Abstract
Context: Nicotine, a bioactive component of tobacco, is highly addictive. Numerous therapies have been developed or are currently under investigation for smoking cessation, and all have met with limited success and/or side effects, indicating the need for additional therapies.
Objective: This study examines the ability of a commerically-available aqueous extract of Passiflora incarnata Linn. (Passifloraceae) to ameliorate the signs of nicotine sensitization using a rat model.
Materials and methods: Rats were administered 0.4 mg/kg nicotine or vehicle once a day for four consecutive days. Nicotine adminstration produces sensitization of locomotor activity, a phenomenon implicated in the development of nicotine dependence. On the fifth day, locomotor activity of the subjects was monitored as rats from each treatment group were administered 800 mg/kg of Passiflora incarnata extract (or its vehicle) followed by a challenge dose of 0.4 mg/kg nicotine.
Results: When given to rats sensitized to nicotine for 4 days, the challenge dose of nicotine increased locomotor activity by more than 2-fold over activity following nicotine challenge in rats treated with vehicle during the sensitization phase. The difference was significant from 15–40 min after nicotine administration. Rats sensitized to nicotine then treated with Passiflora incarnata extract prior to the nicotine challenge exhibited a level of locomotor activity the same as the vehicle-treated controls.
Discussion: Passiflora incarnata extract did antagonize the expression of nicotine locomotor sensitization.
Conclusion: Passiflora incarnata extract should be examined in future studies to evaluate its potential for treating nicotine addiction in humans.
Introduction
Tobacco is the single greatest cause of preventable disease and premature death in the United States today and is responsible for more than 440,000 deaths each year. Despite the morbidity, mortality and cost due to smoking, nearly 21% of all adult Americans continue to smoke (CitationMoeller & Sun, 2010).
Nicotine, the active ingredient responsible for the addictive nature of cigarette smoking, exerts excitatory and inhibitory pharmacologic effects which account for its stimulating yet calming effects. The compound binds to central nicotinic receptors causing release of the neurotransmitter dopamine in the mesolimbic area, the corpus striatum, and the frontal cortex (CitationBenowitz, 1996). An increase in dopamine levels in the nucleus accumbens has been shown to be related to the addictive properties of nicotine (CitationDani & De Biasi, 2001).
Nicotine replacement therapy (NRT), bupropion and varenicline, are currently FDA approved as smoking cessation aids in the United States and are all considered effective, but no pharmacotherapy appears to be effective for more than about 1/3 of patients (CitationFiore et al., 2008). Thus, there is still a great need to find new therapies for this addiction. NRT acts by replacing approximately 50% of the nicotine that a smoker would receive from a cigarette, thereby reducing cravings following abrupt cessation (CitationRennard & Daughton, 2000). Varenicline is a partial nicotine agonist that activates nicotine receptors while also blocking the effects of nicotine (CitationObach et al., 2006). Bupropion is a dopamine and norepinephrine reuptake inhibitor which acts to increase the synaptic concentrations of dopamine and norepinephrine (CitationPaterson et al., 2007).
Preparations of passion flower [Passiflora incarnata Linn. (Passifloraceae)] have been used in traditional and herbal medicines to treat anxiety, insomnia, and seizures, but controlled clinical studies of passion flower extract for these indications are limited (CitationWerneke et al., 2006). Passion flower extract has been examined in double blind studies and shown to treat generalized anxiety disorder similar to oxazepram (CitationAkhondzadeh et al., 2001b), to help patients through opiate withdrawal (CitationAkhondzadeh et al., 2001a), and to help alleviate presurgical anxiety (CitationMovafegh et al., 2008). In laboratory rodents, passion flower extract or single chemical constituents of passion flower have been shown to be sedative, anxiolytic (CitationSoulimani et al., 1997; CitationZanoli et al., 2000; CitationKrenn, 2002) and anticonvulsant (CitationNassiri-Asl et al., 2007). Moreover, several studies in laboratory rodents by CitationDhawan and colleagues (2002, CitationDhawan, 2003) have demonstrated that whole extracts or an undisclosed benzoflavone moiety from passion flower are anxiolytic, antitussive and aphrodisiac, and may reduce nicotine dependence as well as dependence on other drugs. They have shown that the benzoflavone drug, given together with the addictive agent during the induction of dependence, reduced the symptoms of antagonist-precipitated withdrawal to morphine, alcohol, nicotine, diazepam and Δ9-tetrahydrocannabinol (CitationDhawan, 2003).
Previous studies have demonstrated that an acute injection of nicotine to rats results in increased locomotion, and daily injections result in sensitization to this effect (CitationKsir et al., 1987; CitationDiFranza & Wellman, 2007; CitationLi et al., 2008). This sensitization is believed to play an essential role in the development of addiction (CitationKayir et al., 2009). Identifying agents that block the expression of locomotor sensitization may be used to predict agents that may promote smoking cessation in humans (CitationKayir et al., 2009).
Previous studies by CitationDhawan and colleagues (2002) used a model where passion flower extracts were used to antagonize the induction of locomotor sensitization by nicotine in mice, and dependence was assessed by precipitating withdrawal using an antagonist. The present study examines whether the test agent can antagonize the expression of locomotor sensitization by nicotine in rats, and does not depend on the use of an antagonist to assess dependence. Both approaches involve assaying passion flower extracts for their ability to prevent or treat nicotine addiction. The former model involved administering the potential treatment concurrent with the nicotine used to produce the addicted state, while the current study involves administration of the treatment to animals after the initiation of addiction. This model of determining passion flower effect after nicotine addiction appears to be more directly applicable to understanding whether an agent may be useful for treatment of human addiction, though humans would take the preparation orally as opposed to by injection.
Materials and methods
Drug treatment of rats
Male Wistar rats (Charles River, Raleigh, NC) were provided with food and water ad libitum and housed in the Campbell University Animal facility on a 12 h light/dark cycle at approximately 21°C for 2–3 weeks before beginning any treatments. For all experiments, rats were approximately 45–65 days old and weighed between 148 and 195 g at the beginning of an experiment. Injections of nicotine tartrate or its vehicle (water) were subcutaneous (s.c.) at 0.4 mg/kg nicotine base (Sigma Aldrich, St. Louis, MO) and 2 mL/kg. Injections of a proprietary formulation of aqueous passion flower extract (Nature’s Answer, Hauppage, NY) or vehicle (1:1 glycerin:water) were intraperitoneal (i.p.) at 800 mg/kg and 0.16 mL/kg. Commercial Passion Flower herbal extract (Nature’s Answer) was chosen for use in this study. The proprietary herbal formulation was prepared by a cold extraction technique of Passiflora incarnata. The cold extraction technique replaces alcohol with natural vegetable glycerin to yield an alcohol-free 1000 mg/mL formulation with constituents in the same ratios as the plant source. The lot used for this study was tested by the manufacturer’s quality control process and was found to contain only Passiflora incarnata and glycerin and did not contain any contaminants (E. Kamhi, Nature’s Answer technical support, personal communication, August 25, 2011). All experiments were pre-approved by Campbell University’s Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
Assessment of time to habituation and of the effect of acute nicotine on spontaneous locomotor activity
Each spontaneous activity chamber was a polypropylene container 44.5 cm × 33 cm and 38 cm high (Sterilite Corporation, Townsend, MA), and all testing was done in a closed room under normal lighting and environmental conditions in the Campbell University Animal Facility. Up to four drug-naive rats were each placed in a separate spontaneous activity chamber for simultaneous determination of locomotor activity using the Limelight video tracking system (ActiMetrics, Wilmette, IL). In order to mimic the conditions of the main set of experiments (described in the next section), this experiment was designed to assess whether stimulation of locomotion by nicotine could be measured after an extended period in the test chambers with injections occurring in the middle of the observation period. Each rat was monitored for 30 min to determine the time course of habituation. Immediately afterward, each rat was injected with vehicle that was subsequently used for nicotine injection at 2 mL/kg and the rats were monitored for 50 min. Then each rat was removed from the chamber briefly to be injected s.c. with 0.4 mg/kg nicotine and monitored for another 90 min. A total of 6 rats were used in these experiments.
Assessment of the effect of passion flower extract on nicotine sensitization
To produce nicotine sensitization, rats were injected once/day for 4 consecutive days with either 0.4 mg/kg nicotine or an equal volume of vehicle. On day 5, known as the test day, rats were divided into four groups; half of those that had received vehicle on days 1–4 were pre-treated with passion flower extract (vehicle-passion flower group) and the other half with vehicle (vehicle-vehicle group). Analogously, half of the rats that received nicotine on days 1–4 received a pre-treatment of i.p. 1:1 water:glycerin (nicotine-vehicle group) on day 5, while the other half was given a pre-treatment of i.p. passion flower extract (nicotine-passion flower group). Each group contained 8 (vehicle-vehicle), 9 (nicotine-passion flower) or 10 (vehicle-passion flower and nicotine-vehicle) rats. Each rat was monitored in the spontaneous activity chamber for 100 consecutive min, starting approximately 23 h after the day 4 nicotine treatment. Before any injections on day 5, 4 rats at a time (1 from each treatment group) were placed in the activity chambers at time zero. An injection of passion flower extract or vehicle was administered at 25 min, and the animals were monitored for another 25 min. At that point all rats received an injection of nicotine and were monitored for another 50 min.
Data analysis
Mean velocity (in cm/s) over each 1 second period was calculated by the LimeLight software using images captured 6 times/sec, and that data was used to calculate the average and SEM of the mean velocities for each 5 min interval for each animal. The results for each 5 min interval for each animal in the same treatment group were averaged and the SEM was determined. Using mean velocity each second as opposed to total distance moved over a time interval allows the user to identify anomalous data points. When such a data point was discovered, the captured video of the session was examined for software errors in the tracking, and if such points were determined to be erroneous, they were omitted from the calculation of the average velocity for that 5 min interval. This happened for at most 6 values per session (over all 5 min intervals) or no more than 0.1% of all the values used for each animal. The values obtained using this method are the average velocity for each second of the 300 s interval, so the total distance moved in each interval (in cm) would be 300 sec × mean velocity (in cm/s). Thus, using average velocity over a specified time interval is the same mathematically as total distance moved as far as statistical analysis is concerned. Figures show the time at the end of the 5 min period (e.g., 10 min, is the 5–10 min interval). In the preliminary assay of nicotine versus vehicle, there were 6 rats, and in the chronic nicotine study there were 8–10 rats in each group. SAS (SAS Institute, Cary, NC) was used to model the data using repeated measures, mixed effect model, with independent factors treatment, time, and time by treatment interaction. The within-rat variance structure was modeled using the autoregressive heteroscedastic variance structure; the variance structure was determined by optimizing Akaike’s Information Criteria. Comparisons among treatments and within time points were performed on adjusted means using Fisher’s Protected Least Significant Difference multiple comparison procedure. In all cases, significance was determined as p < 0.05.
To assess the effect of the interaction between treatment and time, the 3 treatment degrees of freedom were partitioned into two orthogonal components: (a) Nicotine-vehicle vs remaining treatment groups (called comparator component, consisting of 1 degree of freedom, p < 0.0001), and treatment nested within comparator (consisting of 2 degrees of freedom, p = 0.4571). In the partition, the time by treatment interaction is partitioned into time by comparator component (p = 0.0364) and time by treatment within comparator component (p = 0.9808). The model suggests that the nicotine-vehicle treatment regimen is statistically significantly different than the remaining three treatment regimens, but that there was no significant difference, either in mean level or in time course, of the non-nicotine-vehicle treatment groups. Additionally, a simpler model of analysis of variance by time point was also constructed, with comparisons among the four treatment groups made by Tukey’s multiple comparison procedure. Results by time point were similar to the repeated measures mixed effect modeling.
Results
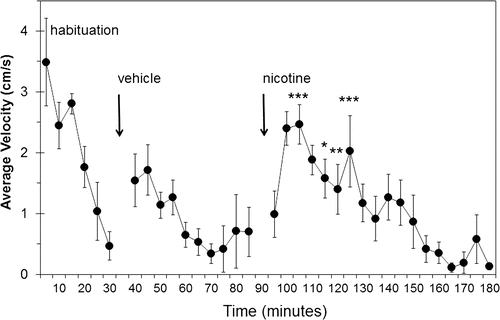
In the first assays, the length of time that it took for the rats to habituate to the spontaneous activity chambers, and the time course of the effects of a single dose of nicotine on locomotor activity were determined. These parameters were determined in order to have data on which to base the design of the subsequent experiment with regard to how long to leave the rats in the chamber before and after the injection of each agent. Drug-naïve rats were placed in the spontaneous activity chambers and their activity recorded until their locomotor activity had largely subsided. The amount of locomotor activity in the first 5 min was high (3.5 cm/s average velocity) and decreased to low levels (0.5 cm/s) during the 25–30 min interval (). Each rat was then given a s.c. injection of vehicle and immediately returned to a spontaneous activity chamber for another 50 min. Locomotor activity was initially higher than at the end of the habituation period (1.5–1.7 cm/s), but not as high as when first introduced to the chamber. It took about 25 min for the level of locomotor activity to decrease to between 0.5 and 0.7 cm/s where it remained for the rest of the session. At the end of this 50 min period, rats were injected s.c. with 0.4 mg/kg nicotine and again returned to the chambers for another 90 min (). While the amount of locomotor activity initially rose from the first to the second 5-min interval, from that point it generally declined from 2.5 to 0.8 cm/s over the first 60 min. During the last 30 min, the level of locomotor activity remained low at between 0.6 and 0.1 cm/s. The amount of locomotor activity was initially similar to, and not significantly different from, that seen in the vehicle-treatment phase. Activity after nicotine treatment peaked from 5–15 min after injection (intervals of 5–10 and 10–15 min), and was significantly different from vehicle treatment in the intervals between 10 and 35 min after injections (with the exception of the interval from 15–20 min) by 2-way ANOVA (treatment p < 0.0001) followed by Bonferroni’s posttest at p < 0.05. After nicotine treatment, rats moved between 2.5 and 1.4 cm/s (an average of 1.9 ± 0.2 cm/s) during the 10-35 min intervals, while during the same period after vehicle treatment the values were between 1.3 and 0.3 cm/s (an average of 0.8 ± 0.1 cm/s).
Figure 1. Spontaneous locomotor activity of rats following no treatment (habituation), or s.c. injections of vehicle or nicotine. Drug-naive rats were monitored in the spontaneous activity chamber for 30 min to determine the time course of habituation (habituation). Immediately afterward, each rat was injected s.c. with vehicle and monitored for 50 min (vehicle). Finally, each rat was injected s.c. with 0.4 mg/kg nicotine and monitored for another 90 min (nicotine). Data shown are the average ± standard error of the mean (SEM) of the mean velocity of each rat over each 5 min period of monitoring (n = 6). Gaps at 30–35 min and 90–95 min were due to interruption of data collection when animals were removed from the chambers for injection. Significant differences were determined by analysis of variance by time point, with comparisons among the four treatment groups made by 2-way analysis of variance followed by Bonferroni’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001 indicate time intervals at which nicotine was different from vehicle at the same time interval after the initiation of the respective phase of the experiment; that is, starting at 40 min for vehicle and at 95 min for nicotine.
An additional preliminary experiment examined whether the passion flower extract at the dose to be used had any effect on spontaneous activity of the rats by itself. Four rats/group were injected with 800 mg/kg passion flower extract before introduction to the spontaneous activity chambers and activity was monitored from the end of the previously-determined habituation period to the end of an hour (from 25–60 minutes after injection and introduction to the spontaneous activity chambers). Average spontaneous activity of the two groups was essentially the same (0.5–0.9 cm/s in each group) through the 35 minute period (data not shown).
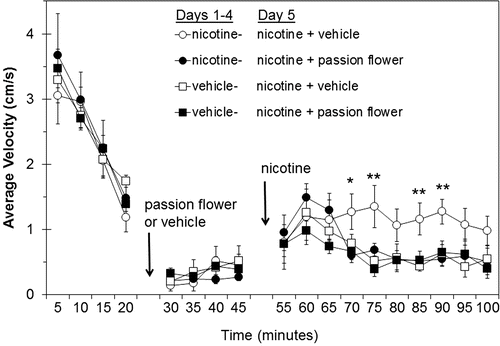
Once the habituation period and time to the peak effect of this dose of nicotine were determined, the nicotine sensitization experiments were begun. Based on the time course shown in , it was determined to allow a habituation period of 25 min before injection of the passion flower extract and to measure locomotion for at least 50 min after nicotine injection. In these experiments, rats were sensitized to nicotine by the administration of 0.4 mg/kg s.c. nicotine or vehicle (as a control) on days 1–4, and the testing was done on day 5. Similar to the preliminary study above, the rats began at a high level of spontaneous activity of approximately 3.5 cm/s then habituated to the spontaneous activity chambers (). At the last measurement during the habituation phase taken at 15–20 min, the activity for the four groups averaged 1.4 ± 0.1 cm/s (). Passion flower extract or its vehicle was administered i.p. during the 20 to 25 min interval and activity was assessed while allowing time for the extract to be absorbed. The activity of all groups remained low during this period with an average of 0.32 ± 0.03 cm/s from 25–45 min (). Between 45 and 50 min, nicotine was injected s.c. into all rats. Activity increased and peaked in all four groups at 55–60 min (). By 65–70 min the nicotine-vehicle group exhibited significantly greater activity than the other three, and this difference persisted up to the 90 min time point (note that the difference at 75–80 min was just not statistically significant). Significance was determined by repeated measures, mixed effect linear model where the effect of treatment was p < 0.0001. To determine differences between groups at specific time points, one-way ANOVAs comparing all four groups were performed at each time interval. In each case where a difference was noted above, the nicotine-vehicle group had significantly greater activity from the other three, while none of the other groups were different from each other.
Figure 2. Spontaneous locomotor activity of rats on the day of the pretreatment and nicotine challenge (day 5). Separate groups of 8–10 rats were treated once/day for four days (Days 1–4) with 0.4 mg/kg nicotine or vehicle. On day 5, each rat was monitored for spontaneous activity for approximately 25 min before i.p. pretreatment with passion flower or vehicle, and were then monitored for another 25 min before receiving an s.c. challenge dose of nicotine. Data shown are the average ± SEM of the mean velocity of each rat over each 5 min period of monitoring. Gaps at 20–25 min and 45–50 min were due to interruption of data collection when animals were removed from the chambers for injection. Significant differences between groups were determined by analysis of variance by time point, with comparisons among the four treatment groups made by Tukey’s multiple comparison procedure. *p < 0.05, **p < 0.01 indicates at each time point when the activity of the groups was different from each other. The nicotine-vehicle group was the only one that was different from any of the others at any time point.
Discussion
The overall goal of these studies was to determine whether an aqueous passion flower extract was able to decrease the signs of nicotine locomotor sensitization using rats. Consistent with a nicotine addiction model, administration of nicotine to rats resulted in increased locomotor activity and chronic administration resulted in sensitization, as indicated by a further increase in locomotion over what was seen after a single dose. Sensitization to consecutive doses of stimulants like nicotine is believed to play a role in addictive behaviors, and may be central to drug seeking and relapse (CitationKayir et al., 2009). Thus, sensitization may be a suitable target for testing therapies for treating or preventing addiction. Antagonism of nicotine locomotor sensitization in rats has been validated as a model for testing pharmacotherapies that may be useful in human smoking cessation (CitationKayir et al., 2009).
The first goal of the preliminary experiment (see ) was to determine how much time was necessary to allow the rats to habituate to the spontaneous activity chambers. It was important to determine the habituation period in order to assure that spontaneous activity would be low enough in the subsequent experiment to detect the nicotine-induced increase in locomotion. It was determined that a habituation period of 25–30 min was sufficient to decrease spontaneous locomotion to approximately 0.5 cm/s. This amount of time has previously been reported as a sufficient habituation time for these types of studies (CitationKayir et al., 2009). Moreover, the level of activity was the same as that seen after longer times in the activity chambers after vehicle injection, and thus was determined to be the level of spontaneous activity in habituated rats under these conditions.
The second goal of the first experiment was to determine the time course of the locomotor effects of the same dose of nicotine to be used in the subsequent experiments. It can be seen in that locomotor activity after 0.4 mg/kg nicotine was significantly greater than vehicle from 10 to 35 min after injection of each. The time course was similar to what was seen after the nicotine injection in the nicotine sensitization experiment shown in . In those studies the increases in activity occurred from 15–40 min after nicotine, as seen in the nicotine vehicle group.
The primary hypothesis of this study was that passion flower extract would antagonize the expression of nicotine locomotor sensitization, and that hypothesis was confirmed. Following the daily nicotine treatments that produced sensitization, the challenge dose of nicotine on the test day caused an increase in locomotion above that seen after an acute or first dose of nicotine. There are two ways in which pharmacotherapies for nicotine addiction are tested in motor sensitization models. One is antagonism of motor sensitization, and involves giving the test compound concurrent with nicotine during the induction of dependence, but not on the day of the challenge dose of nicotine. The other is known as antagonism of the expression of motor sensitization, and involves giving the test compound only once shortly before the administration of the challenge dose of nicotine. If a test drug treatment blocks the increased response to the challenge dose of nicotine in this model, it indicates antagonism of sensitization.
In these studies, the nicotine-vehicle group (control nicotine-sensitized group) showed significantly greater activity than the vehicle-vehicle group following the challenge dose of nicotine, reflecting nicotine sensitization. Comparison of the nicotine-vehicle and nicotine-passion flower groups illustrates that passion flower extract antagonized the expression of motor sensitization, since the nicotine-passion flower group showed the same level of activity as the non-sensitized vehicle-vehicle control group following nicotine challenge. Comparison of the vehicle-passion flower group with the vehicle-vehicle group in the period between the administration of passion flower extract and nicotine and after the administration of nicotine did not show any effect of passion flower alone on locomotor activity. Finally, none of the groups varied from one another in locomotor activity in the habituation or passion flower extract phases of the experiment, showing that the groups were similar in the basal levels of spontaneous activity, despite the fact that half of the rats had been injected for each of the previous four days with 0.4 mg/kg nicotine and the other half with vehicle.
The choice of an 800 mg/kg dose of Passiflora extract was based on a previous study (CitationSoulimani et al., 1997). This study showed a dose-response relationship for a hydroalcoholic Passiflora incarnata extract that plateaued at 800 mg/kg for antianxiolytic and sedative effects. As the current study was among the first to examine such a preparation for ameliorating signs of nicotine sensitization, we chose to use the dose at the higher end of the dose-response range so that effects, if present, could be detected. It must be noted that the preparation used in the current study is not the same as that used in the previous study (CitationSoulimani et al., 1997), and the concentration of the unidentified active constituents may have varied between studies.
Previous studies have demonstrated that chronic co-administration of a benzoflavone from passion flower with nicotine ameliorated nicotine withdrawal signs that were precipitated by the opioid antagonist naloxone in mice (CitationDhawan et al., 2002). In those studies, the passion flower extract was given together with the nicotine during the development of addiction. The present study differs from the previous in that it shows the effect of a single dose of a passion flower extract in a model of sensitization in rats. These factors make the present results more directly relevant to treatment of human addiction. First, smoking cessation therapies are administered after individuals are already addicted to nicotine to help ameliorate craving and prevent relapse. The present study used the model that more closely mimics human therapy, since the treatment was given to the rats after they had already developed sensitization (an indicator of developing dependence) to nicotine. This is not to suggest that humans would take passion flower extract i.p., since this preparation is intended for use orally. Finally, this study utilized a model more analogous human addiction, since humans do not take antagonist drugs to precipitate withdrawal from nicotine (as was done with the animals in the previous studies). Future studies planned include determining the composition and active chemical component(s) of the extract and human trials exploring the effectiveness of an oral dosage of complete passion flower extract or of active component(s).
Conclusions
The goal of these studies was to determine whether an aqueous passion flower extract may be able to treat nicotine sensitization. In the rat subjects, the extract of Passiflora incarnata did antagonize the expression of motor sensitization. This model used a paradigm analogous to how humans might use such an extract to alleviate nicotine seeking and help prevent relapse. Thus, a protocol similar to this one is worthy of further study in clinical trials of human smokers.
Acknowledgments
Christian Chiamulera, PharmD, MSc for expert guidance in conduct of the nicotine-induced hypermotility model, Campbell University students Tina Lee and Richard DeBenedetto for technical assistance, and Russell Reeve, PhD for review of statistical methodology.
Declaration of interest
The authors would like to thank the Departments of Pharmaceutical Sciences and Clinical Research of the Campbell University College of Pharmacy & Health Sciences for financial support. The authors report no declarations of interest.
References
- Akhondzadeh S, Kashani L, Mobaseri M, Hosseini SH, Nikzad S, Khani M. (2001a). Passionflower in the treatment of opiates withdrawal: A double-blind randomized controlled trial. J Clin Pharm Ther, 26, 369–373.
- Akhondzadeh S, Naghavi HR, Vazirian M, Shayeganpour A, Rashidi H, Khani M. (2001b). Passion flower in the treatment of generalized anxiety: A pilot double-blind randomized controlled trial with oxazepam. J Clin Pharm Ther, 26, 363–367.
- Benowitz NL. (1996). Pharmacology of nicotine: Addiction and therapeutics. Annu Rev Pharmacol Toxicol, 36, 597–613.
- Dani JA, De Biasi M. (2001). Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav, 70, 439–446.
- Dhawan K, Kumar S, Sharma A. (2002). Nicotine reversal effects of the benzoflavone moiety from Passiflora incarnata Linneaus in mice. Addict Biol, 7, 435–441.
- Dhawan K. (2003). Drug/substance reversal effects of a novel tri-substituted benzoflavone moiety (BZF) isolated from Passiflora incarnata Linn.–a brief perspective. Addict Biol, 8, 379–386.
- DiFranza JR, Wellman RJ. (2007). Sensitization to nicotine: How the animal literature might inform future human research. Nicotine Tob Res, 9, 9–20.
- Fiore M, Jaen CR, Baker TB. Bailey WC, Benett G. (2008). A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med, 35, 158–176.
- Kayir H, Goktalay G, Yildirim M, Uzbay TI. (2009). Clozapine inhibits development and expression of nicotine-induced locomotor sensitization in rats. Synapse, 63, 15–21.
- Krenn L. (2002). [Passion Flower (Passiflora incarnata L.)–a reliable herbal sedative]. Wien Med Wochenschr, 152, 404–406.
- Ksir C, Hakan RL, Kellar KJ. (1987). Chronic nicotine and locomotor activity: influences of exposure dose and test dose. Psychopharmacology (Berl), 92, 25–29.
- Li Z, DiFranza JR, Wellman RJ, Kulkarni P, King JA. (2008). Imaging brain activation in nicotine-sensitized rats. Brain Res, 1199, 91–99.
- Moeller DW, Sun LS. (2010). Chemical and radioactive carcinogens in cigarettes: Associated health impacts and responses of the tobacco industry, U.S. Congress, and federal regulatory agencies. Health Phys, 99, 674–679.
- Movafegh A, Alizadeh R, Hajimohamadi F, Esfehani F, Nejatfar M. (2008). Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: A double-blind, placebo-controlled study. Anesth Analg, 106, 1728–1732.
- Nassiri-Asl M, Shariati-Rad S, Zamansoltani F. (2007). Anticonvulsant effects of aerial parts of Passiflora incarnata extract in mice: Involvement of benzodiazepine and opioid receptors. BMC Complement Altern Med, 7, 26.
- Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O’Connell TN, Zandi KS, Miller S, Coe JW. (2006). Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos, 34, 121–130.
- Paterson NE, Balfour DJ, Markou A. (2007). Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. Eur J Neurosci, 25, 3099–3108.
- Rennard SI, Daughton DM. (2000). Smoking cessation. Chest, 117, 360S–364S.
- Soulimani R, Younos C, Jarmouni S, Bousta D, Misslin R, Mortier F. (1997). Behavioural effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. J Ethnopharmacol, 57, 11–20.
- Werneke U, Turner T, Priebe S. (2006). Complementary medicines in psychiatry: Review of effectiveness and safety. Br J Psychiatry, 188, 109–121.
- Zanoli P, Avallone R, Baraldi M. (2000). Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia, 71 Suppl 1, S117–S123.