Abstract
Context: Extracellular matrix (ECM) synthesis regulation by sympathetic nervous system (SNS) or angiotensin II (ANG II) was widely reported, but interaction between the two systems on ECM synthesis needs further investigation.
Objective: We tested implication of SNS and ANG II on ECM synthesis in juvenile rat aorta.
Materials and methods: Sympathectomy with guanethidine (50 mg/kg, subcutaneous) and blockade of the ANG II AT1 receptors (AT1R) blocker with losartan (20 mg/kg/day in drinking water) were performed alone or in combination in rats. mRNA and protein synthesis of collagen and elastin were examined by Q-RT-PCR and immunoblotting.
Results: Collagen type I and III mRNA were increased respectively by 62 and 43% after sympathectomy and decreased respectively by 31 and 60% after AT1R blockade. Combined treatment increased collagen type III by 36% but not collagen type I. The same tendency of collagen expression was observed at mRNA and protein levels after the three treatments. mRNA and protein level of elastin was decreased respectively by 63 and 39% and increased by 158 and 15% after losartan treatment. Combined treatment abrogates changes induced by single treatments.
Discussion and conclusion: The two systems act as antagonists on ECM expression in the aorta and combined inhibition of the two systems prevents imbalance of mRNA and protein level of collagen I and elastin induced by single treatment. Combined inhibition of the two systems prevents deposit or excessive reduction of ECM and can more prevent cardiovascular disorders.
Introduction
Collagen types I and III and elastin are the major extracellular matrix (ECM) proteins in vascular wall (CitationVelleman et al., 2001). Under physiological conditions, the integrity of the blood vessel wall is maintained by equilibrium between ECM synthesis and degradation process (CitationJacob, 2003; CitationFondard et al., 2005). Imbalance between synthesis and degradation of ECM contribute to development of cardiovascular disorders such as atherosclerosis (CitationSpinetti et al., 2008).
Much less is known about the role of the sympathetic nervous system (SNS) and interaction between SNS and renin angiotensin system (RAS) in the regulation of cardiovascular ECM synthesis in blood vessels. This is an important question because the SNS and RAS are activated in disease states affecting these tissues. Therefore adrenergic receptors and AT1R antagonists were largely used in treatment and prevention of cardiovascular diseases (CitationKopecky, 2006).
Overexpression and higher circulating levels of angiotensin II (ANG II) are associated with many cardiovascular disorders such as primary hypertension (CitationLee et al., 1993). ANG II exert their effects on cells through AT1R either directly, by activation of phospholipase C, or indirectly, through the endothelin system (CitationBalakrishnan et al., 1996; Citationd’Uscio et al., 1998). Neuro-humoral interaction between the SNS and the RAS has been described by the stimulator action of ANG II on the SNS via presynaptic AT1R (CitationDendorfer et al., 1998; CitationBalt et al., 2001) which facilities norepinephrine (NE) release from peripheral sympathetic fibers acting on blood vessels (CitationCox et al., 1995). However, interactions between SNS and RAS have been more often illustrated in the heart (CitationZucker et al., 2001) than in peripheral vessels.
There are fewer investigations of a single effect of ANG II and SNS interaction between the two systems on ECM remodeling in the rat heart and vasculature. Thus, we have previously investigated the effect of SNS and ANG II on ECM synthesis and degradation in rat heart (CitationDab et al., 2009a,Citationb), vessels from adult rats (CitationDab et al., 2011a) and from juvenile rats (CitationDab et al., 2011b). We found that ANG II and SNS can act in synergy especially on matrix metalloproteinases expression or as antagonist system on ECM synthesis.
This study investigated interactions between SNS and ANG II and effect of the combined blocking of the two systems on the aortic ECM synthesis in rat. mRNA and proteins levels of elastin, collagen type I and III have been determined by RT-PCR and immunoblotting.
Methods
Animals
The animal protocols used for this study were approved by the University Animal Care and Use Committee of University of Claude Bernard, Lyon 1, France and were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
New-born Wistar–Kyoto rats (n = 40), were divided into four groups: a sympathectomised group (n = 10), a losartan-treated group (n = 10), a sympathectomised and losartan-treated group (n = 10) and a non-treated group (sham group) (n = 10). The 1 week aged rats were sympathectomised with guanethidine sulfate (50 mg/kg i.p.; 5 days/week) for 3 weeks (CitationJohnson, 1975). Animal sex was determinate at 2 weeks of age and only male rats were used for this study. The effect of guanethidine administration was evaluated by the development of ptosis (CitationDwyer et al., 2004).The losartan-treated group received 20 mg/kg/day of losartan (Sigma, St. Louis, MO, USA), in drinking water (CitationRocha et al., 2007) until 6 weeks of age. The water consumption and rat body weight were measured daily and the concentration of losartan was adjusted each day according to water consumption and animal body weight. The sham group received injections of saline (i.p.) for the same time period than sympathectomy group and received tap water.
At the age of 6 weeks, the rats were deeply anesthetized with pentobarbital; aorta was removed carefully and placed in ice-cold physiological salt solution to remove blood and surrounding tissue. Aorta was then rapidly frozen in liquid nitrogen and frozen immediately in liquid nitrogen, and stored at −80°C.
Total RNA extraction and real-time RT-PCR analysis
The protocol of RNA extraction and real-time RT-PCR analysis is described in detail in our previous study (CitationDab et al., 2009a). Briefly, total RNA was extracted from aorta using Trizol Reagent (Invitrogen, Carlsbad, CA). After DNase treatment, 2 µg of total RNA was reverse-transcripted with superscript II transcriptase (Invitrogen, France) using random hexamers as primers (pdN6; Amersham Biosciences, Piscataway, NJ). Quantitative real-time RT-PCR was performed for selected gene transcripts with a MyiQ thermal cycler (Bio-Rad Laboratories, Hercules, CA). Real-time RT-PCR was performed using the resulting cDNA as template, iQ SYBR Green Supermix (Bio-Rad Laboratories), and the appropriate set of primers (Invitrogen, France) specific to collagen type I (sense: CGAGACCCTTCTCACTCCTG; anti-sense: GGCATCCATAGTGCATCCTT), collagen III (sense: AGCTGGACCAAAAGGTGATG; anti-sense: GACCTCGTGCTCCAGTTAGC), elastin (sense: AGCTCCCTTGTTCTTGTGGA; anti-sense: GGTGTGCCTAGCCAGACAGT). 18S ribosomal RNA (18S rRNA) are used as housekeeping gene internal controls (sense: AGTCGGCATCGTTTATGGTC; anti-sense: TGAGGCCATGATTAAGAGGG). Two-step RT-PCR real-time amplifications were carried out as follows: 3 min at 95°C followed by 10 s at 95°C and 45 s at 60°C. For each sample, PCR was performed in duplicate. Control templates for each target gene were amplified by semi-quantitative RT-PCR and amount of cDNA was quantified by spectroscopy at 260 nm. Gene quantity was expressed in (nano-grams) for each target gene and in micro-grams for housekeeping gene (18S rRNA). Dilutions of these control cDNA were used to generate the standard curves for each target gene parallel to the amplification of samples issued from all groups. Cycle threshold values and relative amount of each studied gene (ng/µg 18S rRNA) were calculated with Optical System Software v1.0 (Bio-Rad Laboratories).
Protein extraction and western blotting
The protocol of protein extraction and immunoblotting is described in detail in our previous study (CitationDab et al., 2009b). Briefly, frozen aortic tissue (100 mg) was homogenized in hydrolysis buffer containing protease inhibitor cocktail (Complete mini, Roche, Munich, Germany). After centrifugation, the protein concentrations were measured in the supernatants with the BCA kit (Pierce, Rockford, IL, USA). The protein extract (50 µg) was loaded on to a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred to nitrocellulose membranes in a transfer medium. After blockage with 0.1% Tris Buffer Saline Tween-20 (TBST) buffer containing powdered goat milk, membranes were incubated overnight at 4°C with specific mouse monoclonal IgG antibodies to collagen type I and III and elastin (1:2000) or 2 h at room temperature with monoclonal IgG anti-β-actin (1:5000). After washing with 0.1% TBST, membranes were incubated with the horseradish peroxidase-conjugated goat anti-mouse IgG antibody (1:5000) for 1 h. All antibodies were from Sigma (Sigma, St. Louis, MO, USA). Immunoreactivity of proteins was visualized by chemiluminescent detection with an enhanced chemiluminescent reagent (ECL, Amersham, Buckinghamshire, UK).
To control and correct protein loading error, we have used β-actin as an internal loading control. Thus, β-actin was largely used as internal control in western blotting (CitationChavez & LaManna, 2002; CitationChan & Leung, 2007). In our experimental conditions, β-actin was used firstly because densitometric quantification of the bands did not show any change after all treatments since the same amount of protein was loaded in electrophoresis gel, and secondly β-actin (42–44) kDa will not interfere with our target protein (type I collagen (95 kDa), type III collagen (70 kDa) and elastin (66 kDa).
Statistical analysis
The observed data were subjected to the parametric 1 way ANOVA followed by the Honestly Significant Difference Tukey procedure to perform pair-wise comparisons between all groups using statistica software (Statsoft, France). This test allow to compare and declare any two treatments to be significantly different at level α = 0.05. Results are expressed as mean ± SEM.
Results
shows body weight variation and mean water intake of rats during the experiments. There are no significant differences recorded between all groups.
Table 1. Body weight, and mean water uptake during the experiments.
ECM synthesis
mRNA of collagen I and collagen III was significantly increased respectively by 62% (p < 0.05) and 43% (p < 0.01) in the sympathectomy group but decreased by 31% (p < 0.01) and 60% (p < 0.01) in the losartan-treated group. Combined treatment increases collagen III mRNA by 36% (p < 0.01) and did not change collagen I expression (). mRNA expression of elastin was significantly decreased by 63% (p < 0.05) after sympathectomy and increased by 185% (p < 0.01) after losartan treatment but remained unchanged in the combined-treated group ().
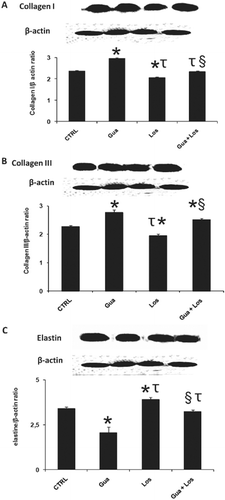
We observe the same tendency on transcriptional level and protein level for the three studied targets after each treatment. Collagen type I and III protein level was respectively increased after sympathectomy by 25 and 22% (p < 0.05) and decreased by 13 and 14% (p < 0.05) after losartan treatment. Combined treatment enhances collagen I protein level by 11% (p < 0.05) but not affect collagen I protein ( and ). Elastin protein level was decreased after sympathectomy by 39% (p < 0.05), increased in the losartan-treated group by 15% (p < 0.05) and was not modified after combined treatment ().
Figure 1 . Quantitative real-time RT-PCR analysis of collagen type I and III (A) and elastin (B) in the aorta from control and treated rats. Values are presented as ratio of mRNA to 18S rRNA. Data are mean ± SEM, n = 10 in each group. *p < 0.05, **p < 0.01 (versus CRTL), τp < 0.05, ττp < 0.01 (versus Gua group), §p < 0.05, §§p < 0.01 (versus Los group). CTRL: control, Gua: sympathectomy group, Los: losartan-treated group, Gua + Los: combined treatment group.
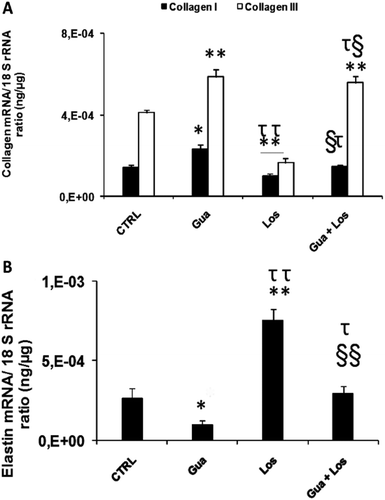
Figure 2 . Immunoblot analysis and densitometric quantification of collagen I (A) and III (B) and elastin (C) in the aorta from control and treated rats. Values are presented here as ratio of collagen I, collagen III or elastin content to β-actin. Data are mean ± SEM; n = 10 in each group. *p < 0.05 (versus CRTL), τp < 0.05 (versus Gua group), §p < 0.05 (versus Los group). CTRL: control group, Gua: sympathectomy group, Los: losartan-treated group, Gua + Los: combined treatment group.
Discussion
In the present study, we tested the contribution of SNS and ANG II on regulation of ECM synthesis after sympathectomy with guanethidine and blockade of AT1R with losartan that have been conducted separately or in combination. The main conclusion deducted from our experimental approach was the differential effect of SNS and ANG II on collagen and elastin synthesis. Accordingly, we found that the two systems act as antagonists on collagen and elastin expression in the aortic wall and combined inhibition of the two systems prevents imbalance of mRNA and protein level of collagen I and elastin induced by single inhibition.
Guanethidine induces a dramatic loss of catecholamine in the circulation and all tissues (CitationAberdeen et al., 1990, CitationVillanueva et al., 2003) and is highly selective for sympathetic fibers. We choose guanethidine from other substances used for sympathectomy such as 6-hydroxydopamine, because its destructive effect is more efficient in the rat (CitationJohnson, 1975). In our study, the efficacy of guanethidine was inspected by the manifestation of ptosis (an index of sympathectomy).
We investigated the effect of SNS and ANG II in aorta from juvenile rats under normal condition for three reasons: firstly to expand our previous investigation in heart and aorta issued from adult rats, secondly to provide a better understanding of the implication of the two systems on ECM synthesis because there are a few studies performed in vascular bed and under normal conditions (CitationHilgers et al., 1993; CitationWang et al., 2005), and thirdly to exclude interaction between effect of our several treatments and hemodynamic factors such as hypertension which are frequently associated with cardiovascular pathologies.
ANG II was described as a powerful actor of the pathogenesis of vascular hypertrophy and arterial stiffness by increasing the collagen content of the arterial wall involved in hypertension and fibrotic remodeling (CitationIzzo, 2000; CitationIzzo & Shykoff 2001).
Contradictory previous investigations showed that sympathectomy increases collagen expression in the vasculature (CitationFronek et al., 1978) and decreases total collagen content in blood vessels from hypertensive rats (CitationIwatsuki et al., 1979). This can be related to the variety of used protocols and to homodynamic factors. Thus in our present study, collagen type I and III, the two major components of ECM in vessels, was evaluated using highly sensitive mRNA and immunoblotting techniques.
SNS and RAS interaction was demonstrated by the stimulator effect of ANG on SNS neurotransmission through AT1R expressed by the SNS terminal axons innervating the cardiovascular tissue (CitationBalt et al., 2001). The use of AT1R antagonists such as losartan reduces the SNS neurotransmission by decreasing the NE release and improving her reuptake (CitationGironacci et al., 1994). Consequently, action of ANG II through AT1R dependent simultaneously from activities of AT1R expressed on vascular cells (direct action) and AT1R expressed in sympathetic fibres (indirect action) (CitationSenzaki et al., 2000). Therefore, our experimental approach could dissociate direct and indirect (via SNS) action of ANG II and allow investigation effects of single and combined inhibition of SNS and ANG II.
Discussion
Sympathectomy removes the indirect pathway of ANG II (CitationSimon & Csiky, 1998), but AT1R could be either open (in the sympathectomy group) or blocked (in the combined treatment group) in vascular tissue. The direct effect of ANG II can be deducted by comparison of the sympathectomy and combined treatment groups. The analysis of SNS and ANG II actions reveals that SNS acts as stimulator and ANG II as inhibitor through direct pathway on collagen type I and III at both the transcriptional and protein levels. Therefore, we found that ANG II and SNS act differently on elastin synthesis, which are enhanced trough SNS action and inhibited by ANG II.
The single inhibition of SNS and ANG II is commonly used to reduce cardiovascular mortality and morbidity. There are several comparisons between effects of adrenergic antagonists and AT1R antagonists (CitationKizer et al., 2005), but the use of combined treatments was less investigated. In this study, we tested the effect of single and combined treatments in expressions of the major compounds of ECM and demonstrates that combined inhibition can be considered like a new therapeutic approach to prevent excessive ECM imbalance to improve a better prevention and treatment of cardiovascular disorders.
ECM level
We have evaluated local synthesis of collagen type I and type III, the two major collagen subtypes in the blood vessels, by quantitative RT-PCR and western blotting.
There is evidence that vascular ECM is an active and dynamic structure that has a fundamental role in the regulation of cardiovascular function under normal and pathological conditions. Our results showed that sympathectomy increases collagen expression at transcriptional and protein level, and confirm previous studies which showed an increase of absolute collagen deposition in blood vessels (CitationFronek et al., 1978) and heart (CitationLurie et al., 1988; CitationFacoetti et al., 2006) after sympathectomy. Collagen was expressed more when AT1R are accessible (sympathectomy group) than when AT1R are blocked (combined-treated group), this indicates a stimulatory action of ANG II on collagen synthesis through AT1R. Thus, we can hypothesize that enhanced collagen expression after sympathectomy was due to the stimulatory effect of ANG II. Paradoxically previous studies report that stimulation of vascular smooth muscle cells (VSMCs) with NE increases collagen production by activation of α1-adrenoceptor (CitationO’Callaghan & Williams, 2002). This in vivo response can be linked to activation of the endothelin system which can stimulate collagen synthesis by VSMCs after norepinephrine treatment (CitationDao et al., 2001) and it was abrogated by endothelin receptors antagonists. On the other hand, sympathectomy increases release of endothelin from the rat mesenteric arterial bed (CitationRalevic et al., 1995) and enhances endothelin-1 receptor in rat thoracic aortic (CitationAliev et al., 1996).
The blocking of AT1R by losartan decreases collagen expression at mRNA and protein levels, this corroborates clinical investigations indicating a benefit effects of losartan in patients with vascular diseases and in experimental model of aortic pathology, since it reduces aortic hypertrophy and collagen accumulation in rat (CitationBenetos et al., 1997). Accordingly, ANG II was previously reported as a stimulator of collagen synthesis in many cells lines like mesanglial cells, rat, porcine and human VSMCs (CitationKato et al., 1991; CitationRizvi et al., 1996). Accumulation of interstitial collagen is a structural feature of vascular disease because it can occupy most of the volume of occlusive atherosclerotic plaque and contribute to hypertension and arterial stiffness (CitationFord et al., 1999).
Our results showed that combined treatment abrogates changes of collagen I and elastin expression induced by SNS and ANG II at transcriptional and protein levels.
Interestingly, we found a discrepancy between collagen expression after sympathectomy. It’s very likely that collagen type I and III expression had a different intracellular transduction signals pathways, an aspect that warrants further investigation, since no previous investigations discussed this findings in rat aorta under normal conditions.
Elastin expression was regulated differently by SNS and ANG II than collagen. In fact, sympathectomy reduces and losartan treatment enhances mRNA and protein level of elastin. Elastin was more expressed in combined-treated group (AT1R blocked) than in sympathectomy group (AT1R open). This indicates that ANG II reduces elastin expression through AT1R expressed on aortic cells. Theses results were in accordance with previous studies indicating that NE enhances (CitationDao et al., 2001) and ANG II reduces elastin content trough AT1R (CitationTokimitsu et al., 1994).
Conclusions
This study was performed to test effect of single or combined blockage of SNS and ANG II and to specify the role of ANG II and SNS in synthesis of ECM in juvenile rat aorta. The major finding was the antagonist action of ANG II and SNS on collagen and elastin synthesis. Combined treatment also abrogates opposite effects of single treatments on collagen I, collagen III and elastin in aorta. These resultsdemonstrate that under physiological conditions activity of SNS and ANG II can mediate structural and functional features of the arterial wall. Previous investigations were especially focused on the use of inhibitor of ANG converting enzyme, inhibition of AT1R blockade and adrenergic receptors antagonists to reduce morbidity and mortality of cardiovascular diseases (CitationWerner et al., 2008). Our present study allow a best proposal to investigate the effect of combined inhibition of SNS and ANG II action on ECM remodeling witch is the key feature of cardiovascular disorders. Our investigation demonstrates combined treatments can prevent deposit or excessive reduction of ECM protein after blockage of SNS or ANG II.
Acknowledgements
We wish to thank the association “Autour de Williams” for their participation in the financing of reagents used in our experiences. Houcine Dab received a grant from the Ministry of higher education, scientific research and technology (Tunisia) to work on this study in the EA4173, INSERM ERI-22, Faculté Rockefeller, Université Claude Bernard, Lyon 1, France.
Declaration of interest
The authors report no conflicts of interest.
References
- Aberdeen J, Corr L, Milner P, Lincoln J, Burnstock G. (1990). Marked increases in calcitonin gene-related peptide-containing nerves in the developing rat following long-term sympathectomy with guanethidine. Neuroscience, 35, 175–184.
- Aliev G, Ralevic V, Burnstock G. (1996). Depression of endothelial nitric oxide synthase but increased expression of endothelin-1 immunoreactivity in rat thoracic aortic endothelium associated with long-term, but not short-term, sympathectomy. Circ Res, 79, 317–323.
- Balakrishnan SM, Wang HD, Gopalakrishnan V, Wilson TW, McNeill JR. (1996). Effect of an endothelin antagonist on hemodynamic responses to angiotensin II. Hypertension, 28, 806–809.
- Balt JC, Mathy MJ, Nap A, Pfaffendorf M, van Zwieten PA. (2001). Effect of the AT1-receptor antagonists losartan, irbesartan, and telmisartan on angiotensin II-induced facilitation of sympathetic neurotransmission in the rat mesenteric artery. J Cardiovasc Pharmacol, 38, 141–148.
- Benetos A, Lacolley P, Safar ME. (1997). Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol, 17, 1152–1156.
- Chan YC, Leung PS. (2007). Angiotensin II type 1 receptor-dependent nuclear factor-κB activation-mediated proinflammatory actions in a rat model of obstructive acute pancreatitis. J Pharmacol Exp Ther, 323, 10–18.
- Chavez JC, LaManna JC. (2002). Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: Potential role of insulin-like growth factor-1. J Neurosci, 22, 8922–8931.
- Cox SL, Ben A, Story DF, Ziogas J. (1995). Evidence for the involvement of different receptor subtypes in the pre- and postjunctional actions of angiotensin II at rat sympathetic neuroeffector sites. Br J Pharmacol, 114, 1057–1063.
- Dab H, Hachani R, Hodroj W, Sakly M, Bricca G, Kacem K. (2009a). Differential control of MMP and t-PA/PAI-1 expressions by sympathetic and renin-angiotensin systems in rat left ventricle. Auton Neurosci, 150, 27–32.
- Dab H, Hachani R, Hodroj W, Sakly M, Bricca G, Kacem K. (2009b). Differential control of collagen synthesis by the sympathetic and renin-angiotensin systems in the rat left ventricle. Auton Neurosci, 151, 106–110.
- Dab H, Hachani R, Hodroj W, Sakly M, Bricca G, Kacem K. (2011b). Interaction between sympathetic nervous system and renin angiotensin system on MMPs expression in juvenile rat aorta. Gen Physiol Biophys, 30, 271–277.
- Dab H, Hachani R, Dhaouadi N, Hodroj W, Sakly M, Randon J, Bricca G, Kacem K. (2011a). Physiological regulation of MMPs and tPA/PAI in the arterial wall of rats by noradrenergic tone and angiotensin II. J Renin Angiotensin Aldosterone Syst. DOI: 10.1177/1470320311414752.
- Dao HH, Lemay J, de Champlain J, deBlois D, Moreau P. (2001). Norepinephrine-induced aortic hyperplasia and extracellular matrix deposition are endothelin-dependent. J Hypertens, 19, 1965–1973.
- Dendorfer A, Raasch W, Tempel K, Dominiak P. (1998). Interactions between the renin-angiotensin system (RAS) and the sympathetic system. Basic Res Cardiol, 93 Suppl 2, 24–29.
- d’Uscio LV, Shaw S, Barton M, Lüscher TF. (1998). Losartan but not verapamil inhibits angiotensin II-induced tissue endothelin-1 increase: Role of blood pressure and endothelial function. Hypertension, 31, 1305–1310.
- Dwyer KW, Provenzano PP, Muir P, Valhmu WB, Vanderby R Jr. (2004). Blockade of the sympathetic nervous system degrades ligament in a rat MCL model. J Appl Physiol, 96, 711–718.
- Facoetti A, Fallarini S, Miserere S, Bertolotti A, Ferrero I, Tozzi R, Gatti C, Palladini G, Perlini S, Nano R. (2006). Histochemical study of cardiac mast cells degranulation and collagen deposition: Interaction with the cathecolaminergic system in the rat. Eur J Histochem, 50, 133–140.
- Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, Hvass U, Couetil JP, Henin D, Michel JB, Vahanian A, Jacob MP. (2005). Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J, 26, 1333–1341.
- Ford CM, Li S, Pickering JG. (1999). Angiotensin II stimulates collagen synthesis in human vascular smooth muscle cells. Involvement of the AT(1) receptor, transforming growth factor-β, and tyrosine phosphorylation. Arterioscler Thromb Vasc Biol, 19, 1843–1851.
- Fronek K, Bloor CM, Amiel D, Chvapil M. (1978). Effect of long-term sympathectomy on the arterial wall in rabbits and rats. Exp Mol Pathol, 28, 279–289.
- Gironacci MM, Adler-Graschinsky E, Peña C, Enero MA. (1994). Effects of angiotensin II and angiotensin-(1-7) on the release of [3H] norepinephrine from rat atria. Hypertension, 24, 457–460.
- Hilgers KF, Veelken R, Rupprecht G, Reeh PW, Luft FC, Mann JF. (1993). Angiotensin II facilitates sympathetic transmission in rat hind limb circulation. Hypertension, 21, 322–328.
- Iwatsuki K, Cardinale GJ, Spector S, Udenfriend S. (1979). Effect of guanethidine on collagen biosynthesis in blood vessels of hypertensive rats. Arch Int Pharmacodyn Ther, 240, 278–284.
- Izzo JL Jr. (2000). Systolic hypertension, arterial stiffness, and vascular damage: Role of the renin-angiotensin system. Blood Press Monit, 5 Suppl 2, S7–11.
- Izzo JL Jr, Shykoff BE. (2001). Arterial stiffness: Clinical relevance, measurement, and treatment. Rev Cardiovasc Med, 2, 29–34, 37.
- Jacob MP. (2003). Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother, 57, 195–202.
- Johnson BM. (1975). The use of radioactive microspheres to compare the effects of hydralazine, guanethidine and SK & F 24260 on the redistribution of cardiac output in anaesthetized rabbits. Br J Pharmacol, 55, 393–402.
- Kato H, Suzuki H, Tajima S, Ogata Y, Tominaga T, Sato A, Saruta T. (1991). Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens, 9, 17–22.
- Kizer JR, Dahlöf B, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, Wachtell K, Edelman JM, Snapinn SM, Harris KE, Devereux RB. (2005). Stroke reduction in hypertensive adults with cardiac hypertrophy randomized to losartan versus atenolol: The Losartan Intervention For Endpoint reduction in hypertension study. Hypertension, 45, 46–52.
- Kopecky SL. (2006). Effect of β blockers, particularly carvedilol, on reducing the risk of events after acute myocardial infarction. Am J Cardiol, 98, 1115–1119.
- Lee MA, Böhm M, Paul M, Ganten D. (1993). Tissue renin-angiotensin systems. Their role in cardiovascular disease. Circulation, 87, IV7–I13.
- Lurie KG, Bristow MR, Minobe WA, Masek M, Billingham ME. (1988). 6-Hydroxydopamine mediated cardiotoxicity in rabbits. Am J Cardiovasc Pathol, 2, 181–191.
- O’Callaghan CJ, Williams B. (2002). The regulation of human vascular smooth muscle extracellular matrix protein production by α- and β-adrenoceptor stimulation. J Hypertens, 20, 287–294.
- Ralevic V, Milner P, Burnstock G. (1995). Augmented flow-induced endothelin release from the rat mesenteric arterial bed after long-term sympathectomy. Endothelium 3, 67–73.
- Rizvi MA, Katwa L, Spadone DP, Myers PR. (1996). The effects of endothelin-1 on collagen type I and type III synthesis in cultured porcine coronary artery vascular smooth muscle cells. J Mol Cell Cardiol, 28, 243–252.
- Rocha FL, Carmo EC, Roque FR, Hashimoto NY, Rossoni LV, Frimm C, Anéas I, Negrão CE, Krieger JE, Oliveira EM. (2007). Anabolic steroids induce cardiac renin-angiotensin system and impair the beneficial effects of aerobic training in rats. Am J Physiol Heart Circ Physiol, 293, H3575–H3583.
- Senzaki H, Paolocci N, Gluzband YA, Lindsey ML, Janicki JS, Crow MT, Kass DA. (2000). β-Blockade prevents sustained metalloproteinase activation and diastolic stiffening induced by angiotensin II combined with evolving cardiac dysfunction. Circ Res, 86, 807–815.
- Simon G, Csiky B. (1998). Effect of neonatal sympathectomy on the development of structural vascular changes in angiotensin II-treated rats. J Hypertens, 16, 77–84.
- Spinetti G, Kraenkel N, Emanueli C, Madeddu P. (2008). Diabetes and vessel wall remodelling: From mechanistic insights to regenerative therapies. Cardiovasc Res, 78, 265–273.
- Tokimitsu I, Kato H, Wachi H, Tajima S. (1994). Elastin synthesis is inhibited by angiotensin II but not by platelet-derived growth factor in arterial smooth muscle cells. Biochim Biophys Acta, 1207, 68–73.
- Velleman SG, McCormick RJ, Ely D, Jarrold BB, Patterson RA, Scott CB, Daneshvar H, Bacon WL. (2001). Collagen characteristics and organization during the progression of cholesterol-induced atherosclerosis in Japanese quail. Exp Biol Med (Maywood), 226, 328–333.
- Villanueva I, Piñón M, Quevedo-Corona L, Martínez-Olivares R, Racotta R. (2003). Epinephrine and dopamine colocalization with norepinephrine in various peripheral tissues: Guanethidine effects. Life Sci, 73, 1645–1653.
- Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. (2005). Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol, 167, 1429–1442.
- Werner C, Baumhäkel M, Teo KK, Schmieder R, Mann J, Unger T, Yusuf S, Böhm M. (2008). RAS blockade with ARB and ACE inhibitors: Current perspective on rationale and patient selection. Clin Res Cardiol, 97, 418–431.
- Zucker IH, Wang W, Pliquett RU, Liu JL, Patel KP. (2001). The regulation of sympathetic outflow in heart failure. The roles of angiotensin II, nitric oxide, and exercise training. Ann N Y Acad Sci, 940, 431–443.