Abstract
Context: Euterpe oleracea Mart. (Arecaceae) fruits and their dietary supplements are gaining much popularity internationally. Anthocyanins and their aglycons are responsible for the dense color of açaí fruit and are associated with a wide spectrum of health promoting effects.
Objective: Quantitative analysis of anthocyanins in açaí dietary supplement raw materials; processed açaí powder (ADSR-1), organic açaí powder (ADSR-2), and nonorganic açaí powder (ADSR-3) by quadrupole-time-of-flight liquid chromatography/mass spectrometry (Q-TOF LC/MS) have been reported in this study.
Materials and methods: The chromatographic separation for anthocyanins was achieved using a C-18 column with a gradient of 0.1% formic acid in water and 0.1% formic acid in methanol and acetonitrile (50:50, v/v). MS and MS/MS experiments were carried out on an electrospray ionization-Q-TOF LC/MS.
Results: Except for ASDR-2, all the açaí samples were found to have cyanidin 3-glucoside (1), cyanidin 3-sambubioside (2), cyanidin 3-rutinoside (3), and peonidin 3-rutinoside (4). ASDR-2 contained anthocyanins 1 and 3. Among the açaí samples quantified, ADSR-3 showed higher concentration of anthocyanins compared to other raw materials and capsules tested in this study.
Discussion and conclusion: The anthocyanins 1–4 present in ADSR-3 were 27.13 ± 0.37, 1.76 ± 0.04, 31.07 ± 0.49, and 3.46 ± 0.08 mg/100 g dry wt, respectively. The LOQ values for anthocyanins 1–4 were in the range of 2.44–9.76 ng/mL. Accuracy of the method was assessed by performing a recovery experiments. The intraday and interday variations (RSDs) were <10%. This is the first report on quantitation of anthocyanins in açaí dietary supplement raw materials and capsules.
Introduction
Over the past few decades, dietary supplements of botanical origin have gained global importance, with both medical and economic implications (CitationRapaka, 2006). Epidemiological studies have already shown that the dietary intake of berry fruits has several positive and profound effects on human health (CitationSeeram, 2010). Hence, there is a continuous growth in exploring new and exotic types of berries in the recent years. Euterpe oleracea Mart. (Arecaceae) fruits have been consumed in the form of beverages, energy bars, and capsules (CitationSchauss et al., 2006a; CitationTaylor, 2010). The açaí fruits are also known for their strong antioxidant activity and considered as functional foods (CitationSchauss et al., 2006b; CitationPacheco-Palencia et al., 2007).
The frozen pulp of açaí is largely consumed all over the world due to its nutritive and medicinal qualities. The major phytochemicals reported from açaí are anthocyanins, proanthocyanidins, and flavonoids (CitationBobbio et al., 2000; CitationPozo-Insfran et al., 2004; CitationGallori et al., 2004; CitationLichtenthaler et al., 2005; CitationSchauss et al., 2006a,b; Citationde Rosso et al., 2008; CitationPacheo-Palencia et al., 2009; CitationKang et al., 2010). In addition, compounds including lipids, amino acids, sterols, and lignans have also been documented (CitationChin et al., 2008; Citationde Souza et al., 2010). Among these phytochemicals, anthocyanins and their aglycons (anthocyanidins) are responsible for the dense color of açaí fruit and are associated with a wide spectrum of health promoting effects. Cyanidin 3-arabinoside, cyanidin 3-arabinosylarabinoside, cyanidin 3-glucoside, pelargonidin 3-glucoside, peonidin 3-rutinoside, peonidin 3-glucoside, and cyanidin 3-sambubioside have been reported from the pulp of açaí so far (CitationBobbio et al., 2000; CitationGallori et al., 2004; CitationPozo-Insfran et al., 2004; CitationLichtenthaler et al., 2005; Citationde Rosso et al., 2008; CitationPacheo-Palencia et al., 2009; CitationKang et al., 2010). In particular, cyandin 3-glucoside and cyanidin 3-rutinoside were found to be predominant in açaí samples (CitationLichtenthaler et al., 2005).
Açaí anthocyanins were evaluated for their medicinal activities such as antioxidant (CitationLichtenthaler et al., 2005; CitationSchauss et al., 2006b; CitationChin et al., 2008; Citationde Rosso et al., 2008; CitationMertens-Talcott et al., 2008; CitationPacheo-Palencia et al., 2009; Citationde Souza et al., 2010; CitationKang et al., 2010), antiinflammatory (CitationMathues et al., 2006), and antiproliferative effects. (CitationPacheo-Palencia et al., 2008, Citation2010a; CitationHogan et al., 2010). The medicinal impact of açaí dietary ingredients in their formulations to improve health has increased their widespread use leading to public health challenges globally in terms of quality, safety, and efficacy. In this context, it is important to characterize and quantify the bioactive compounds accurately. Also, the variation of anthocyanin content among the different açaí berries and their processed products emphasizes the need for more selective, accurate, and sensitive analytical detection and quantification methods to determine the exact content of anthocyanins and their composition in dietary botanical supplements. Numerous studies have been reported in the literature for characterization and quantification of anthocyanins in açaí fruits by HPLC-UV (CitationBobbio et al., 2000; CitationPozo-Insfran et al., 2004; CitationPacheco-Palencia et al., 2007) and HPLC-MS (CitationGallori et al., 2004; CitationLichtenthaler et al., 2005; CitationSchauss et al., 2006a; Citationde Rosso et al., 2008; CitationPacheo-Palencia et al., 2009, Citation2010; CitationHogan et al., 2010) methods. Liquid chromatography coupled with quadrupole time of flight mass spectrometry (LC-Q-TOF) has become a useful technique for the rapid and sensitive quantification of anthocyanins (CitationKosir et al., 2004; CitationMontoro et al., 2006; CitationLing et al., 2009). Various methods using atmospheric pressure photoionization and electrospray ionization (ESI) for detection and identification of anthocyanins in plants and foods have been developed (CitationGomez-Ariza et al., 2006; CitationCavaliere et al., 2008; CitationMaldini et al., 2009; CitationPati et al., 2009). In this study, we report quantitative analysis of anthocyanins in açaí dietary supplement raw materials and capsules.
Materials and methods
Chemicals and reagents
Three açaí raw materials that are used in the preparation of dietary supplements and formulations were used to study their anthocyanin content. The processed (ADSR-1, Nature’s Sunshine Products, Spanish Fork, UT, Lot No. Q166545), organic (ADSR-2, Live Superfoods, Bend, OR, Lot No. LSF077), and nonorganic açaí dietary supplement raw materials (ADSR-3, FutureCeuticals, Momence, IL, Lot No. N424) were kindly provided by Dr. William Hurst from the Hershey Company, Hershey, PA. The açaí capsules (NATROL, Chatsworth, CA; Lot No. 2040068) were a kind gift from Walgreens, Auburn, AL. Anthocyanin standards (purity > 95%), cyanidin 3-glucoside, cyanidin 3-rutinoside, cyanidin 3-sambubioside, and peonidin 3-rutinoside were purchased from INDOFINE Chemical Company, Inc. (Hillsborough, NJ). Internal standard (IS) reserpine (purity > 99%) and formic acid of LC/MS grade were bought from Sigma-Aldrich (Allentown, PA). All the solvents used are of LC/MS grade and were purchased from Fischer-Scientific (Atlanta, GA). Deionized water was purified by a Milli-Q reagent water system (Millipore, MA). All the samples were filtered through 0.2 µm nylon membranes (VWR International, Suwanee, GA). All the dietary supplement raw materials were stored at +4°C until extraction.
Açaí dietary supplement raw materials and capsules
Processed açaí powder (ADSR-1): Açaí powder was prepared from the fruits of Euterpe oleracea from Brazil at Nature’s Sunshine Products, Spanish Fork, UT. It was processed by defrosting the açaí fruits and adding chlorinated potable water. The mixture was prepared in a homogenization tank. Then it was filtered and stored in an equilibration tank and pasteurized at 90–100°C and dried in a spray drier. It contains 25% maltodextrin from non-GMO corn starch. Organic açaí powder (ADSR-2): Freshly grown Brazilian organic açaí fruits were harvested, freeze-dried within 24 h of the fruit being picked without preservatives, and stored in air-tight bags at cold temperature until use. Nonorganic açaí powder (ADSR-3): Fresh nonorganic whole açaí fruits from Brazil were freeze-dried and powdered. The açaí capsules (NATROL, Chatsworth, CA; Lot No. 2040068) contained açaí extract 1000 mg per serving.
Sample preparation
The samples of processed açaí powder (ADSR-1, 1.0599 g average dry wt), organic açaí powder (ADSR-2, 1.0112 g average dry wt), nonorganic açaí powder (ADSR-3, 1.0808 g average dry wt) and capsules (4.2584 g average dry wt) were weighed accurately and transferred to a mortar and pestle individually. The powder of each açaí sample was mixed thoroughly and transferred to a 50-mL polypropylene centrifuge tube and extracted with 25 mL of acidic methanol: water (70:30, 0.1% HCl, v/v). The samples were sonicated in a Bransonic® Model 2510 ultrasonic bath (VWR International, Atlanta, GA) for 20 min at room temperature. The samples were centrifuged at 4000 rpm for 10 min using a Beckmann Coulter Allegra® 6 Series centrifuge (VWR International, Atlanta, GA). The extraction procedure was repeated twice, and the combined supernatants were filtered through a 0.2 μm PTFE membrane (VWR International, Atlanta, GA). All the extracts were dried under high vacuum at 40°C using a Büchi rotavapor R-210 equipped with Büchi recirculating chiller B-740 and Buchi vacuum pump V-700 (VWR International, Atlanta, GA). A total number of eight extracts per açaí sample were prepared. Four extracts of each açaí sample were dissolved in 3 mL of methanol and water (70:30, 0.1% formic acid v/v), transferred, and made up to the volume in a 5 mL volumetric flask using the same solvent. The samples were spiked with IS reserpine (50 ng/mL), prepared in methanol and water (70:30, 0.1% formic acid v/v), and analyzed on the same day in triplicate. The remaining extracts (four extracts each) of açaí samples were stored at −80°C, and the samples were prepared in the same way as described earlier and analyzed on the 30th day.
The extracts of açaí samples for recovery study were prepared in a similar procedure on two different days. The açaí dietary supplement raw materials and capsules after extraction and drying were spiked with 5 µg/mL of standard anthocyanins 1 and 2, extracted, and analyzed to study intraday (0 day) and interday (day 5) precision and accuracy of the method. All the samples were prepared in duplicate and analyzed in quadruplicate.
Calibration standards preparation
Individual stock solutions of cyanidin 3-glucoside (1), cyanidin 3-sambubioside (2), cyanidin 3-rutinoside (3), and peonidin 3-rutinoside (4) were prepared in methanol at a concentration of 1 mg/mL in a volumetric flask. Serial dilutions of standard stock solutions for 1, 2, 3, and 4 were prepared in methanol and water (70:30, 0.1% formic acid) to afford concentrations ranging from 0.24–31.25, 0.49–15.62, 0.12–15.62, and 0.06–15.62 µg/mL, respectively. All the samples were spiked with a fixed amount of IS, reserpine (50 ng/mL). The samples were stored at −80°C until use.
Liquid chromatography mass spectrometry conditions
Quantitative analysis of anthocyanins was performed using an Agilent 6520 Q-TOF mass spectrometer equipped with a 1220 RRLC system (Agilent Technologies, Little Falls, DE). Liquid chromatography analysis was carried out on a 2.1 × 100 mm, 1.8-µm ZORBAX Eclipse plus C18 column (Agilent Technologies, New Castle, DE) using a mobile phase consisting of (A) 0.1% formic acid in water and (B) 0.1% formic acid in methanol and acetonitrile (50:50, v/v) at 25°C. The gradient was performed at 0.2 mL/min with an initial condition of 1% B for 2 min. The mobile phase was linearly increased to 99% B at 25 min and maintained same until 27 min. The system was subsequently returned to the initial conditions at 30 min and equilibrated further for 5 min. The sample injection volume was 10 µL. Protonated molecules of anthocyanins were detected using electrospray MS in positive mode. The complete mass scanning range was from m/z 100–1000. The experiments were conducted using capillary voltage of 3400 V. Nitrogen was supplied as nebulizing and drying gas at flow rates of 25 and 600 L/h, respectively. The drying gas temperature was 350°C. The fragmentor voltage was optimized to 175 eV. MS/MS experiments were conducted with similar LC-MS conditions, and the collision energy was set at 30 eV. All the extracts and standard solutions were analyzed in triplicate. Quantitative analysis of anthocyanins was performed using full-scan mode MS experiments as described under experimental conditions. Reserpine was used as an IS at a concentration of 50 ng/mL. The structural identification of anthocyanins was carried out using MS/MS experiments and by comparison of retention times with those of commercially available standards. The data was acquired and analyzed using Agilent MassHunter Workstation Qualitative Analysis software, version B.02.00.
Method validation
The LC/MS method developed was validated in terms of linearity, accuracy, and precision. The linearity of the method was evaluated by triplicate analyses of standard solutions with concentrations from 1 to 1000 ng/mL with a fixed amount of reserpine (50 ng/mL) as an internal standard. A calibration curve for each standard was constructed by plotting concentration against peak area ratio of the analyte of interest versus the internal standard. The limit of detection (LOD) and limit of quantitation (LOQ) were determined by injecting a series of dilute solutions with known concentrations. Sensitivity of the method was evaluated by determining the LOD and LOQ, which were defined as the signal-to-noise ratio equal to 3 and 10, respectively. Accuracy of the method was assessed by performing recovery experiments. All the dietary supplement raw materials and capsules after successive extraction and drying were spiked with 5 µg/mL of standard anthocyanins 1 and 2 and analyzed under optimized conditions to study intraday (0 day) and interday (5 day) precision and accuracy of the method. Accuracy was assessed by comparison of the calculated mean concentrations to nominal concentrations. The percent (%) accuracy of the method was determined by the formula: Mean of measured concentration/nominal concentration × 100. Recoveries were calculated by comparing the observed concentration with the spiked concentration using the formula: % Recovery = observed concentration/spiked concentration × 100.
Results
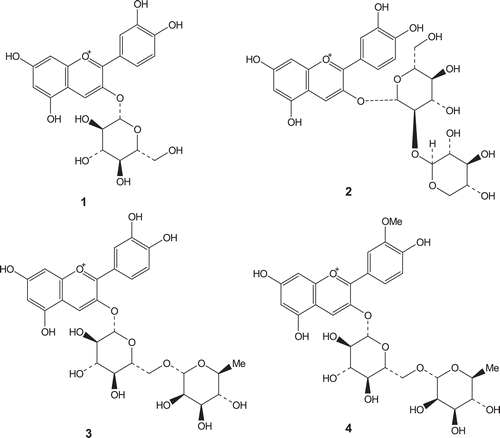
The chemical structures of anthocyanins 1–4 are shown in . Under the chromatography conditions listed in “Materials and Methods” section, anthocyanins 1, 2, 3, and 4 eluted at 9.65, 9.43, 9.81, and 10.54 min, respectively. The quantitation of anthocyanins was performed using reserpine as an IS, which was eluted at 17.44 min under similar mobile-phase conditions. Quantitation of structurally different compounds using reserpine as an internal standard has been reported in the literature (CitationLu et al., 2006; CitationSleno and Volmer, 2006). Reserpine is a compound with similar character (multiple aromatic rings) but different enough to assure that an unidentifed anthocyanin would not interfere in the LC–MS analysis.
Figure 1. Structures of. anthocyanins, cyanidin 3-glucoside (1), cyanidin 3-sambubioside (2), cyanidin 3-rutinoside (3), and peonidin 3-rutinoside (4).
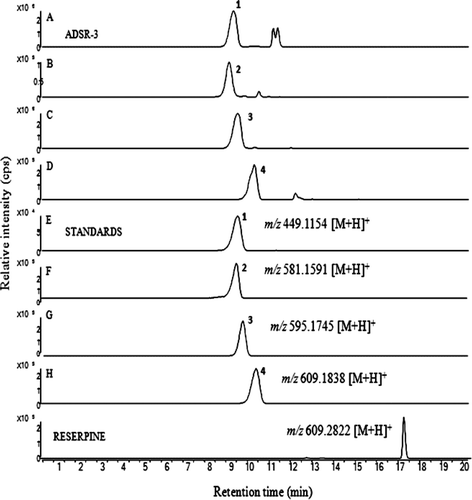
ESI–LC/MS analysis revealed that anthocyanins 1 and 3 were found to be common in all the açaí samples. ADSR-1, ADSR-3, and capsules showed anthocyanins 1–4. In case of ADSR-2, only anthocyanins 1 and 3 were found. The peak intensities of anthocyanins are higher in ADSR-3 than ADSR-1, ADSR-2, and capsules. We have illustrated the chromatograms of anthocyanins present in ADSR-3 in comparison with the chromatograms developed for anthocyanin standards under the similar LC conditions in . Anthocyanins (1–4) were detected by electrospray ion source in positive mode. The MS conditions were optimized with a capillary voltage of 3400 V; drying gas temperature 350°C; fragmentor voltage 175 V. Nitrogen was supplied as a nebulizing and drying gas at flow rates of 25 and 600 L/h, respectively.
Figure 2. Extracted ion chromatogram of anthocyanins 1 (A), 2 (B), 3 (C), and 4 (D) present in nonorganic açaí powder (ADSR-3), anthocyanin standards 1–4 (E–H) and internal standard reserpine.
The anthocyanins are reported to have relatively low stability, and they are reported to be sensitive to temperature, pH, and light (CitationPacheo-Palencia and Talcott, 2010b). In our method, anthocyanin extraction was optimum when the samples were extracted with acidic methanol and water (70:30, v/v, 0.1% HCl). There was a significant increase in separation and improvement of peak shape in the anthocyanin LC chromatograms when the samples were prepared in methanol and water mixture containing 0.1% formic acid. Further dilutions of samples were carried out before LC/MS analysis when the concentration of the anthocyanins exceeded the linear range of the calibration. The use of an internal standard is essential for LC/MS analysis to achieve acceptable precision and accuracy. Obviously, stable isotope-labeled compounds will represent the ideal choice, but they are not always available, especially in natural products drug discovery. Reserpine was found to be the most suitable IS for this study because of the multicomponent nature of the samples; it was convenient to use a single internal standard for several compounds. Also, reserpine gave reliable MS response throughout the analysis.
Positive full-scan MS in the range 100–1000 was used for anthocyanin quantification. The mass spectra of anthocyanins 1–4 obtained in positive ESI/MS experiments showed protonated molecular ions [M + H]+ at m/z 449.1154, 581.1591, 595.1745, and 609.1838, respectively (, ). Whereas the protonated molecular ion of IS reserpine was detected at m/z 609.2820. Further structural confirmations of anthocyanins were performed by positive ion ESI–MS/MS experiments with collision energy of 30 eV to obtain the characteristic fragment ions corresponding to each anthocyanin. The structure identification of anthocyanins 1–4 was confirmed by MS/MS experiments. The results have been illustrated in . The MS/MS fragmentation pattern of anthocyanins was in agreement with those of literature data (CitationTian et al., 2005). Further confirmation of anthocyanins 1–4 was made by comparison of retention times with their respective standards.
Table 1. ESI-LC/MS and MS/MS data of anthocyanins in ADSR-3 and internal standard reserpine.
Quantification was performed by preparing the six-point calibration curves for anthocyanin standards (1–4) using reserpine as IS. Peak area ratios of the analyte/IS were used for quantitation. From the LC/MS results obtained, the linearity of the analytical response across the studied range was excellent with correlation coefficients (r2) higher than 0.99 as shown in . The linearity range for anthocyanins 1, 2, 3, and 4 was 0.24–31.25, 0.49–15.62, 0.12–15.62, and 0.06–15.62 µg/mL, respectively, as shown in . The quantitative LC/MS results indicated that açaí dietary supplement raw materials ADSR-1, ADSR-3, and capsules contained anthocyanins, cyanidin 3-glucoside (1), cyanidin 3-sambubioside (2), cyanidin 3-rutinoside (3), and peonidin 3-rutinoside (4). Whereas açaí sample ADSR-2 was found to contain cyanidin 3-glucoside (1) and cyanidin 3-rutinoside (3). The data suggest that cyanidin 3-glucoside (1) and cyanidin 3-rutinoside (3) were the common anthocyanins in all the açaí dietary supplement raw materials and capsules. In the literature, cyanidin 3-glucoside and cyanidin 3-rutinoside were reported as the major anthocyanins in açaí samples by CitationGallori et al. (2004) and CitationLichtenthaler et al. (2005). However, cyanidin 3-sambubioside and peonidin 3-rutinoside were reported in minor amounts (CitationSchauss et al., 2006a,b). The quantities of anthocyanins were individually calculated against commercially available anthocyanin external standards and expressed as their equivalents. Among the samples quantified, ADSR-3 had higher anthocyanin content, and quantities of 1, 2, 3, and 4 in ADSR-3 sample were 27.13 ± 0.37, 1.76 ± 0.04, 31.07 ± 0.49, and 3.46 ± 0.08 mg/100 g dry wt, respectively (). Capsules contained lower concentrations of anthocyanins, and the quantities of anthocyanins 1, 2, 3, and 4 detected were 0.08 ± 0.01, 0.12 ± 0.00, 0.22 ± 0.01, and 0.09 ± 0.00 mg/100 g dry wt, respectively. Whereas the quantities of anthocyanins 1 and 3 in ADSR-2 were 8.39 ± 0.23 and 1.40 ± 0.03 mg/100 g dry wt as shown in . The quantities of anthocyanins 1 (0.59 ± 0.04), 2 (0.49 ± 0.01), 3 (1.56 ± 0.06), and 4 (0.37 ± 0.01) in açaí sample ADSR-1 were higher than the anthocyanins present in capsules.
Table 2. Regression equation, correlation coefficient (r2), limit of detection (LOD), limit of quantitation (LOQ), and linearity range for anthocyanins by rapid resolution LC/MS.
Table 3. Anthocyanin content in processed (ADSR-1), organic (ADSR-2), and nonorganic (ADSR-3) açaí dietary supplement raw materials and capsules (n = 4).
Sensitivity of the method was evaluated by determining LOD and LOQ. The LOD and LOQ for anthocyanins 1–4 at a signal-to-noise ratio of 3 and 10 were illustrated in . The LOD and LOQ ranged from 0.01 to 0.12 and 0.06 to 0.49 µg/mL, respectively. The LODs and LOQs values obtained by the developed LC/MS method were found to be much more sensitive than previously published methods. Very low standard error observed during multiple injections indicated that the results are highly reproducible. To study the variation in anthocyanin content, samples of dried açaí extracts (quadruplicate) stored at −80°C were prepared for LC/MS analysis on the 30th day. The results have been summarized in . No significant changes were observed when the anthocyanin content was quantified after 30 days.
The accuracy of the method was determined by recovery studies. The açaí dietary supplement raw materials and capsules were spiked with 5 µg/mL of standard anthocyanins 1 and 2, extracted, and analyzed. Intraday and interday precision and accuracy values were obtained by analyzing samples spiked with known concentration of anthocyanin standards 1 and 3 on 0th day and 5th day. Intraday and interday variations of the method were represented as % RSDs. shows the validation results for intra and interday assay accuracy and precision. Precision was assessed in terms of the relative standard deviation of the measured concentrations, while accuracy was assessed in terms of the mean error in the quadruplicate set. The data showed good precision of the method with an intra and interday assay RSD of below 10%, suggesting that the method was sufficiently accurate. summarizes the validation results for intra and interday assay accuracy and precision.
Table 4. The intraday (0 day) and interday (day 5) accuracy for anthocyanins, cyanidin 3-glucoside (1) and cyanidin 3-rutinoside (3) in processed (ADSR-1), organic (ADSR-2), and nonorganic (ADSR-3) açaí dietary supplement raw materials and capsules (n = 4).
Discussion
Anthocyanins are dietary components of açaí fruits, which have achieved great interest due to their health implications (CitationJagger, 2007; CitationSchauss et al., 2010). Based on the harvesting time, processing, and storage conditions, a great degree of anthocyanin content variation in açaí samples has been reported in literature (CitationLichtenthaler et al., 2005). Because of this variation, the need for robust, rapid, and sensitive analytical methods to detect biologically active chemical constituents in botanical dietary supplements is gaining much interest recently (CitationCardellina, 2002; CitationSalgueiro et al., 2010). Within the aim to demonstrate the ability of LC/Q-TOF/MS for the quality assessment of açaí raw materials and capsules, the quantitative method developed was applied to detect their anthocyanin content. The low LOD and LOQ values reported in this study demonstrate further the sensitivity of the current method. In addition, the variation of anthocyanin content between these dietary supplement raw materials and capsules were shown for the first time. All the açaí samples have shown cyanidin 3-glucoside and cyanidin 3-rutinoside as major anthocyanins, and the results were consistent with those of literature (CitationGallori et al., 2004). The quantitative method developed in our study may be useful to confirm anthocyanin quantity and composition in various processed açaí samples. Furthermore, the method can be used in the selection of raw materials that have high amounts of health beneficial anthocyanins for the preparation of botanical dietary supplements and their formulations.
Conclusions
Our current work describes anthocyanin quantitation in processed açaí dietary supplement raw materials and capsules. Application of this method in quantifying anthocyanins present in various açaí samples may provide the consumers with accurate information concerning health beneficial levels of anthocyanins as well as the quality of açaí dietary ingredients and formulations. This is the first report on quantitation of anthocyanins in açaí dietary supplement raw materials and capsules.
Acknowledgments
We thank Dr. William Hurst from the Hershey Company, Hershey, PA and Walgreens, Auburn, AL for supplying samples of açaí raw materials and capsules.
Declaration of interest
The authors are deeply indebted to United Stated Pharmacopeia (USP) Fellowship Program 2009–2010 for financial support.
References
- Bobbio FO, Druzian JI, Abrao PA, Bobbio PA, Fadelli S. (2000). Identification and quantification of anthocyanins from the açaí fruit (Euterpe oleracea) Mart. Ciên Tecnol Aliment, 20, 388–390.
- Cardellina JH 2nd. (2002). Challenges and opportunities confronting the botanical dietary supplement industry. J Nat Prod, 65, 1073–1084.
- Cavaliere C, Foglia P, Gubbiotti R, Sacchetti P, Samperi R, Laganà A. (2008). Rapid-resolution liquid chromatography/mass spectrometry for determination and quantitation of polyphenols in grape berries. Rapid Commun Mass Spectrom, 22, 3089–3099.
- Chin YW, Chai HB, Keller WJ, Kinghorn AD. (2008). Lignans and other constituents of the fruits of Euterpe oleracea (Açaí) with antioxidant and cytoprotective activities. J Agric Food Chem, 56, 7759–7764.
- Del Pozo-Insfran D, Brenes CH, Talcott ST. (2004). Phytochemical composition and pigment stability of açaí (Euterpe oleracea Mart.). J Agric Food Chem, 52, 1539–1545.
- de Rosso VV, Hillebrand S, Montilla EC, Bobbio FO, Winterhalter P, Mercadante AZ. (2008). Determination of anthocyanins from acerola (Malpighia emarginata DC.) and açaí (Euterpe oleracea Mart.) by HPLC-PDA-MS/MS. J Food Comp Anal, 21, 291–299.
- de Souza MO, Silva M, Silva ME, Oliveira Rde P, Pedrosa ML. (2010). Diet supplementation with açaí (Euterpe oleracea Mart.). pulp improves biomarkers of oxidative stress and the serum lipid profile in rats. Nutrition, 26, 804–810.
- Gallori S, Birla AR, Bergonzi MC, Barbosa WLR, Vincieri FF. (2004). Polyphenolic constituents of fruit pulp of Euterpe oleracea Mart. (Açaí palm). Chromatographia, 59, 739–743.
- Gomez-Ariza JL, Garcia-Barrera T, Lorenzo F. (2006). Anthocyanins profile as fingerprint of wines using atmospheric pressure photoionisation coupled to quadrupole time-of-flight mass spectrometry. Anal Chim Acta, 570, 101–108.
- Hogan S, Chung H, Zhang L, Li J, Lee Y, Dai Y, Zhou K. (2010). Antiproliferative and antioxidant properties of anthocyanin-rich extracts from açaí. Food Chem, 118, 208–214.
- Jagger A. (2007). Amazonian berry. Chem Ind, 6, 24–25.
- Kang J, Li Z, Wu T, Jensen GS, Schauss AG. (2010). Anti-oxidant capacities of flavanoid compounds isolated from açaí pulp. Food Chem, 122, 610–617.
- Kosir IJ, Lapornik B, Andrensek S, Wondra AG, Vrhovsck U, Kidric J. (2004). Identification of anthocyanins in wines by liquid chromatography, liquid chromatography-mass spectrometry and nuclear magnetic resonance. Anal Chim Acta, 513, 277–282.
- Lichtenthäler R, Rodrigues RB, Maia JG, Papagiannopoulos M, Fabricius H, Marx F. (2005). Total oxidant scavenging capacities of Euterpe oleracea Mart. (Açaí) fruits. Int J Food Sci Nutr, 56, 53–64.
- Ling Y, Ren C, Mallery SR, Ugalde CM, Pei P, Saradhi UV, Stoner GD, Chan KK, Liu Z. (2009). A rapid and sensitive LC-MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. J Chromatogr B Analyt Technol Biomed Life Sci, 877, 4027–4034.
- Lu W, Kimball E, Rabinowitz JD. (2006). A high-performance liquid chromatography-tandem mass spectrometry method for quantitation of nitrogen-containing intracellular metabolites. J Am Soc Mass Spectrom, 17, 37–50.
- Maldini M, Montoro P, Piacente S, Pizza C. (2009). ESI-MS, ESI-MS/MS fingerprint and LC-ESI-MS analysis of proathocyanidins from Bursera simaruba Sarg bark. Nat Prod Commun, 4, 1671–1674.
- Mathues ME, de Olivera Fernandes SB, Silveira CS, Rodrigues VP, de Sousa Menezes F, Fernandes PD. (2006). Inhibitory effects of Euterpe oleracea Mart. on nitric oxide production and iNOS expression. J Ethno Pharmacol, 107, 291–296.
- Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST, Derendorf H. (2008). Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem, 56, 7796–7802.
- Montoro P, Tuberoso CI, Perrone A, Piacente S, Cabras P, Pizza C. (2006). Characterisation by liquid chromatography-electrospray tandem mass spectrometry of anthocyanins in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur. J Chromatogr A, 1112, 232–240.
- Pacheo-Palencia LA, Duncan CE, Talcott ST. (2009). Phytochemical composition and thermal stability of two commercial açaí species, Euterpe oleracea and Euterpe precatoria. Food Chem, 115, 1199–1205.
- Pacheco-Palencia LA, Haken P, Talcott ST. (2007). Phytochemical, antioxidant and pigment stability of açaí (Euterpe oleracea Mart.) as affected by clarification, ascorbic acid fortification and storage. Food Res Int, 40, 620–628.
- Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST. (2008). Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from açaí (Euterpe oleracea Mart.). J Agric Food Chem, 56, 4631–4636.
- Pacheo-Palencia LA, Mertens-Talcott SU, Talcott ST. (2010a). In vitro absorption and antiproliferative activities of monomeric and polymeric anthocyanin fractions from açaí fruit (Euterpe oleracea Mart.). Food Chem, 119, 1071–1078.
- Pacheo-Palencia LA, Talcott ST. (2010b). Chemical stability of açaí fruit (Euterpe oleracea Mart.) anthocyanins as influenced by naturally occurring and extremely added polyphenolic cofactors in model systems. Food Chem, 118, 17–25.
- Pati S, Liberatore MT, Gambacorta G, Antonacci D, La Notte E. (2009). Rapid screening for anthocyanins and anthocyanin dimers in crude grape extracts by high performance liquid chromatography coupled with diode array detection and tandem mass spectrometry. J Chromatogr A, 1216, 3864–3868.
- Rapaka RS, Coates PM. (2006). Dietary supplements and related products: a brief summary. Life Sci, 78, 2026–2032.
- Salgueiro L, Martins AP, Correia H. (2010). Raw materials: The importance of quality and safety. A review. Flavour Frag J, 25, 253–271.
- Schauss AG, Jensen GS, Wu X. (2010). Açaí (Euterpe oleracea): An Amazonian palm fruit with broad antioxidant and anti-inflammatory activities. In: Qian M, Rimando A, eds. Flavor and Health Benefits of Small Fruit, American Chemical Society (ACS) Symposium Series. USA: Oxford University Press, Chapter 13, 213–223.
- Schauss AG, Wu X, Prior RL, Ou B, Huang D, Owens J, Agarwal A, Jensen GS, Hart AN, Shanbrom E. (2006). Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (açaí). J Agric Food Chem, 54, 8604–8610.
- Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, Kababick JP. (2006). Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae Mart. (açaí). J Agric Food Chem, 54, 8598–8603.
- Seeram NP. (2010). Recent trends and advances in berry health benefits research. J Agric Food Chem, 58, 3869–3870.
- Sleno L, Volmer DA. (2006). Assessing the properties of internal standards for quantitative matrix-assisted laser desorption/ionization mass spectrometry of small molecules. Rapid Commun Mass Spectrom, 20, 1517–1524.
- Taylor L. (2010). Tropical Plant Database: Açaí. Carson City, NV. 2005. Available at: www.rain-tree.com/açaí.htm. Acessed January 7, 2010.
- Tian Q, Giusti MM, Stoner GD, Schwartz SJ. (2005). Screening for anthocyanins using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with precursor-ion analysis, product-ion analysis, common-neutral-loss analysis, and selected reaction monitoring. J Chromatogr A, 1091, 72–82.