Abstract
Context: The genus Primula (Primulaceae) has been used in traditional medicine to treat convulsion and microbial or viral infections.
Objective: In the present study, we evaluate antioxidant, antihemolytic, and protective effects of flavonoid-rich fractions of endemic Primula heterochroma Stapf. (Primulaceae) against Fe2+-induced lipid peroxidation and oxidative stress in rat brain in vitro.
Materials and methods: Aerial parts of plant were defatted and extracted with 60% acetone. Then, 60% acetone extract was fractionated sequentially with n-hexane, ethyl acetate, and water. Antioxidant activity of fractions was evaluated by employing six different assays, i.e., 1,1-diphenyl-2-picryl hydrazyl (DPPH) and hydrogen peroxide scavenging, metal chelating and reducing power activities and hemoglobin-induced linoleic acid system and Fe2+-induced lipid peroxidation and oxidative stress in rat brain. Also, its antihemolytic activity was determined using 2,2′-azobis(2-amidinopropane) dihydrochloride-induced hemolysis in rat erythrocyts.
Results: Among the flavonoid-rich fractions of Primula heterochroma, aqueous fraction demonstrated the most protective effect through decreasing brain thiobarbituric acid reactive substances (TBARS) levels at a dose 200 μg mL−1 (40%, p < 0.001 versus iron group). Also, the aqueous fraction showed better activity in Fe2+ chelating (89 ± 3.8 μg mL−1) and DPPH radical scavenging (394.4 ± 18.4 μg mL−1) models than other fractions. The probable protective mechanism of flavonoid-rich fractions may be attributed to their Fe2+ chelating, DPPH radical scavenging and reducing power activities. Also, the n-hexane fraction demonstrated a higher protective effect in the hemoglobin-induced linoleic acid system and 2,2′-azobis(2-amidinopropane) dihydrochloride-induced hemolysis (67 ± 2.6 μg mL−1).
Discussion and conclusion: Results of this study demonstrate Primula heterochroma is a rich source of natural antioxidant compounds.
Keywords::
Introduction
Lipid peroxidation inhibition has an important role in neuropathological injuries such as neurodegenerative, aging and other mental diseases (CitationHalliwell & Gutteridge, 1984). Oxidative stress in membrane lipids and proteins has been related to cerebral ischemia (CitationRehncrona et al., 1982). Cerebral tissues are favorable targets for oxidative stress because they have a high amount of polyunsaturated fatty acids (CitationHalliwell & Gutteridge, 1984). Iron ion induces lipid peroxidation in brain tissues through increases in the production of reactive oxygen species under in vitro conditions (CitationAndorn et al., 1996). Fenton reactions, occurring in ischemic reperfusion have been involved in the oxidative stress (CitationRehncrona et al., 1982). In ischemic reperfusion, an increase in production of reactive oxygen species, inducing lipid peroxidation, has been reported previously (CitationYoshida et al., 1985).
Flavonoids are an important class of natural polyphenolic compounds with beneficial effects in some diseases involving physiopathological events such as membrane lipid peroxidation. The ability of flavonoids to interact with membrane lipids and proteins, chelate iron and scavenge free radicals may be responsible for its different pharmacological effects (Citationvan Acker et al., 1996). Therefore, flavonoid topical administration can be useful for protection of iron-induced lipid peroxidation and oxidative stress.
Primula heterochroma Stapf. (Primulaceae) is an endemic member of the wild species of Primula genus in Iran (CitationWendelbo, 1965) which is native of Mazandaran forests, northern Iran. This endemic species is spread across some parts of the northern area (Gilan, Mazandaran, and Golestan) as well as in Talish part of Republic of Azerbaijan. To the best of our knowledge, there is no scientific report on the biological effects of Primula heterochroma.
The Primula genus is well known for its anticonvulsant activity in Iranian traditional medicine (CitationBasbülbül et al., 2008). Numerous biological and pharmacological effects including anxiolytic (CitationSufka et al., 2001), antimicrobial (CitationBasbülbül et al., 2008), antiviral and expectorant activities (CitationKati et al., 2001) have been reported from this genus.
The present study was performed to examine the antioxidant, antihemolytic, and inhibitory effect of flavonoid-rich fractions of Primula heterochroma against iron induced oxidative stress and lipid peroxidation in brain tissues.
Materials and methods
Chemicals
Trichloroacetic acid (TCA), Folin–Ciocalteau, 1,1-diphenyl-2-picryl hydrazyl (DPPH), hydrochloric acid, linoleic acid, ferrozine, butylated hydroxyanisole (BHA), quercetin, thiobarbituric acid (TBA), ascorbic acid, hydrogen peroxide, dinitrophenyl hydrazine (DNPH), gallic acid and methanol were purchased from Sigma-Aldrich Chemical Company. Sodium carbonate, potassium acetate, aluminium chloride (AlCl3), sodium dodecyl sulphate, Tris–HCl buffer, potassium ferricyanide, ferrous sulphate (FeSO4), and ferric chloride (FeCl3) were of analytical grade. Other chemicals were purchased at analytical grade or purer.
Animals
The study was performed on 8–12-week-old male Wistar rats (200–250 g), housed in ventilated animal rooms at 24 ± 2°C with a 12/12 h light/dark cycle and 60 ± 5% humidity. Animals were allowed for a week to acclimatize before starting of this study. This study was performed under the approval of the University of Mazandaran institutional animal care and use committee (Approval number: No.S-2009 UMZ).
Plant material
Fresh aerial parts of Primula heterochroma were collected from Darabkola forests, Mazandaran, Iran during April 2011 and identified by Dr. Alireza Naqinezhad. A voucher of this plant was deposited in the herbarium of the University of Mazandaran under number 1511. The materials were oven dried at 38°C, for 5 days.
Extraction of flavonoids
Plant powder (100 g) was defatted twice with 100 mL of chloroform and extracted twice with 100 mL of 60% acetone for 12 h at 25°C. The extract was then separated from the sample residues by filtration through Whatman No.1 filter paper. The solvent in the combined filtrates was evaporated at 30°C using a rotary evaporator, leaving the 60% acetone extract. After the preparation of a 10% methanol slurry, the extract was fractionated sequentially with 300 mL of each n-hexane, ethyl acetate, and water. The n-hexane, ethyl acetate, and aqueous fractions were used as flavonoid-rich fractions (CitationChung et al., 2009).
Phytochemical content
Determination of total phenolic content
Total phenolic content of flavonoid-rich fractions of Primula heterochroma was determined using the Folin–Ciocalteau method (CitationKuda et al., 2005). Briefly, samples (0.5 mL, 1.6 mg/mL) were mixed with 2.5 mL of 0.2 N of Folin–Ciocalteau reagent for 5 min and 2.0 mL sodium carbonate (75 mg/mL) was then added. The absorbance of reactions mixture was recorded at 760 nm after 2 h of incubation at 25°C. Results were calculated as gallic acid equivalents.
Determination of flavonoid content
Total flavonoid content was determined according to the method of CitationKuda et al. (2005). Briefly, 0.5 mL of samples were mixed with 1.5 mL of methanol, 0.1 mL of aluminum chloride (10%), 0.1 mL of potassium acetate (1 M), and 2.8 mL of distilled water and incubated at 25°C (30 min). The absorbance of the reaction mixture was recorded at 415 nm. Total flavonoid content was calculated as quercetin equivalents.
Antioxidant activity
DPPH radical scavenging
The stable DPPH radical was used for determination of free radical scavenging potential of fractions (CitationOboh et al., 2012). Briefly, different concentrations of samples solution were added to an equal volume of ethanol solution of DPPH (100 μM). After incubation at room temperature (15 min), the absorbances of samples were recorded at 517 nm. Vitamin C was used as standard control.
Reducing power
The reducing power ability of flavonoid-rich fractions of Primula heterochroma was measured according to the method of CitationPulido et al. (2000). Briefly, 2.5 mL of different concentrations of the samples (25–800 μg/mL) were mixed with 0.2 M of phosphate buffer (2.5 mL, pH 6.6) and 2.5 mL of potassium ferricyanide (1%). The reaction mixtures were incubated at 50°C for 20 min. Then, 10% of TCA (2.5 mL) was added to the reaction mixtures, which was centrifuged for 10 min (1000g). The upper layer of the reaction mixtures (2.5 mL) was mixed with distilled water (2.5 mL) and 0.1% of FeCl3 (0.5 mL) and the absorbances of the reaction mixtures were recorded at 700 nm. Vitamin C was used as positive control.
Metal chelating ability
The iron ions chelating by the fractions was measured by the method of CitationDinis et al. (1994). Briefly, 1 mL of different concentrations of samples (0.2–3.2 mg/mL) was added to 2 mM of FeCl2 (0.05 mL). Then, 5 mM of ferrozine (0.2 mL) was added. Reaction mixtures were shaken and incubated for 10 min at room temperature. Absorbance of the reaction mixtures were recorded at 562 nm. Ethylenediaminete traacetic acid (EDTA) was used as positive control.
Antihemolytic activity
Preparation of rat erythrocytes
Rats were anesthetized by administration of ketamine (60 mg/kg) and xylazine (5 mg/kg) and blood was collected from heart puncture. Erythrocytes were isolated and stored by the method described by CitationKuda et al. (2005). Separated erythrocytes were stored at 4°C and used within 6 h for further studies.
2,2′-Azobis(2-amidinopropane) dihydrochloride-induced hemolysis assay
To evaluate antihemolytic activity of the fractions against 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress in rat erythrocytes, an erythrocyte suspension (2% hematocrit, 1 mL) was preincubated with the different concentrations of each fractions (100–800 µg/mL, 1 mL) at 37°C for 30 min, followed by incubation with 2,2′-azobis(2-amidinopropane) dihydrochloride (50 mM, 1 mL). Reaction mixtures were shocked slowly while being incubated for 4 h at 37°C. Hemolysis was evaluated spectrophotometrically by the method of CitationKo et al. (1997). The hemolysis percentage was evaluated by recording the absorbance at 545 nm.
Lipid peroxidation inhibitory effect
Hemoglobin-induced linoleic acid system
Reaction mixtures (2 mL) containing different concentrations of the fractions (100–400 µg/mL), linoleic acid emulsion (1 mM), phosphate buffer (40 mM, pH 6.5), and hemoglobin suspension (0.0016%), were incubated at 37°C for 45 min. Then, an ethanol solution of hydrochloric acid (0.6%, 2.5 mL) was added to stop the lipid peroxidation. The amount of peroxide value was measured by thiocyanate method through recording absorbance at 480 nm after coloring with of FeCl2 (0.02 M, 100 µL) and of ammonium thiocyanate (0.3 g/mL, 50 µL). Ascorbic acid was used as positive control (CitationKuda et al., 2005).
Preparation of brain homogenates
The whole brain tissue was homogenized in 10 volumes of potassium dihydrogen phosphate buffer (100 mM) containing 1 mM EDTA (pH 7.4) and centrifuged at 12000 rpm for 30 min at 4°C.
Lipid peroxidation and thiobarbibutric acid reactions
Lipid peroxidation
Briefly, 100 µL of each fractions was mixed with a reaction mixture containing 30 µL of 0.1 M Tris–HCl buffer (pH 7.4) and 30 µL of FeSO4 solution (250 µM). The volume was made up to 300 µL with water before incubation at 37°C for 1 h. The color reaction was developed by adding 300 µL of sodium dodecyl sulphate (8.1%) to the reaction mixtures containing fractions (CitationNabavi et al., 2012a).
Evaluation TBARS level
Lipid peroxidation in terms of thiobarbituric acid reactive substances (TBARS) formation was determined by the method of CitationNabavi et al. (2012c). Reaction mixtures were incubated with 1 mL of TCA (20%) and 2 mL of thiobarbituric acid (0.67%) and was kept in a boiling water bath for 30 min. After cooling, the precipitate was removed by centrifugation for 15 min (5000g). The amount of TBARS formed was measured by recording the absorbance of the supernatant at 532 nm. Blank was contained all the reagents except samples.
Statistical analysis
The values were presented as means ± SD. Differences between group means were estimated using one-way analysis of variance followed by Duncan’s multiple range tests. Results were considered statistically significant when p < 0.05.
Results
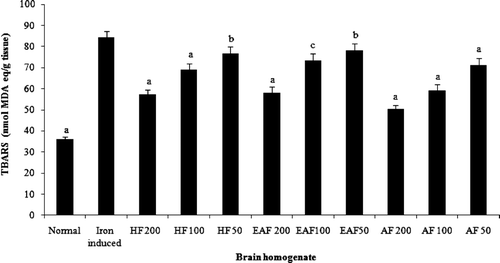
Total phenol and flavonoid content of flavonoid-rich fractions of Primula heterochroma are shown in . The aqueous fraction show better activity in DPPH radical scavenging (394.4 ± 18.4 μg mL−1) and iron chelating (89 ± 3.8 μg mL−1) than other fractions (). In the reducing power assay, recorded absorbance increasing indicates an increase in reducing ability. Reducing power ability of flavonoid-rich fractions is show in . Flavonoid-rich fractions of Primula heterochroma show a good effect in the hemoglobin-induced linoleic acid system (). n-Hexane fraction show better activity than others. Results show that flavonoid-rich fractions of Primula heterochroma inhibit AAPH-induced hemolysis (). The inhibitory role of flavonoid-rich fractions of Primula heterochroma against Fe2+ -induced lipid peroxidation and oxidative stress in rat brain are show in . Results demonstrate that Fe2+ increase TBARS level in rat brain tissues compared to the normal (p < 0.001). Fractions prevent Fe2+ induced oxidative stress through decreasing in TBARS level (). There is no significant difference between normal versus aqueous fractions at 200 µg/mL (p > 0.05).
Table 1. Phenol and flavonoid contents and antioxidant activities of Primula heterochroma fractions.
Figure 1. Reducing power of flavonoid-rich fractions of Primula heterochroma. Vitamin C used as positive control.
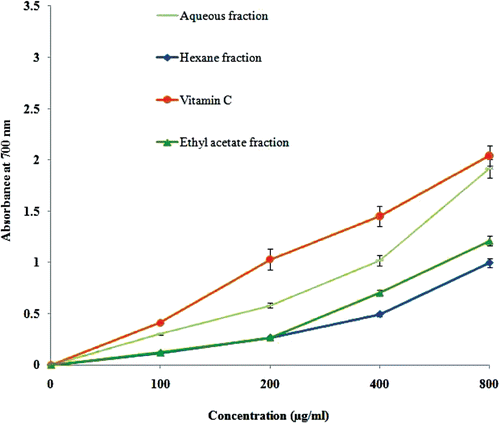
Figure 2. Antioxidant activity of flavonoid-rich fractions of Primula heterochroma against hemoglobin-induced lipid peroxidation. Vitamin C used as positive control.
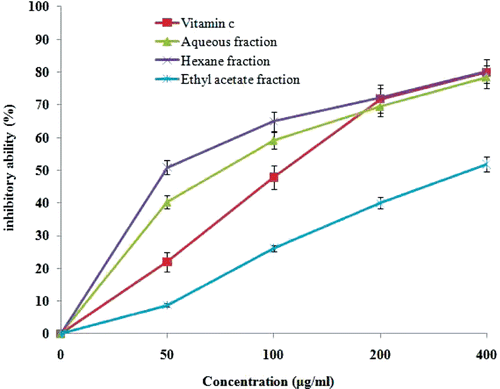
Figure 3. Protective effect of flavonoid-rich fractions of Primula heterochroma against Fe2+ induced lipid peroxidation in rat’s brain. Normal = lipid peroxidation without Fe2+ as pro-oxidants; iron induced = lipid peroxidation with Fe2+ as pro-oxidants alone; AF 200 = aqueous fraction (200 µg mL−1); AF 100 = aqueous fraction (100 µg mL−1); AF 50 = aqueous fraction (50 µg mL−1); HF 200 = hexane fraction (200 µg mL−1); HF 100 = hexane fraction (100 µg mL−1); HF 50 = hexane fraction (50 µg mL−1); EAF 200 = ethyl acetate fraction (200 µg mL−1); EAF 100 = ethyl acetate fraction (100 µg mL−1); EAF 50 = ethyl acetate fraction (50 µg mL−1). ap < 0.001 versus iron group. bp > 0.05 versus iron group. cp < 0.01 versus iron group.
Discussion
Natural product such as phenolics and flavonoids can protect the body against free radical induced toxicity through different antioxidative mechanisms (CitationNabavi et al., 2012b).
Previous reports exhibit that electron donor materials can scavenge DPPH radicals by electron or hydrogen donation and change the color of solutions from violet to yellow (CitationLópez & Calvo, 2011). Iron chelation therapy is a consequential method to diminish iron ion in iron mediated diseases and so improve life quality and generally survival in different iron mediated diseases such as thalassemia major. Lately, scientific research has focused on finding a natural compound as an iron chelator for chelation therapy with lower toxic effects. Furthermore, protective roles of chelator agents against neurodegenerative diseases development have been reported previously. For example, in Parkinson disease, chelated iron cannot contribute to oxidative stress and restrain dopaminergic midbrain neurons decay (CitationJomova et al., 2010). In DPPH and iron chelating models, aqueous fractions show better activity than other fractions and may correlate with its higher phenolic content (CitationLópez & Calvo, 2011).
In reducing power, flavonoid-rich fractions reduce Fe3+ to Fe2+ by electron donor phytochemical compounds. Perl’s Prussian blue color induced by Fe2+ complex can be monitored by absorbance recording at 700 nm (CitationKuda et al., 2005). The aqueous fraction shows better reducing power action than other fraction which may originate from its higher electron donor compounds such as phenols.
Erythrocytes are principal targets of free radicals, because of the presence of both polyunsaturated fatty acid membranes and oxygen associated with redox active hemoglobin, which are important promoters of reactive oxygen species. Lipid peroxidation inhibition by phytochemicals may be the result of their free radical scavenging potential. Lipid peroxidation is launched by superoxide, because superoxide anions act as a precursor of hydroxyl radical and singlet oxygen. Hydroxyl radical assault to membrane lipids and proteins by removing of hydrogen atoms which can induce lipid peroxidation (CitationNabavi et al., 2012c). In this method, n-hexane and aqueous fractions show better activity than the ethyl acetate fraction which may be the result of higher phytochemical content.
Under physiological conditions, AAPH decomposes strongly to produce peroxyl radicals. Peroxyl radicals cause oxidation of polyunsaturated fatty acids of erythrocytes membranes to induce lipid peroxidation. As a result of lipid peroxidation, erythrocyte membrane suffers injury quickly and loses integrity, which leads to hemolysis (CitationBanerjee et al., 2008). CitationBanerjee et al. (2008) exhibit that preincubation of erythrocytes with phytochemicals inhibit AAPH-induced hemolysis. In this study, we found that the n-hexane fraction shows potent antihemolytic effect which can be result of its higher flavonoid content.
Fe2+ caused lipid peroxidation may be associate with catalyzing one-electron transfer reactions effects, which produces reactive oxygen species. Also, Fe2+ generates peroxyl and alkoxyl radicals via lipid peroxides decomposition, which favors lipid oxidation production (CitationZago et al., 2000). However, flavonoid-rich fractions of Primula heterochroma showed a dose-dependent decrease in the TBARS level in brain tissues. The aqueous fraction showed a better protective role than others. Phytochemicals can protect cells by preventing/decreasing the production of reactive oxygen species or by scavenging/inhibiting of these free radicals which are generated in body or by chelating metal ions and reducing metal composition in the human diet (CitationOboh et al., 2012).
The flavonoid-rich fractions of Primula heterochroma were able to protect Fe2+-induced lipid peroxidation and oxidative stress in brain tissues. The good inhibitory role of flavonoid-rich fractions Primula heterochroma may be a result of their antioxidant effect. Also, flavonoid-rich fractions of Primula heterochroma show a good protective effect against AAPH-induced hemolysis in rat erythrocytes. In addition, the possible mechanisms of these protective effects of flavonoid-rich fractions may be through their radical scavenging, metal chelating and reducing power activities.
Conclusion
In this study, antioxidant and antihemolytic activities of flavonoid-rich fractions of Primula heterochroma have been evaluated using different models. Future studies on antioxidant mechanism and toxicological evaluation of these fractions are needed. The results of this study may be useful for further applications of this species in herbal formulations, drug discovery, and development.
Declaration of interest
The authors declare no conflicts of interest.
References
- Andorn AC, Britton RS, Bacon BR. (1996). Ascorbate-stimulated lipid peroxidation in human brain is dependent on iron but not on hydroxyl radical. J Neurochem, 67, 717–722.
- Basbülbül G, Özmen A, Biyik HH, Sen Ö. (2008). Antimitotic and antibacterial effects of the Primula veris L. flower extracts. Caryologia, 61, 88–91.
- Banerjee A, Kunwar A, Mishra B, Priyadarsini KI. (2008). Concentration dependent antioxidant/pro-oxidant activity of curcumin studies from AAPH induced hemolysis of RBCs. Chem Biol Interact, 174, 134–139.
- Chung SK, Chen CY, Blumberg JB. (2009). Flavonoid-rich fraction from Sageretia theezans leaves scavenges reactive oxygen radical species and increases the resistance of low-density lipoprotein to oxidation. J Med Food, 12, 1310–1315.
- Dinis TC, Maderia VM, Almeida LM. (1994). Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys, 315, 161–169.
- Halliwell B, Gutteridge JM. (1984). Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J, 219, 1–14.
- Jomova K, Vondrakova D, Lawson M, Valko M. (2010). Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem, 345, 91–104.
- Kati H, Ertürk Ö, Demirbag Z, Beldüz AO. (2001). Antiviral activity of Primula longipes extracts against baculovirus. Biologia, 56, 633–636.
- Ko FN, Hsiao G, Kuo YH. (1997). Protection of oxidative hemolysis by demethyldiisoeugenol in normal and β-thalassemic red blood cells. Free Radic Biol Med, 22, 215–222.
- Kuda T, Tsunekawa M, Goto H, Araki Y. (2005). Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J Food Comp Anal, 18, 625–633.
- López V, Calvo MI. (2011). White tea (Camellia sinensis Kuntze) exerts neuroprotection against hydrogen peroxide-induced toxicity in PC12 cells. Plant Foods Hum Nutr, 66, 22–26.
- Nabavi SF, Moghaddam AH, Eslami S, Nabavi SM. (2012a). Protective effects of curcumin against sodium fluoride-induced toxicity in rat kidneys. Biol Trace Elem Res, 145, 369–374.
- Nabavi SF, Nabavi SM, Abolhasani F, Moghaddam AH, Eslami S. (2012b). Cytoprotective effects of curcumin on codium fluoride-induced intoxication in rat erythrocytes. Bull Environ Contam ToxicoL, 88, 486–490.
- Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. (2012c). In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem, 132, 931–935.
- Oboh G, Akinyemi AJ, Ademiluyi AO. (2012). Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. rubra) and white ginger (Zingiber officinale Roscoe) on Fe(2+) induced lipid peroxidation in rat brain in vitro. Exp Toxicol Pathol, 64, 31–36.
- Pulido R, Bravo L, Saura-Calixto F. (2000). Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem, 48, 3396–3402.
- Rehncrona S, Westerberg E, Akesson B, Siesjö BK. (1982). Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem, 38, 84–93.
- Sufka KJ, Roach JT, Chambliss WG Jr, Broom SL, Feltenstein MW, Wyandt CM, Zeng L. (2001). Anxiolytic properties of botanical extracts in the chick social separation-stress procedure. Psychopharmacology (Berl), 153, 219–224.
- van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, Bast A. (1996). Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med, 20, 331–342.
- Wendelbo P. (1965). Primulaceae. In: Rechinger KH, ed. Flora Iranica. Vol. 9. Graz: Akademische Druck-U.
- Yoshida S, Busto R, Watson BD, Santiso M, Ginsberg MD. (1985). Postischemic cerebral lipid peroxidation in vitro: Modification by dietary vitamin E. J Neurochem, 44, 1593–1601.
- Zago MP, Verstraeten SV, Oteiza PI. (2000). Zinc in the prevention of Fe2+-initiated lipid and protein oxidation. Biol Res, 33, 143–150.