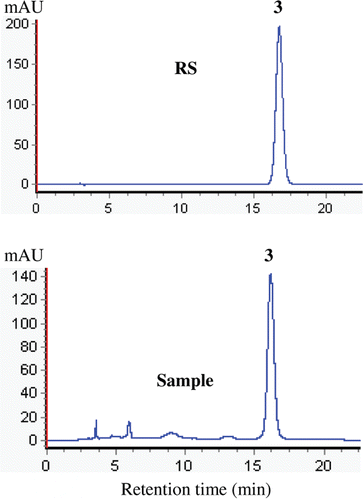
In dried spicas of P. vulgaris, the amount of RA decreased from 5th May to 25th June, and the UA and OA indices declined steadily during this period (). The sample harvested on 5th May had the highest amounts, including RA: 56.81 mg·g−1, UA: 2.77 mg·g−1, and OA: 0.91 mg·g−1. The lowest amounts were obtained from the 25th June harvest (RA: 1.66 mg·g−1, UA: 2.27 mg·g−1, and OA: 0.43 mg·g−1). Total average RA amount in samples harvested on 5th May (56.81 mg·g−1) was significantly higher than that of 25th June (1.66 mg·g−1). Moreover, the average amount of UA and OA in samples harvested on 5th May increased 22 and 111%, respectively, compared with the 25th June samples (). The maximum amounts of RA, UA, and OA occurred on 5th May during the spica formation period. Previous studies have reported that changes in the amount of the major bioactive compounds in spicas of P. vulgaris occur at different harvest times, such as mid-May (CitationBoard of Pharmacopoeia of P.R. China, 1977) and mid-June (CitationLuo et al., 2005; CitationWang et al., 2011). Late June (CitationWang et al., 1993) was reported as the best harvest time. These differences might be affected by environmental stresses (e.g., light, heavy metal, and drought), genetics, climatic conditions, ecotype, cultural practices, or a combination of these factors (CitationGuo et al., 2010; CitationWu et al., 2010; CitationChen et al., 2011; CitationLiao et al., 2010); therefore, it is worthwhile to experimentally determine the optimal time for RA, UA, and OA production for each location and ecotype rather than rely on published data only. Our study reported that the preflowering stage (5th May) had higher RA, UA, and OA amounts compared to the fully developed mature stage (15th June). These results are in agreement with those of CitationZhang et al. (2007) and CitationShou et al. (2009) who observed a decline in RA, UA, and OA with ripening. The decline in RA, UA, and OA from preflowering to fully mature plants could be caused by a dilution effect because the total sample volume increased during this period (CitationJiang et al., 2009). Similar results have occurred in other species as well (CitationChen et al., 2003; CitationWu et al., 2004).
Determination of the most suitable harvest time for spicas of P. vulgaris
There is an increasing interest in the use of natural substances (CitationDeans & Svoboda, 1993) because many herbal medicines are free from side effects and easy to obtain, considered “green” and healthy, and generate income. Chemical markers, however, are often used for quality control of medicinal products. According to the Chinese Pharmacopoeia 2010, RA is the standard component for quality control of Prunellae Spica, and its content should be more than 2.0 mg·g−1. Many pharmacological investigations have shown that active components, such as RA, UA, and OA, have important pharmacological effects (CitationLiu, 1995; CitationPsotova et al., 2003) and different content requirements for the quality control of medicinal products. For example, as a marker for quality control of Xiakucao Oral solution, the content of RA per mL should be more than 1.00 mg (CitationJin et al., 2009). For Jiliukang Oral liquid, the content of UA should be more than 0.50 mg per 10 mL (CitationDeng et al., 2000).
In summary, present study indicates that the content of bioactive components in the spicas of P. vulgaris is closely related to harvest time, which should be determined according to the dynamics of target compound accumulation. Our results suggest that the optimal harvest time for maximum RA, UA, and OA accumulation in the spicas of P. vulgaris is early May.
Acknowledgments
This research was supported by the programs of National Nature Science Foundation of China (No. 30772730 and 81072986).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chen SQ, Dong CM, Zheng XK, Dong SL, Wang L, Feng WS. (2003). The quantitative variety of rosmarinic acid in Rabdosia rubescens from different harvesting time. J Henan Univ Chin Med, 18, 28–30.
- Chen YH, Guo QS, Liu L, Liao L, Zhu ZB. (2011). Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J Med Plant Res, 5, 1749–1755.
- Chen YH, Guo QS, Wang CY. (2010). Textual research on change of medicinal parts and herbal medicine of Prunella vulgaris. Zhongguo Zhong Yao Za Zhi, 35, 242–246.
- Cheung HY, Zhang QF. (2008). Enhanced analysis of triterpenes, flavonoids and phenolic compounds in Prunella vulgaris L. by capillary zone electrophoresis with the addition of running buffer modifiers. J Chromatogr A, 1213, 231–238.
- Board of Pharmacopoeia of P. R. China. (2010). Pharmacopoeia of the People’s Republic of China. Beijing: China Medico-Pharmaceutical Science & Technology Puhlishig House.
- Board of Pharmacopoeia of P.R. China. (1977). Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Press.
- Deans SG, Svoboda KP. (1993). Biological activity of plant volatile oils. In: Hay RKM, Warterman PG, eds. Volatile Oil Crops: Their Biology, Biochemistry and Production. London: Longman Group, 97–112.
- Deng SY, He JJ, Chen QL. (2000). Study on quality standards for Jiliukang oral liquid. Chin Tradi Patent Med, 22, 335–337.
- Fang L, Lin N, Wu Y. (2010). [Simultaneous determination of four active components in Spica Prunellae by HPLC]. Zhongguo Zhong Yao Za Zhi, 35, 616–619.
- Guo Q, Zhou L, Gong W, Wu X. (2010). [Effect of different water treatments on quality and yield of spadix in Prunella vulgaris]. Zhongguo Zhong Yao Za Zhi, 35, 1795–1798.
- Jiang SJ, Zhao LZ, Yu YG, Duan LD, Tan BX. (2008). Study on optimization of supercritical fluid extraction conditions of ursolic acid from Prunella vulgaris Linn. leaves. Food Sci, 29, 294–297.
- Jiang XZ, Wu YZ, Zhang DR, Liu W. (2009). Cultivated techniques of Prunella vulgaris under good agriculture practice. J Jiangxi Univ Tradit Chin Med, 21, 79–82.
- Jin RY, An WX, Pan XH, Zhao H, Ren MT. (2009). HPLC determination of rosmarinic acid in Xiakucao oral solution. Chin J Pharm Anal, 29, 1010–1012.
- Laranjinha JA, Almeida LM, Madeira VM. (1994). Reactivity of dietary phenolic acids with peroxyl radicals: Antioxidant activity upon low density lipoprotein peroxidation. Biochem Pharmacol, 48, 487–494.
- Liao L, Liu L, Guo QS, Wang ZY, Chen YH. (2010). Morphological and chemical variation of Prunella vulgaris populations from different locations in China. Chin Herb Med, 2, 305–311.
- Liu H, Shi Y, Wang D, Yang G, Yu A, Zhang H. (2003). MECC determination of oleanolic acid and ursolic acid isomers in Ligustrum lucidum Ait. J Pharm Biomed Anal, 32, 479–485.
- Liu J. (1995). Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol, 49, 57–68.
- Liu P, Yuan BG, Yin DD, Mian F. (2010). Accumulation laws of main medicinal ingredients in different part of Prunella vulgaris L. Acta Agri Boreali-Occidentalis Sin, 19, 137–140.
- Luo MH, Zhou RB, Tong QZ, Qu WH. (2005). A study on the optimum picking period for Prunella vugaris L.cultivated in a standardized way. J Hunan Univ Tradit Chin Med, 25, 12–14.
- Osakabe N, Takano H, Sanbongi C, Yasuda A, Yanagisawa R, Inoue K, Yoshikawa T. (2004). Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors, 21, 127–131.
- Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. (2002). Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in d-galactosamine (D-GalN)-sensitized mice. Free Radic Biol Med, 33, 798–806.
- Pinkas M, Trotin F, Peng M, Trock M. (1994). Use chemistry and pharmacology of the Chinese medicinal plants. Fitoterapia, 55, 343–353.
- Psotová J, Kolár M, Sousek J, Svagera Z, Vicar J, Ulrichová J. (2003). Biological activities of Prunella vulgaris extract. Phytother Res, 17, 1082–1087.
- Ryu SY, Oak MH, Yoon SK, Cho DI, Yoo GS, Kim TS, Kim KM. (2000). Anti-allergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med, 66, 358–360.
- Shou D, Zhang JM, Yu ZM. (2009). Dynamic quantitative variety of main constituent in fruit of Cornus officinalis during the process of fruit maturation. Chin Arch Tradit Chin Med, 27, 1418–1420.
- Wang HB, Zhang ZY, Zia ZX, Su Z, Li CK. (1993). [Qualitative analysis of Chinese drug xiakucao (Prunella)]. Zhongguo Zhong Yao Za Zhi, 18, 655–7, 702.
- Wang Y, Yin J, Guo Q, Xiao Y. (2011). [Dynamic change of active component content in different parts of Prunella vulgaris]. Zhongguo Zhong Yao Za Zhi, 36, 741–745.
- Wang ZJ, Zhao YY, Wang B, Ai TM, Chen YY. (2000). Depsides from Prunella vulgaris. Chin Chem Letters, 11, 997–1000.
- Wu JF, Liu B, Zhu CC, Lai XP. (2004). Determination of 2α-hydroxy-ursolic acid from Herba Rabdosiae Serrae in various collecting periods. Chin Tradit Herbal Drugs, 35, 81–83.
- Wu Z, Guo Q, Wang Q, Zhou L, Zhang Z, Zhang L, Huang T. (2010). [Effects of lead, copper and cadmium stresses on growth and inherent quality of Prunalla vulgaris]. Zhongguo Zhong Yao Za Zhi, 35, 263–267.
- Xu L. (2001). The standardized culture of Chinese Rare Medicinal herbs and industrialization Development New Technology. Beijing: Publishing House of Peking Union Medical College.
- Zhang LZ, Qin W, Zhang XH. (2007). Assay method for contents of caffeic acid and rosmarinic acid in the different parts of Prunella vulgaris L. Beijing Univ Trad Chin Med, 30, 343–345.