Abstract
Context: The activation of peroxisome proliferator-activated receptor α (PPARα) target genes promotes hepatic oxidation of fatty acids. We hypothesized that Gyeongshingangjeehwan 18 (GGEx18), a mixture of three herbs, Laminaria japonica Aresch (Laminariaceae), Rheum palmatum L. (Polygonaceae), and Ephedra sinica Stapf (Ephedraceae), can regulate high-fat diet-induced hepatic steatosis through PPARα activation in the liver.
Objective: To investigate the effects of GGEx18 on obesity-related hepatic steatosis and the responsible mechanism.
Materials and methods: The effects of GGEx18 on hepatic lipid accumulation, serum lipid profiles, and the expression of PPARα target genes were studied in high-fat diet-induced obese mice. The effects of GGEx18 on the expression of the PPARα targets and PPARα reporter gene activation were measured in NMu2Li liver cells.
Results: GGEx18 administration to obese mice for 9 weeks markedly (p < 0.05) decreased hepatic lipid accumulation compared with that in obese control mice. Serum triglyceride and total cholesterol levels were significantly (p <0.05) decreased by GGEx18. GGEx18 treatment increased the messenger RNA levels of PPARα target genes, which are responsible for fatty acid oxidation, in liver tissues. Consistent with the in vivo data, similar activation of genes was observed in GGEx18-treated NMu2Li liver cells. GGEx18 also elevated PPARα reporter gene expression in NMu2Li cells.
Discussion and conclusion: These results suggest that GGEx18 prevents hepatic steatosis and hyperlipidemia in high-fat diet-induced obese mice, and this process may be mediated through PPARα activation in the liver.
Introduction
Gyeongshingangjeehwan 18 (GGEx18), an herbal drug composed of three medicinal plants, Rheum palmatum L. (Polygonaceae), Laminaria japonica Aresch (Laminariaceae), and Ephedra sinica Stapf (Ephedraceae), has already been used as an antiobesity drug in local clinics in Korea, although the mechanism of its action remains unknown. These three herbs are reported to have antiobesity, antidiabetic, and lipid-lowering effects (CitationBoozer et al., 2001; CitationYou et al., 2009; CitationHuang et al., 2010; CitationXue et al., 2010). These reports led to the speculation of whether high-fat diet-induced obesity in mice can be regulated more efficiently when treated with a mixture of the above three herbal extracts (GGEx18) at the same time. In our previous study, we found that obesity in mice could be regulated more efficiently when treated with GGEx18 (CitationYoon et al., 2010), supporting the belief that GGEx18 may regulate obesity-related metabolic disorders including type 2 diabetes, atherosclerosis, hyperlipidemia, and hepatic steatosis.
Steatosis is one of the most common liver diseases and a major contributor to obesity-related morbidity and mortality (CitationAngulo, 2002). The mechanisms underlying liver steatosis are complex and multifunctional. However, a line of evidence suggests that peroxisome proliferator-activated receptor α (PPARα), which regulates lipid metabolism (CitationYoon, 2009, Citation2010), is involved in its pathogenesis (CitationReddy, 2001). Systemic agonists for PPARα have been widely used as lipid-lowering agents; thus, steatosis can be treated via PPARα modulation (CitationCostet et al., 1998; CitationStaels et al., 1998; CitationBasaranoglu et al., 1999). PPARα-null mice develop fatty liver, which is aggravated by a high-fat diet (CitationKersten et al., 1999). PPARα is highly expressed in the liver, where it regulates a number of enzymes critical for fatty acid catabolism. The target genes of PPARα include those involved in fatty acid uptake, fatty acid binding, and fatty acid oxidation in microsomes, peroxisomes, and mitochondria (CitationYoon, 2009, Citation2010). The activation of PPARα target genes, therefore, promotes increased hepatic oxidation of fatty acids, as well as the increased degradation, reduced synthesis, and secretion of triglycerides. We, therefore, hypothesized that GGEx18 can regulate high-fat diet-induced hepatic steatosis through PPARα activation in the liver.
We treated both high-fat diet-induced obese mice and NMu2Li liver cells with GGEx18. We demonstrate that hepatic lipid accumulation and hyperlipidemia were significantly reduced in GGEx18-treated mice compared with the findings in obese controls. Concomitantly, GGEx18 treatment increased the messenger RNA (mRNA) expression of PPARα and its target genes, such as 3-keotacyl-CoA thiolase (thiolase), very long chain acyl-CoA dehydrogenase (VLCAD), and uncoupling protein 2 (UCP2). Similar to the in-vivo data, in addition to increasing the mRNA levels of PPARα target genes, GGEx18 also elevated PPARα reporter gene activation in NMu2Li cells. These data suggest that GGEx18 can inhibit hepatic steatosis and dyslipidemia through PPARα activation in the livers of obese mice.
Materials and methods
Preparation of GGEx18
GGEx18 was prepared from food-grade aqueous extracts of the three herbs (40% L. japonica, 20% E. sinica, and 20% R. palmatum − expressed as % dry weight, Hwalim, Busan, Korea) (CitationShin et al., 2011). Briefly, three dried herbs with their contents weighed were boiled together in distilled water for 22 h at 95°C. The aqueous extracts were then filtered through a membrane filter (6 μm) and freeze-dried under vacuum.
Animal treatments
Eight-week-old male, wild-type C57BL/6J mice (n = 8/group) were purchased from Central Lab Animal (Seoul, Korea) and randomly divided into five groups. Mice were fed a low-fat diet (normal group; 13% kcal fat, CJ, Incheon, Korea), a high-fat diet (control group; 45% kcal fat, Research Diets, New Brunswick, NJ, USA), or the high-fat diet supplemented with one of three doses of GGEx18 (125, 250, or 500 mg/kg/day) for 9 weeks. At the end of the study, mice were sacrificed under diethyl ether anesthesia, and liver tissues were harvested, weighed, snap-frozen in liquid nitrogen, and stored at −80°C until use. Additional sections of liver were prepared for histological analyses. Blood was collected from the saphenous vein after a 12 h fast, and serum was separated and stored at −80°C until analysis. All animal experiments were approved by the Institutional Animal Care and Use Committee of Dongeui University and conducted according to National Research Council Guidelines.
Blood analysis
Serum concentrations of triglycerides, total cholesterol, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured using an automatic blood chemical analyzer (CIBA Corning, Oberlin, OH, USA).
Histological analysis
The right lobe of the liver was fixed in 10% phosphate-buffered formalin for 1 day and processed in a routine manner for paraffin sectioning. Five-micrometer-thick sections were stained with hematoxylin and eosin for microscopic examination.
Reverse transcription-polymerase chain reaction
Total cellular RNA from liver tissues and NMu2Li murine liver cells was prepared using Trizol reagent (Invitrogen, Carlsbad, CA, USA). After 2 μg total RNA were reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (MMLV-RT; Promega, Madison, WI, USA) and an antisense primer, complementary DNA was generated. The RNA was denatured for 5 min at 72°C and immediately placed on ice for 5 min. Denatured RNA was mixed with MMLV-RT, MMLV-RT buffer, and a deoxyribonucleotide triphosphate mixture and incubated for 1 h at 42°C. Synthesized cDNA fragments were amplified by polymerase chain reaction (PCR) in an MJ Research Thermocycler (Waltham, MA, USA). The PCR primers used for gene expression analysis are shown in . The cDNA was mixed with PCR primers, Taq DNA polymerase (Nanohelix, Daejon, Korea), and a dNTP mixture. The reaction consisted of 28–45 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 56–60°C, and elongation for 1 min at 72°C. The PCR products were analyzed by electrophoresis on a 1% agarose gel. Relative expression levels are presented as a ratio of target gene cDNA vs. β-actin cDNA. PCR products were quantified from agarose gels using GeneGenius (Syngene, Cambridge, UK).
Table 1. PCR primers and conditions used for cDNA synthesis by RT-PCR.
Transient transfection assay
The expression vectors pSG5-mPPARα and PPRE3-tk-luc reporter gene were generously provided by Dr. Frank J. Gonzalez (National Cancer Institute, NIH, Bethesda, MD, USA). NMu2Li cells were routinely cultured in DMEM containing 10% fetal bovine serum (Invitrogen), penicillin G (100 U/mL), streptomycin sulfate (100 μg/mL), amphotericin B (0.25 μg/mL), and 2-mercaptoethanol (50 μM). Cells were seeded in six-well tissue culture plates (2 × 104 cells/well) 24 h prior to transfection. For all transfections, 200 ng/well of each of the appropriate plasmids were used. Transfections were performed using Lipofectamine (Invitrogen) according to the manufacturer’s instructions. After 6h, the culture medium was changed, and cells were exposed to different concentrations of GGEx18 or fenofibrate for 24 h, after which cells were washed twice with phosphate-buffered saline and assayed for luciferase and β-galactosidase activity using commercial kits according to the manufacturer’s instructions (Promega).
Statistical analysis
All values are expressed as the mean ± standard deviation. Statistical analysis was performed by analysis of variance followed by Turkey’s multiple comparison test. A p value < 0.05 was considered statistically significant.
Results
Inhibition of hepatic lipid accumulation by GGEx18 in nutritionally obese mice
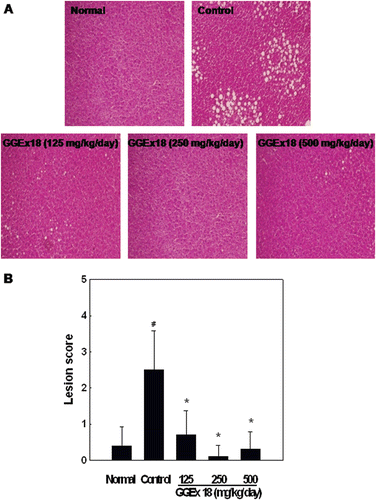
High-fat diet-induced obese controls exhibited considerable hepatic lipid accumulation compared with the accumulation in chow diet-fed normal mice. However, we found that hepatic lipid accumulation was markedly lower in GGEx18-treated obese mice than in obese controls (. When obese mice were treated with GGEx18 at a dose of 250 mg/kg/day, there was a complete inhibition of hepatic lipid accumulation, as indicated by the decreased hepatic lesion scores (.
Figure 1. Effects of Gyeongshingangjeehwan 18 (GGEx18) on hepatic lipid accumulation in high-fat diet-induced obese mice. Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 125, 250, or 500 mg/kg/day GGEx18 for 9 weeks. (A) Representative hematoxylin and eosin-stained sections of livers are shown (original magnification, ×100). (B) Histological analyses of hepatic lipid accumulation. Pathological scores of hepatic lipid accumulation are as follows: 0, no lesion; 1, mild; 2, moderate; 3, severe; 4, very severe. All values are expressed as the mean ± standard deviation of four independent experiments. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group.
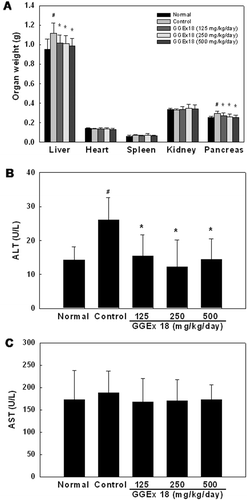
GGEx18 also significantly decreased liver and pancreas weights that were increased by high-fat diet (. Serum ALT levels were also significantly reduced to the levels of lean normal mice after GGEx18 administration although AST levels were not altered in GGEx18-treated obese mice ( and ). In addition, organ weights, such as heart, spleen, and kidney, were not changed by GGEx18 in obese mice.
Figure 2. Effects of Gyeongshingangjeehwan 18 (GGEx18) on organ weights, serum alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels in high-fat diet-induced obese mice. Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 125, 250, or 500 mg/kg/day GGEx18 for 9 weeks. (A) Organ weights. (B) Serum ALT levels. (C) Serum AST levels. All values are expressed as the mean ± standard deviation. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group.
Improvement of lipid profiles by GGEx18 in nutritionally obese mice
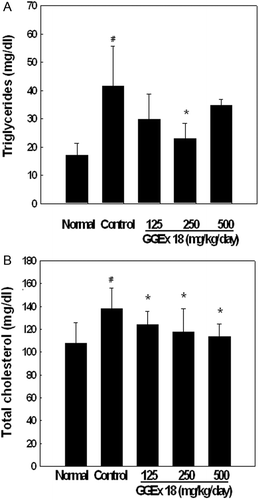
To determine whether GGEx18 has similar beneficial effects on circulating lipid profiles, its effects on serum triglycerides and total cholesterol were examined. After 9 weeks, obese controls had significantly higher serum levels of triglycerides and total cholesterol than normal mice. However, 44 and 17% reductions of plasma triglycerides and total cholesterol levels, respectively, were observed in the GGEx18 (250 mg/kg/day) group ().
Figure 3. Effects of Gyeongshingangjeehwan 18 (GGEx18) on circulating (A) triglycerides and (B) total cholesterol in high-fat diet-induced obese mice. Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 125, 250, or 500 mg/kg/day GGEx18 for 9 weeks. Serum concentrations of triglycerides and total cholesterol were measured, and all values are expressed as the mean ± standard deviation. #p < 0.05 compared with the normal group, *p <0.05 compared with the control group.
Regulation of hepatic PPARα target gene expression by GGEx18 in nutritionally obese mice
To evaluate whether the effects of GGEx18 on hepatic lipid accumulation and circulating lipid metabolism in obese mice are caused by changes in the expression of genes involved in PPARα activation in the liver, we measured the mRNA levels of PPARα target enzymes, which are involved in fatty acid β-oxidation, in the liver. GGEx18 (250 mg/kg/day) administration to obese mice upregulated the mRNA levels of liver thiolase and VLCAD by 31 and 23%, respectively, compared with their levels in obese controls ().
Figure 4. Effects of Gyeongshingangjeehwan 18 (GGEx18) on PPARα target gene mRNA levels in high-fat diet-induced obese mice. (A)Adult male C57BL/6 mice (n = 8/group) were fed a low-fat diet (Normal), a high-fat diet (Control), or the high-fat diet supplemented with 250 mg/kg/day GGEx18 for 9 weeks. Total cellular RNA was extracted from liver tissue, and mRNA levels were measured using RT-PCR. Data are reported as the mean ± standard deviation of relative density units (R.D.U.) using β-actin as a reference. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group. (B) Representative RT-PCR bands from one of four independent experiments are shown.
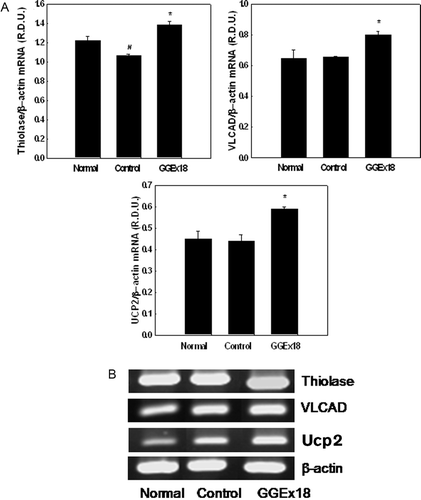
We also examined the effects of GGEx18 on hepatic UCP2 gene expression, which is regulated by a selective PPARα activator, fenofibrate that upregulates fatty acid oxidation (CitationNakatani et al., 2002). GGEx18 administration increased UCP2 mRNA levels by 32% compared with those in control mice ().
Regulation of the expression of PPARα target genes by GGEx18 in NMu2Li liver cells
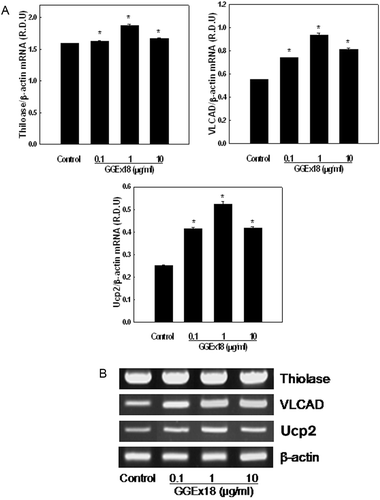
Next, we measured the mRNA expression of PPARα target enzymes in liver cells. Similar to the in-vivo data, treatment of cells with GGEx18 upregulated the mRNA levels of thiolase, VLCAD, and UCP2 at all concentrations used in this study (). Maximal levels of thiolase, VLCAD, and UCP2 mRNA expression were achieved at a concentration of 1 μg/mL, which resulted in 18, 69, and 109% increases, respectively, compared with control levels.
Figure 5. Effects of Gyeongshingangjeehwan 18 (GGEx18) on PPARα target gene mRNA levels in NMu2Li liver cells. (A) NMu2Li cells were transiently transfected with pSG5-mPPARα, the reporter plasmid PPRE-TK-Luc, the β-galactosidase gene, and pBSK. Cells were treated with GGEx18 at concentrations of 0.1, 1, and 10 μg/mL. After incubation for 24 h, cells were harvested. RNA was extracted from cells, and mRNA levels were measured using RT-PCR. Data are reported as the mean ± standard deviation of relative density units (R.D.U.) using β-actin as a reference. #p < 0.05 compared with the normal group, *p < 0.05 compared with the control group. (B) Representative RT-PCR bands from one of four independent experiments are shown.
Modulation of PPARα reporter gene expression by GGEx18 in NMu2Li liver cells
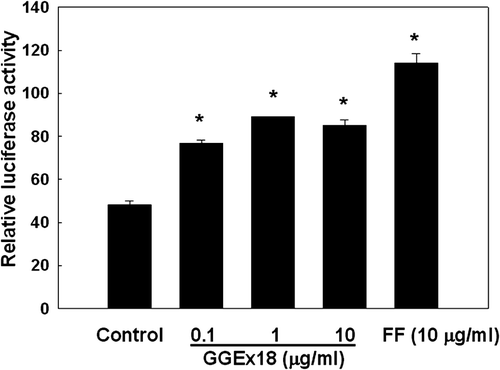
To examine the mechanism by which GGEx18 increases the mRNA expression of PPARα target genes, NMu2Li cells were cotransfected with PPARα expression constructs and the luciferase reporter construct PPRE3-tk-luc, which contains three copies of the PPAR response element (PPRE) from the rat acyl-CoA oxidase (ACOX) gene. The transfected cells were treated with GGEx18 at concentrations found to be nontoxic by Trypan blue exclusion. Treatment of the transfected cells with GGEx18 caused a significant increase in the magnitude of PPARα reporter gene activation (). GGEx18 increased PPARα reporter activity by 60–85% at concentrations of 0.1–10 μg/mL compared with control levels. Similarly, the PPARα activator fenofibrate (10 μM) also increased PPARα luciferase activity by 138%.
Figure 6. Effects of Gyeongshingangjeehwan 18 (GGEx18) on PPARα reporter gene expression in NMu2Li liver cells. NMu2Li cells were transiently transfected with pSG5-mPPARα, the reporter plasmid PPRE-TK-Luc, the β-galactosidase gene, and pBSK. Cells were treated with GGEx18 at concentrations of 0.1, 1, and 10 μg/mL or 10 μM fenofibrate (FF). After incubation for 24 h, cells were harvested and subsequently assayed for luciferase and β-galactosidase activities. Data are expressed as the mean ± standard deviation of relative luciferase units/β-galactosidase activity for three experiments. *p < 0.05 compared with the control group.
Discussion
Nonalcoholic fatty liver disease (NAFLD) is a common hepatic disease that can present as simple steatosis or nonalcoholic steatohepatitis and eventually progress to cirrhosis (CitationAngulo, 2002). The pathogenesis of NAFLD is not well understood, and effective therapy for NAFLD has not been established. As NAFLD is deeply associated with obesity (CitationFabbrini et al., 2010), this study was undertaken to verify whether hepatic steatosis can be regulated by GGEx18, which decreases body weight gain and adipose tissue mass in high-fat diet-induced obese mice (CitationShin et al., 2011), and to clarify the molecular mechanism by which GGEx18 exerts these effects.
Our results demonstrate that GGEx18 decreased high-fat diet-induced hepatic steatosis and hyperlipidemia in mice. Excessive intrahepatic triglycerides, or steatosis, has been histologically defined when 5% or more hepatocytes contain visible intracellular triglycerides (CitationKleiner et al., 2005). In this study, high-fat diet-fed control mice had much higher triglyceride droplet levels than normal low-fat diet-fed mice. However, GGEx18 administration to control mice for 9 weeks significantly reduced lipid droplet levels by 72–96%. Of the three doses of GGEx18, 250 mg/kg/day GGEx18 almost completely prevented obesity-induced hepatic lipid accumulation. This finding is supported by a previous animal study demonstrating that GGEx18 administration (250 mg/kg/day) to obese mice for 9 weeks significantly reduced both body weight gain and total fat mass (CitationShin et al., 2011). This finding suggests that GGEx18 is beneficial for treating obesity-related hepatic steatosis. In accordance with reduced hepatic lipid accumulation, serum triglyceride and total cholesterol levels were also significantly reduced by GGEx18, indicating a role of GGEx18 in lipid metabolism. This result suggests that GGEx18 can be used to treat obesity-related hepatic steatosis and hyperlipidemia.
To gain insight into the molecular mechanisms underlying the effects described above, we measured the liver mRNA levels of PPARα target enzymes, which are responsible for fatty acid oxidation. PPARα is a ligand-activated transcription factor that regulates the expression of a number of genes critical for lipid and lipoprotein metabolism, thereby leading to lipid homeostasis. PPARα is known to initiate lipid-lowering effects through the transcriptional activation of mitochondrial, peroxisomal, and microsomal fatty acid-metabolizing enzymes (CitationAoyama et al., 1998; CitationKroetz et al., 1998; CitationYan et al., 1998; CitationJeong et al., 2004). Our previous study demonstrated that the PPARα ligand fenofibrate increases hepatic fatty acid oxidation and thus decreases the levels of plasma triglycerides due to PPARα activation in the liver (CitationYoon et al., 2003; CitationJeong et al., 2004). This finding is supported by a report that PPARα-deficient mice exhibited abnormalities in triglyceride and cholesterol metabolism and became obese with age (CitationCostet et al., 1998). Fatty acid oxidative PPARα target genes and PPARα expression levels are suppressed in the adipose tissue and skeletal muscle of obese mice (CitationKamei et al., 2005). PPARα also upregulates the expression of UCP2, which contains the PPRE in its promoters and participates in fatty acid oxidation (CitationLentes et al., 1999; CitationNakatani et al., 2002). In this study, GGEx18 administration to obese mice significantly increased hepatic mRNA levels of peroxisomal and mitochondrial PPARα targets, such as thiolase and VLCAD, respectively. Similar gene activation was observed in GGEx18-treated NMu2Li liver cells. These results are in accordance with our data on the substantial reduction of hepatic lipid droplet, serum triglyceride, and total cholesterol levels by GGEx18. These results suggest that GGEx18 increases PPARα-dependent fatty acid oxidation in the livers of obese mice.
To determine the mechanism by which GGEx18 increases PPARα target gene expression and to examine whether GGEx18-induced PPARα activation prevents hepatic lipid accumulation and hyperlipidemia in obese mice, we examined the effects of GGEx18 on PPARα transactivation using NMu2Li cells. Consistent with the in-vivo data, our reporter assay data also suggest that GGEx18 significantly increased luciferase activity compared with that in control cells, although the luciferase activity was lower in cells treated with GGEx18 than in cells treated with fenofibrate, suggesting that GGEx18 can act as a potential agonist of PPARα and stimulate PPARα-dependent fatty acid catabolism.
Although ephedra-containing dietary supplements and ephedrine have been reported to have toxicity (CitationHaller & Benowitz, 2000; CitationFleming, 2008), this study showed that significant side effects were not observed in GGEx18-treated mice. Treatment of mice with GGEx18 did not affect organ weights, such as heart, spleen, and kidney. Interestingly, GGEx18 decreased liver and pancreas weights that were increased by high-fat diet. Moreover, GGEx18 also reduced circulating ALT levels to the levels of normal mice. Thus, it seems that GGEx18 may be a safe and effective drug for hepatic steatosis and hyperlipidemia.
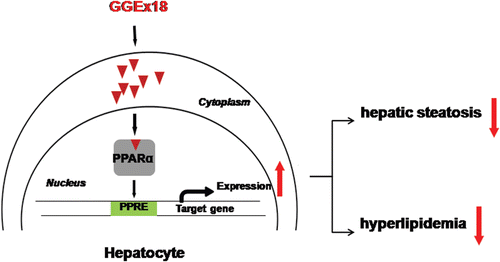
In conclusion, these studies demonstrate that GGEx18 may prevent hepatic steatosis and hyperlipidemia, such as hypertriglyceridemia and hypercholesterolemia, in obese mice, by activating hepatic PPARα (). Further investigation will be necessary to identify bioactive compounds from GGEx18 to develop natural therapeutic agents or dietary supplements to treat obesity-related metabolic disease.
Figure 7. Possible mechanism of Gyeongshingangjeehwan 18 (GGEx18) to inhibit hepatic steatosis and hyperlipidemia. GGEx18 may activate PPARα target genes responsible for fatty acid oxidation in the livers of obese mice, leading to decreased hepatic steatosis, hypertriglyceridemia, and hypercholesterolemia.
Declaration of interest
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MEST) (NRF-2010-0027498 and NRF-2011-0003703), Korea. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Angulo P. (2002). Nonalcoholic fatty liver disease. N Engl J Med, 346, 1221–1231.
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. (1998). Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem, 273, 5678–5684.
- Basaranoglu M, Acbay O, Sonsuz A. (1999). A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol, 29, 495–501.
- Boozer CN, Nasser JA, Heymsfield SB, Wang V, Chen G, Solomon JL. (2001). An herbal supplement containing Ma Huang-Guarana for weight loss: A randomized, double-blind trial. Int J Obes Relat Metab Disord, 25, 316–324.
- Costet P, Legendre C, Moré J, Edgar A, Galtier P, Pineau T. (1998). Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. J Biol Chem, 273, 29577–29585.
- Fabbrini E, Sullivan S, Klein S. (2010). Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology, 51, 679–689.
- Fleming RM. (2008). Safety of ephedra and related anorexic medications. Expert Opinion on Drug Safety, 7, 749–759.
- Jeong S, Kim M, Han M, Lee H, Ahn J, Kim M, Song YH, Shin C, Nam KH, Kim TW, Oh GT, Yoon M. (2004). Fenofibrate prevents obesity and hypertriglyceridemia in low-density lipoprotein receptor-null mice. Metab Clin Exp, 53, 607–613.
- Haller CA, Benowitz NL. (2000). Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med, 343, 1833–1838.
- Huang L, Wen K, Gao X, Liu Y. (2010). Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm Biol, 48, 422–426.
- Jeong S, Kim M, Han M, Lee H, Ahn J, Kim M, Song YH, Shin C, Nam KH, Kim TW, Oh GT, Yoon M. (2004). Fenofibrate prevents obesity and hypertriglyceridemia in low-density lipoprotein receptor-null mice. Metab Clin Exp, 53, 607–613.
- Kamei Y, Suzuki M, Miyazaki H, Tsuboyama-Kasaoka N, Wu J, Ishimi Y, Ezaki O. (2005). Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J Nutr Sci Vitaminol, 51, 110–117.
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. (1999). Peroxisome proliferator FJ, Desvergne B, W alpha mediates the adaptive response to fasting. J Clin Invest, 103, 1489–1498.
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. (1999). Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest, 103, 1489–1498.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ: Nonalcoholic Steatohepatitis Clinical Research Network. (2005). Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology, 41, 1313–1321.
- Kroetz DL, Yook P, Costet P, Bianchi P, Pineau T. (1998). Peroxisome proliferator-activated receptor alpha controls the hepatic CYP4A induction adaptive response to starvation and diabetes. J Biol Chem, 273, 31581–31589.
- Lentes KU, Tu N, Chen H, Winnikes U, Reinert I, Marmann G, Pirke KM. (1999). Genomic organization and mutational analysis of the human UCP2 gene, a prime candidate gene for human obesity. J Recept Signal Transduct Res, 19, 229–244.
- Nakatani T, Tsuboyama-Kasaoka N, Takahashi M, Miura S, Ezaki O. (2002). Mechanism for peroxisome proliferator-activated receptor-alpha activator-induced up-regulation of UCP2 mRNA in rodent hepatocytes. J Biol Chem, 277, 9562–9569.
- Reddy JK. (2001). Nonalcoholic steatosis and steatohepatitis. III. Peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am J Physiol Gastrointest Liver Physiol, 281, G1333–G1339.
- Shin SS, Park D, Lee HY, Hong Y, Choi J, Oh J, Lee H, Lee HR, Kim MR, Shen ZB, Cui HH, Yoon M. (2012). The herbal composition GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica reduces obesity via skeletal muscle AMPK and PPARa. Pharm Biol, 50, 506–515.
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. (1998). Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation, 98, 2088–2093.
- Xue J, Ding W, Liu Y. (2010). Anti-diabetic effects of emodin involved in the activation of PPARgamma on high-fat diet-fed and low dose of streptozotocin-induced diabetic mice. Fitoterapia, 81, 173–177.
- Yan ZH, Karam WG, Staudinger JL, Medvedev A, Ghanayem BI, Jetten AM. (1998). Regulation of peroxisome proliferator-activated receptor alpha-induced transactivation by the nuclear orphan receptor TAK1/TR4. J Biol Chem, 273, 10948–10957.
- Yoon KH, Lee HY, Jung YS, Seo BI, Park KY, Yoon M, Shin SS. (2010). Modulation of obesity by Gyeongshingangjeehwan 18 in ob/ob mice. Kor J Herb, 25, 1–9.
- Yoon M. (2009). The role of PPARalpha in lipid metabolism and obesity: Focusing on the effects of estrogen on PPARalpha actions. Pharmacol Res, 60, 151–159.
- Yoon M. (2010). PPARa in Obesity: Sex Difference and Estrogen Involvement. PPAR Res, 2010, 584296.
- Yoon M, Jeong S, Lee H, Han M, Kang JH, Kim EY, Kim M, Oh GT. (2003). Fenofibrate improves lipid metabolism and obesity in ovariectomized LDL receptor-null mice. Biochem Biophys Res Commun, 302, 29–34.
- You JS, Sung MJ, Chang KJ. (2009). Evaluation of 8-week body weight control program including sea tangle (Laminaria japonica) supplementation in Korean female college students. Nutr Res Pract, 3, 307–314.