Abstract
Context: Acrostichum aureumL. (Pteridaceae), a mangrove fern, has been used as a Bangladeshi traditional medicine for a variety of diseases including peptic ulcer.
Objective: Isolation and structural elucidation of cytotoxic secondary metabolites from the methanol extract of the aerial parts of A. aureum.
Materials and methods: Compounds were isolated using HPLC. The compound structures were elucidated by 1D and 2D NMR, MS and other spectroscopic methods using published data. The compounds were tested for their cytotoxic activity against healthy and cancer cells using the MTT assay. Active compounds were further evaluated for apoptosis–and necrosis-inducing potential against gastric cancer cells (AGS) using the FITC Annexin V apoptosis assay.
Results and discussion:Seven known compounds, patriscabratine, tetracosane and 5 flavonoids (quercetin-3-O-β-d-glucoside, quercetin-3-O-β-d-glucosyl-(6→1)-α-l-rhamnoside, quercetin-3-O-α-l-rhamnoside, quercetin-3-O-α-l-rhamnosyl-7-O-β-d-glucoside and kaempferol) were isolated. Patriscabratine was found moderately cytotoxic against AGS, MDA-MB-231 and MCF-7 cells with IC50 values ranging from 69.8 to 197.3 μM. Tetracosane showed some cytotoxic activity against AGS, MDA-MB-231, HT-29 and NIH 3T3 cells with IC50 values ranging from 128.7 to >250 μM. Patriscabratine and tetracosane displayed an apoptotic effect (10%) on AGS cells within 24 h which was increased (20%) after 48 h, and was comparable to, if not greater, than the positive control, cycloheximide.
Conclusion:Except for quercetin-3-O-β-d-glucoside and kaempferol; compounds were isolated for the first time from this plant and evaluated for their cytotoxic activity. The results highlight the potential of this plant as a source of bioactive compounds and provide a rationale for its traditional use in peptic ulcer treatment.
Introduction
Acrostichum aureum L. (Pteridaceae) is a mangrove fern that occurs in tropical and subtropical areas worldwide (CitationMomtaz, 2008). In Bangladesh, it is found in the Sundarban and other coastal belts as ‘Tiger fern’, because it provides a suitable hiding place for the famous carnivorous Royal Bengal Tiger of the Sundarban (CitationZafrul, 2000). In Bangladesh, preparations from rhizomes and leaves of A. aureum are used to treat wounds, peptic ulcers and boils (CitationMomtaz, 2008). In China, the rhizome is used to treat worm infections (CitationMomtaz, 2008), whereas, Fijian people use it to treat asthma, constipation, elephantiasis, febrifuge, and chest pain (CitationCoambie & Ash, 1994). The native people of Costa Rica use leaves as emollients, whereas, the Cuna people (Panama and Colombia) use the young fiddleheads to extract fish bones from the throat and as a medicinal bath for infants (CitationNatural Resources Conservation Service, 2010). The crude extract of a Japanese A. aureum specimen is reported to possess anti-oxidant, tyrosinase inhibiting activity (CitationLai et al., 2009), while a Hainan specimen reported anti-tumor activity against cervical cancer cell line (CitationDai et al., 2005). Recently, we reported the cytotoxic effect of water and methanol extracts from a Bangladeshi specimen of A. aureum on gastric, colon and breast cancer cells (CitationUddin et al., 2011). So far, a total of 19 compounds have been isolated from A. aureumbelonging to different natural product classes, such as sterols, flavonoids, fatty acids and long-chain hydrocarbons (CitationMei et al., 2006; CitationNobutoshi et al., 1981; CitationSultana et al., 1986).
Here we report on the isolation and cytotoxic activity evaluation of seven known compounds (1-7), five of which were isolated for the first time from A. aureum. Two compounds, namely tetracosane and patriscabratine, were further evaluated for their apoptosis–and necrosis-inducing potential on gastric adenocarcinoma cells (AGS).
Materials and methods
General experimental procedures
Optical rotations were measured on a Jasco P-1010 polarimeter. IR spectra were recorded on a Bruker Optics alpha-QuickSnap FT-IR spectrophotometer. NMR spectra were recorded on either a Bruker Avance 300 or 600 MHz spectrometer in CD3OD. LR-MS were obtained on a Bruker Daltonics esquire 3000 mass spectrometer. Analytical HPLC was performed on a Varian Prostar instrument with a 335 DAD using a RP (Luna C18, 5 μm, 250 × 4.6 mm) column. Preparative HPLC was performed on a Waters instrument equipped with a Waters 600E pump, Rheodyne 7725i injector, using a RP (Luna C18, 5 μm, 150 × 21.2 mm) column. SPE cartridges (Alltech, 10 g, RP-C18) were used to fractionate the extract.
Plant material
The aerial parts of A. aureum were collected from tidal forests in the coastal Sundarbans (a swamp region in the Ganges delta) of Bangladesh in February, 2007. The plant material was identified by Dr. Momtaz Mahal Mirza, Principle Scientific Officer, Bangladesh National Herbarium, Dhaka, and shade-dried. A specimen was deposited in the Bangladesh National Herbarium, Dhaka (Voucher no.: DACB 31538).
Preparation of plant extract and isolation of cytotoxic compounds
The dried and pulverized whole plant material of A. aureum (150 g) was extracted with methanol (1 L) by soaking it overnight at room temperature with continuous stirring. The extract was filtered and the residue was further extracted with methanol (3 × 1 L) for 1 h under sonication. All extracts were combined and concentrated under reduced pressure to give 7.57 g extract (5.04% w/w). The resulting methanol extract was partitioned between n-hexane and methanol (1:1) to give two fractions. The methanol fraction (6.70 g) was further sub-fractionated into four fractions using reverse-phase SPE columns with a H2O:MeOH stepwise gradient (100:0 (SPE1), 80:20 (SPE2), 60:40 (SPE3) and 0:100 (SPE4)).
The SPE3 (40% methanol) and SPE4 (100% methanol) fractions were further analyzed by preparative RP-HPLC using a H2O:MeOH gradient containing 0.05% TFA. The SPE3 fraction (0.42 g) yielded compound 1 (40 mg) and SPE4 fraction (0.60 g) yielded compounds 2 (20.0 mg), 3 (5.0 mg), 4 (0.60 mg), 5/6 as mixture (2.90 mg) and 7 (9.0 mg).
Spectroscopic properties of isolated compounds
The structures of the isolated compounds were elucidated by the comparison of 1D and 2D NMR, MS and other spectroscopic data with published data.
In vitro assay for cytotoxic activity
Cell lines
All cell lines (NIH 3T3, ATCC: CRL-1658; AGS, ATCC: CRL-1739; HT-29, ATCC: HTB-38; MCF-7, ATCC: HTB-22; and MDA-MB-231, ATCC: HTB-26) were purchased from ATCC, Manassas, VA 20108, USA. Cell lines were cultured in Advanced Dulbecco’s modified Eagle’s medium supplemented with 10% inactivated newborn calf serum and 5 mMl-glutamine, and grown at 37°C in a humidified atmosphere of 5% CO2in air.
MTT cytotoxicity assay
The cytotoxicity of the compounds was tested against normal mouse fibroblast cells and four human cancer cell lines using the MTT assay according to the method described by CitationUddin et al. (2011) and references therein. The IC50 values were calculated with Probit analysis software (Bakr, Citation2010). Cycloheximide was used as a positive control generating IC50 values of 1.1, 3.6, 12.8, 1.2, and 218.2 μM against NIH 3T3, AGS, HT29, estrogen receptor negative (ER-) MDA-MB-231 and estrogen receptor positive (ER+) MCF-7 cells, respectively.
Annexin V-FITC apoptosis measurement
The FITC Annexin V apoptosis assay was used to measure apoptosis of the isolated cytotoxic compounds from A. aureum against a human gastric adenocarcinoma (AGS) cell line according to CitationJason et al. (2008). Cells were treated with tetracosane (500 μg/mL) and patriscabratine (100 μg/mL) for 24 and 48 h and analyzed with the BD FACS Calibur Flow Cytometer (BD Bioscience, San Jose, California, USA). Cells with no treatment served as a negative control and cycloheximide (150 μg/mL) served as a positive control.
Results and discussion
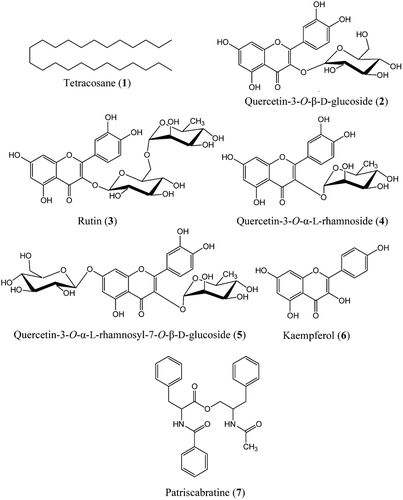
A total of seven compounds (1-7), namely, tetracosane (1) (CitationYamaji et al., 2010); quercetin-3-O-β-d-glucoside or hyperoside (2) (CitationMatsuura et al., 2002); quercetin-3-O-β-d-glucosyl-(6→1)-α-l-rhamnoside or rutin (3) (CitationAbdullah et al., 2008); quercetin-3-O-α-l-rhamnoside (4) (CitationFossen et al., 1999; CitationIorizzi et al., 2001); quercetin-3-O-α-l-rhamnosyl-7-O-β-d-glucoside (5); kaempferol (6) (CitationImperato, 2008; CitationXiao et al., 2006); and patriscabratine (7) (CitationGu et al., 2002) (), were isolated from the methanol extract of A. aureum following subfractionation using SPE C18 cartridges. Only compound 2 and 6 have been reported from this plant previously.
Tetracosane (1) showed significant cytotoxicity (IC50 128.7 μM) only against HT-29 colon cancer cells. It (1) showed some toxicity against the estrogen-dependent breast cancer (MDA-MB-231) cells (IC50 >250 μM) and gastric cancer cells (AGS; IC50>250 μM) but no toxicity against estrogen-independent breast cancer (MCF-7) cells. Tetracosane has been identified as a component in many volatile oils from plants, however to-date there are no reports on the pharmacological activity of tetracosane itself. Although a variety of pharmacological activities, including cytotoxicity, have been reported for volatile oils containing tetracosane (CitationAl Ashaal et al., 2010; CitationKansoh et al., 2009) this study is the first report on the cytotoxicity of tetracosane itself.
Patriscabratine (7) showed significant cytotoxicity against gastric (AGS) (IC50133.6 μM) and breast cancer cells (MDA-MB-231; IC5069.8 μM and MCF-7; IC50197.3 μM), but no cytotoxicity against normal mouse fibroblasts (NIH3T3) and colon cancer cells (HT-29). Previous research showed that synthetic patriscabratine has cytotoxic activity against breast carcinoma cell lines (ER- MDA-MB-231) (CitationYuan et al., 2010), with the IC50value for MDA-MB-231 being slightly higher (IC50 >100 μM) than in our study (IC50 69.8 μM). This is, however, the first report of patriscabratine’s (7) effects on ER+ MCF-7 breast cancer cells, as well as colon cancer cells.
None of the five known flavonoids (2, 3, 4, 5 and 6) isolated from A. aureum showed detectable cytotoxic activity (IC50>500 μM) against the cell lines tested in this study confirming no or low cytotoxicity against various cancer cell lines in previous studies for the flavonoids (2, 3, 4 and 6) (CitationAfifi et al., 2009; CitationLi et al., 2009; CitationMei et al., 2006; CitationYang & Liu, 2009). However, our study is the first report on the evaluation of cytotoxicity for quercetin-3-O-α-l-rhamnosyl-7-O-β-d-glucoside (5).
No report exists to-date on the apoptotic potential of pure tetracosane and patriscabratine in human cancer cells. In this study, tetracosane (1) and patriscabratine (7) caused early apoptosis (AV+/PI−) to a similar degree and with similar time dependence. Both compounds showed approximately 10 and 20% apoptosis following 24 and 48 h of treatment, respectively (). For both compounds early apoptosis was induced to a similar degree as cycloheximide at 24 and 48 h, however both compounds showed greater late apoptosis/necrosis effects than the positive control cycloheximide following 48 h treatment.
Figure 2. Flow cytometry results for AGS cells following treatment with and without tetracosane (1) (500 μg/mL) and patriscabratine(7) (100 μg/mL) in comparison to cycloheximide (150 μg/mL)after 24 and 48 h incubation. A represents AV+/PI- (early apoptosis) and B represents AV+/PI+ (late apoptosis or necrosis) after 24 and 48 h treatment, respectively.
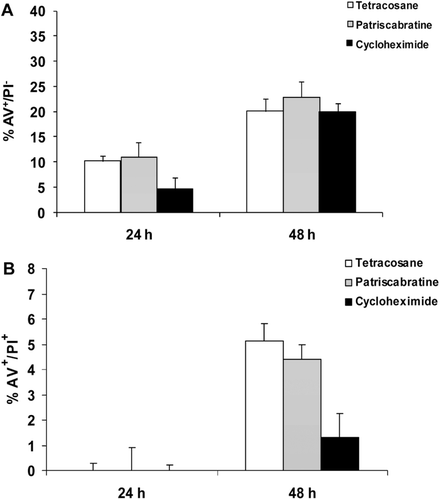
Although the concentration of tetracosane (1) was approximately 3× higher than cycloheximide (150 μg/mL) the apoptosis inducing potential of patriscabratine (7) can certainly be seen as stronger than the positive control, having shown apoptosis at lower concentration (100 μg/mL) than cycloheximide.
In conclusion, this study describes for the first time the isolation of tetracosane, patriscabratine and five flavonoids from A. aureum. The cytotoxic potential of isolated tetracosane (1), quercetin-3-O-α-l-rhamnosyl-7-O-β-d-glucoside (5) and patriscabratine (7) against different cancer cell lines was evaluated for the first time, and the mode of cytotoxicity for (1) and (7) against gastric cancer (AGS) cells was revealed as induction of apoptosis.
A. aureum is traditionally used against peptic ulcer, which is a risk factor for the development of gastric cancer (CitationRayburn et al., 2009). Interestingly, patriscabratine (7), isolated from this plant, had significant effects against gastric cancer cells which could contribute to the reported traditional medicinal effects of the plant.
Declaration of interest
Shaikh J. Uddin was funded by a Griffith University Postgraduate Research Scholarship (GUPRS) and a Griffith University International Postgraduate Research Scholarship (GUIPRS). The authors report no conflicts of interest.
References
- Abdullah Y, Schneider B, Petersen M. (2008). Occurrence of rosmarinic acid, chlorogenic acid and rutin in Marantaceae species. Phytochem Lett, 1, 199–203.
- Afifi MS, Kansoh AL, Abdulla MM. (2009). Flavonoids and bioactivity of Myriophyllum spicatum L. (Haloragaceae) growing in Egypt. Bull Fac Pharm, 47, 67–75.
- Al Ashaal HA, Farghaly AA, Abd El Aziz MM, Ali MA. (2010). Phytochemical investigation and medicinal evaluation of fixed oil of Balanites aegyptiaca fruits (Balantiaceae). J Ethnopharmacol, 127, 495–501.
- Bakr E, 2010. LdP Line. Ehab Bakr, Plant Protection Research Institute, Cairo, Egypt [Online]. Available at: http://embakr.tripod.com/ldpline/ldpline.htm. Accessed on 12 January, 2010.
- Coambie RC, Ash J. (1994). Fijian medicinal plants. Melbourne, Australia: CSIRO Publisher.
- Dai H, Mei W, Hong K, Zeng Y, Zhuang L.. (2005).Screening of the tumor cytotoxic activity of sixteen species of mangrove plants in Hainan. Zhongguo Haiyang Yaowu, 24, 44–46.
- Fossen T, Larsen Å, Kiremire BT, Andersen OM. (1999). Flavonoids from blue flowers of Nymphaèa caerulea. Phytochemistry, 51, 1133–1137.
- Gu ZB, Yang GJ, Liu WY, Li TZ, Qiu Y, Zhang WD. (2002). A new alkaloid from Patrinia scabra. Chin Chem Lett, 13, 957–958.
- Imperato F. (2008). A new flavone glucoside, apigenin 7-O-glucoside-4-acetate and a new fern constituent, quercetin 3-O-rhamnoside-7-O-glucoside from Dryopteris villarii. Am Fern J, 98, 251–253.
- Iorizzi M, Lanzotti V, De Marino S, Zollo F, Blanco-Molina M, Macho A, Muñoz E. (2001). New glycosides from Capsicum annuum L. var. acuminatum. Isolation, structure determination, and biological activity. J Agric Food Chem, 49, 2022–2029.
- Jason TL, Figueredo R, Ferguson PJ, Vincent MD, Berg RW, Koropatnick J. (2008). ODN 491, a novel antisense oligodeoxynucleotide that targets thymidylate synthase, exerts cell-specific effects in human tumor cell lines. DNA Cell Biol, 27, 229–240.
- Kansoh AL, Afifi MS, Elgindi OD, RO B. (2009). Chemical composition, antimicrobial and cytotoxicity activities of essential oil and lipoidal matter of the flowers and pods of Tipuana tipu growing in Egypt. Can J Pure Appl Sci, 3, 661–668.
- Lai HY, Lim YY, Tan SP. (2009). Antioxidative, tyrosinase inhibiting and antibacterial activities of leaf extracts from medicinal ferns. Biosci Biotechnol Biochem, 73, 1362–1366.
- Li L, Henry GE, Seeram NP. (2009). Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J Agric Food Chem, 57, 7282–7287.
- Matsuura H, Amano M, Kawabata J, Mizutani J. (2002). Isolation and measurement of quercetin glucosides in flower buds of Japanese butterbur (Petasites japonicus subsp. gigantea Kitam.). Biosci Biotechnol Biochem, 66, 1571–1575.
- Mei W, Zeng Y, Ding Z, Dai H. (2006). Isolation and identification from mangrove plant of the chemical constituents from mangrove plant Acrostichum aureum. Zhongguo Yaowu Huaxue Zazhi, 16, 46–48,64.
- Momtaz MM. (2008). Encyclopedia of Flora and Fauna of Bangladesh. Dhaka, Bangladesh: Asiatic Society of Bangladesh.
- Natural Resources Conservation Service (2010). Plants Database. Natural Resources Conservation Sevice, United States Department of Agriculture (USDA), USA [Online]. Available at: http://plants.usda.gov. Accessed on 25 October, 2010.
- Nobutoshi T, Takao M, Yasuhisa S, Chiu Ming C, Gomez P, Luis D. (1981). Chemical and chemotaxonomical studies of ferns. XXXVII. Chemical studies on the constituents of Costa Rican fern. Chem Pharm Bull, 29, 3455–3463.
- Rayburn ER, Ezell SJ, Zhang R. (2009). Anti-inflammatory agents for cancer therapy. Mol Cell Pharmacol, 1, 29–43.
- Sultana S, IIyas M, Shaida WA. (1986). Chemical investigation of Acrostichum aureum Linn. J Indian Chem Soc, 63, 1074–1075.
- Uddin SJ, Grice ID, Tiralongo E. (2011). Cytotoxic effects of Bangladeshi medicinal plant extracts. Evid Based Complement Alternat Med, 2011, 1–7.
- Xiao Z, Wu H, Wu T, Shi H, Hang B, Aisa H. (2006). Kaempferol and quercetin flavonoids from Rosa rugosa. Chem Nat Compd, 42, 736–737.
- Yamaji T, Saito T, Hayamizu K, Yanagisawa M, Yamamoto O (2010). 1H and 13C NMR spectra of tetracosane. “Spectral Database for Organic Compounds SDBS”, National Institute of Advanced Industrial Science and Technology (AIST) [Online]. Available at: http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi. Accessed on 15 November, 2010.
- Yang J, Liu RH. (2009). Synergistic effect of apple extracts and quercetin 3-β-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J Agric Food Chem, 57, 8581–8586.
- Yuan L, Wang JH, Sun TM. (2010). Total synthesis and anticancer activity studies of the stereoisomers of asperphenamate and patriscabratine. Chin Chem Lett, 21, 155–158.
- Zafrul DH. (2000). Some plants of Sundarbans. Chittagong, Bangladesh: Tectona Publisher.