Abstract
Context: Currently, natural products have been shown to present interesting biological and pharmacological activities and are used as chemotherapeutic agents. Plants have historically been used in treating cancer and are recognized for their ability to produce secondary metabolites. Juglans regia L. (Juglandaceae) has medicinal applications to treat a wide range of diseases such as cancer.
Objective: The current study was designed to evaluate the antiproliferative activity of total extract as well as several fractions from the leaves of J. regia.The total phenolics, flavonoids, and condensed tannins content of these extracts were also determined to obtain further information on the correlation between the contents of phenolic compounds and antiproliferative effects as well as the leaf developmental stages.
Materials and methods: Antiproliferative activity was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and flow cytometry methods against human oral cancer, breast adenocarcinoma and colon adenocarcinoma cell lines. The total phenolics, flavonoids, and condensed tannins were determined by Folin-Ciocalteu, aluminum chloride and butanol-HCl colorimetric methods.
Results: Our present study has shown that chloroform fraction has the lowest IC50 values (0.36–0.81 mg/mL) and also induces cell cycle arrest (G0\G1 phase) after a 24 h treatment. The colorimetric methods showed the highest amount of total phenolics, flavonoids, and condensed tannins in the methanol fraction (120.28 ± 2.32, 59.44 ± 0.87, 227.00 ± 4.91 mg/g of dry weight of extract).
Discussion and conclusion: The results obtained herein indicate that walnut chloroform fraction may contain effective compounds which can be used as a chemotherapeutic agent.
Introduction
Cancer is one of the most eminent human diseases which have encouraged researchers worldwide to discover new anticancer agents. Plants have historically been used in treating cancer. It is important that over 60% of currently used anticancer agents are derived from natural sources including plants (CitationCragg & Newman, 2005). Plants are recognized for their ability to produce secondary metabolites (CitationCragg et al., 1999). Several of these natural products have been shown to present interesting biological and pharmacological activities and are used as chemotherapeutic agents (CitationVerpoorte, 1998, Citation2000). Epidemiological studies have consistently shown that there is a clear significant positive association between regular consumption of fruits, nuts, and vegetables, and a reduced incidence of some types of cancer, such as oral cavity, pharynx, and colon cancers (CitationBlock et al., 1992; CitationHeimendinger et al., 1996; CitationReddy et al., 2003; CitationJenab et al., 2004; CitationMathew et al., 2004).
The Juglans genus (Juglandaceae) includes several species and is widely distributed throughout the world. Juglans regia L. (walnut trees) is a well-known member found in temperate areas and cultivated commercially throughout southern Europe, northern Africa, Asia, United States, and western South America (CitationOliveira et al., 2008). It has long been known that walnut has medicinal applications to treat a wide range of diseases such as cancer (CitationKaur et al., 2003; CitationHardman & Ion, 2008). Some walnut extracts have shown antiproliferative activity against some cancer cell lines (CitationYang et al., 2009). Walnut leaves contain several therapeutically active constituents such as several phenolic compounds, flavonoids, and terpenoid substances (CitationNahrstedt et al., 1981; CitationPereira et al., 2007). Previous studies demonstrated that antiproliferative properties of walnut leaf extracts are likely related to its phenolic constituents and introduced methanol extract as an excellent source of natural antioxidant and chemopreventive agent (CitationCarvalho et al., 2010).
Interest in natural anticancer sources prompted us to continue investigating the cytotoxic activity of J. regialeaves. To our knowledge there are no published reports on the anticancer activity of different walnut leaf extracts including hexane, chloroform, ethyl acetate, and methanol in human oral, breast and colon carcinoma cell lines. Therefore, several fractions were assayed with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and flow cytometry methods in human oral squamous carcinoma (BHY), colon adenocarcinoma (HT-29) and breast adenocarcinoma (MCF7) cell lines. We also determined the total phenolics, flavonoids, and condensed tannins content of these extracts by Folin-Ciocalteu, aluminum chloride and butanol-HCl colorimetric methods to obtain further information on the correlation between the contents of phenolic compounds and antiproliferative effects as well as the leaf developmental stages.
Materials and methods
Chemicals and reagents
Hexane, chloroform, ethyl acetate, methanol, ethanol, and dimethyl sulfoxide (DMSO) were purchased from Merck (Darmstadt, Germany). MTT, quercetin, gallic acid, and Folin-Ciocalteu’s phenol reagent were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle Medium (DMEM), Roswell Park Memorial Institute medium (RPMI), fetal bovine serum (FBS), and penicillin-streptomycin were purchased from Gibco (Life Technologies Ltd, Paisley, UK). RNase was prepared from Fermentas (Fermentas Inc., Glen Burnie, MD, USA). All other chemicals were obtained from Merck.
Plant material and extraction procedure
J. regia leaves were collected from Tehran in June 2009. A voucher specimen (No. 6727 THE) was deposited at Herbarium of Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. The young and mature leaves were separated and dried in the dark for 3 days. Total extracts were prepared by thoroughly mixing 30 g of dried leaf powder with ethanol:water (80:20) (3 × 200 mL) at room temperature. In addition, 30 g of powder was extracted sequentially by solvents with different polarities including hexane, chloroform, ethyl acetate, and methanol using a maceration method. The partitioned extracts were filtered and concentrated to dryness under reduced pressure. The yield of extraction was 21.66% for both young and mature leaf extracts, while hexane, chloroform, ethyl acetate and methanol fractions yields were 8.66, 2.13, 2.68 and 12.01%, respectively.
Estimation of total phenolics, flavonoids, and condensed tannins
Phenolic extract solutions of all samples were prepared according to Wang’s method (CitationWang et al., 2007) with some modifications. Total phenolics, flavonoids, and condensed tannins content in the phenolic extract solutions were estimated by Folin-Ciocalteu, aluminium chloride and butanol-HCl colorimetric methods, respectively (CitationPorter et al., 1986; CitationMakkar et al., 1993; CitationChang et al., 2002). The results are expressed as mg of gallic acid and quercetin equivalent/g of dry leaf extract. Condensed tannins (% in dry leaf extract) are expressed as leukocyanidin equivalent.
Cell culture
The human cancer cells HT-29, MCF7, BHY, and normal cell mouse Swiss embryo fibroblast (NIH3T3) were obtained from the cell bank of Pasture Institute of Iran (NCBI) and DSMZ (German Collection of Microorganisms and Cell Cultures). Cells were cultured in RPMI 1640 or DMEM supplemented with 10% FBS, 2 mmol/L L-glutamine, 1% penicillin (100 U/mL) and streptomycin (100 µg/mL), and maintained at 37°C with 5% CO2 in a humidified atmosphere.
In vitro cytotoxicity assay
Total extracts and fractions were tested for their cytotoxic effects toward cancer and normal cell lines using the MTT assay. Cells were added in 96-well plates to yield 105 cells/well, then sample solutions were added at concentrations ranging from 0.25 to 1.5 mg/mL to each well and incubated for 24 h. DMSO (0.5%) treated cells served as the solvent control. Treated cells were incubated with MTT (0.5 mg/mL in phosphate buffered saline) for 4 h at 37°C. The medium was removed and dye crystal formazan was solubilized in DMSO. The absorbance was measured at 545 nm. Cell viability was measured as the percentage of absorbance compared with control. The 50% inhibitory concentration (IC50) value, defined as the amount of extract that inhibits 50% of cell growth, was calculated from concentration-response curves following a 24 and 48 h exposure times. Three independent experiments, performed in triplicate, were used for these calculations.
Flow cytometric analysis
Cell cycle phase distribution was determined by analytical DNA flow cytometry. MCF7 and BHY cells were incubated for 24 h with 0.25 and 0.5 mg/mL of chloroform fraction. Cells were harvested and adjusted to 7×105 cells/plate in 24-well plates and stained with propidium iodide (PI) reagent at 37°C for 15 min in the dark. PARTEC flow cytometer (Partec GmbH, Munster, Germany) with Flowjo software was used to analyze DNA content using UV light at FL2-A. The percentage of cells in the various phases was determined and statistical analysis of data from flow cytometric experiments was carried out.
Statistical analysis
Statistical analysis was performed using the SPSS version 16 software. Multiple comparisons between more than two groups were performed by one-way ANOVA supplemented with Tukey’s post hoc test. Significance was accepted at p < 0.05.
Results
Total phenolics, flavonoids, and condensed tannins content
Results of total phenolics, flavonoids and condensed tannins for total extracts and different fractions are displayed in . The total phenolics, flavonoids, and condensed tannins were significantly higher in total extracts than other fractions. also clearly indicates that the contents of these compounds are closely related to the age of walnut leaves. The amounts of phenolic compounds in total extracts from mature and young leaves of J. regia were in order of total phenolics (182.70 ± 2.60, 267. 30 ± 2.19 mg/g of dry leaf extract), flavonoids (96.89 ± 2.44, 149 ± 2.55 mg/g of dry leaf extract), and condensed tannins (237.42 ± 2.2, 950.56 ± 4.5 mg/g of dry leaf extract). According to these data, the contents of the phenolic compounds in young leaves were significantly (p < 0.05) () higher than their amounts in the mature leaves, which might be explained by synthesis of secondary metabolites. These results are in agreement with the observations made by CitationSalgado et al. (2008), who also found a relation between the amount of phenolic compounds and plant organ, age and phenologic stage as well as weather condition. Such a behavior may be explained by the morphology of young leaves, which are more vulnerable to external factors due to a lower level lignification that may require greater secondary metabolites.
Table 1. Contents of total phenolics, flavonoids and condensed tannins in J. regia leaf extracts.
Figure 1. Contents of total phenolics, flavonoids and condensed tannins in (A) young and mature leaf total extracts, and (B) hexane, chloroform, ethyl acetate and methanol fractions. Significant differences are indicated by *p< 0.05 in each group when total mature leaf extract was compared with young extract and various fractions were compared with methanol fraction. Values are presented as mean ± SE of three independent experiments.
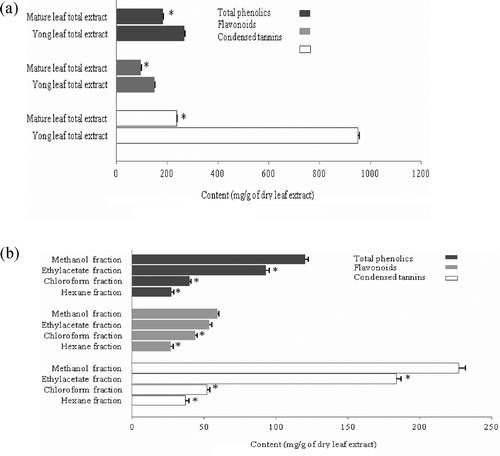
To further understand the difference in content of phenolic compounds between various fractions, a well-differentiated distribution diagram was obtained by determining the amounts of these compounds in various extracts. As shown in , the total phenolic value is much less in chloroform and hexane fractions in comparison with the methanol fraction which is the same for flavonoids and condensed tannins.
Human cancer cell antiproliferative activity of walnut leaf extracts
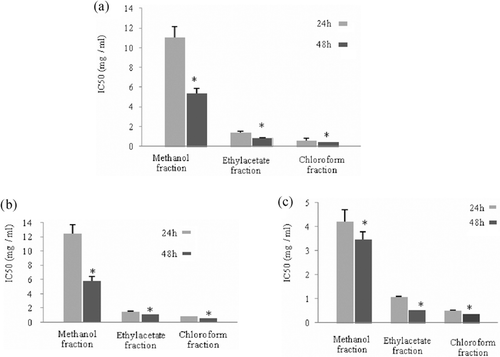
In this study, growth inhibitory activity of total extracts and different fractions from walnut leaves were screened against a panel of human cancer cell lines representing different histological types including BHY, MCF7, and HT-29 cells. For this purpose, cultured BHY, MCF7 and HT-29 cells were incubated in the absence and presence of 0.25–1.5 mg/mL of different leaf extracts for 24 and 48 h. These extracts caused loss of cell viability in a concentration-dependent manner. IC50 values for various extracts in three different cell lines were calculated (,). A considerable difference in sensitivity of the cell lines to total (mature and young) walnut leaf extracts was evident. Our results indicated that MCF7 and BHY cells were best inhibited by total extracts after 48-h treatment; however HT-29 cells were not inhibited by them. There was no significant difference (p < 0.05) between IC50 values of young and mature extracts in the three cell lines at 24 and 48 h ().
Table 2A. IC50 values (mg of total extracts/mL) for antiproliferative activity of young and mature leaf extracts towards BHY, MCF7, and HT-29 cells. Values are presented as mean ± SE of three independent experiments, performed in triplicate.
Table 2B. IC50 values (mg of fractions/mL) for antiproliferative activity of different fractions towards BHY, MCF7, and HT-29 cells. Values are presented as mean ± SE of three independent experiments, performed in triplicate.
In addition, it was our main observation that growth of three cell lines was inhibited after 24 and 48 h exposure to various leaf fractions. The IC50 values obtained for antiproliferative activities of these fractions in BHY, MCF7 and HT-29 cell lines are presented in . Some differences were observed in terms of cell line and fraction. On the other hand, MCF7 showed a better inhibitory activity toward different fractions and chloroform fraction indicated more remarkable antiproliferative efficiency at 24 and 48 h in the three cell lines. In addition, there was a significant difference between 24 and 48 h treatments of the three cell lines with various fractions. According to , the lowest IC50 values belong to the 48 h treatment. These results are also indicative of a weak correlation between the phenolic content and cytotoxicity activity. On the other hand, certain classes of phytochemicals may notably influence antiproliferative activity.
Figure 2. Evaluation of time effect on IC50 values of methanol, ethyl acetate and chloroform fractions in (A) human oral cancer, (B) breast adenocarcinoma, and (C) colon adenocarcinoma cells. Antiproliferative activity of fractions was evaluated after 24 and 48 h treatments. Values are presented as mean ± SE of three independent experiments, performed in triplicate. Significant differences are indicated by *p< 0.05 in each group relative to 24 h samples.
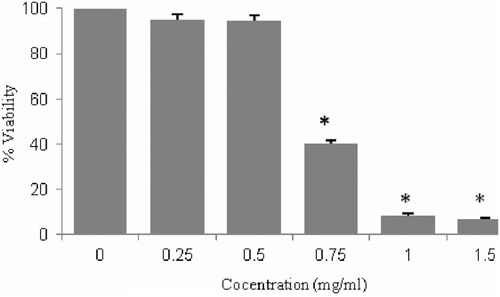
In order to evaluate cell cytotoxicity of the chloroform fraction in normal cell lines, the viability assay of this fraction was performed on NIH3T3 as normal cells after 48 h incubation. No effect on cell viability was observed in cells treated with 0.5 mg/mL of the chloroform fraction compared to control cells ().
Figure 3. Concentration effectiveness of chloroform fraction on proliferation of mouse Swiss embryo fibroblast cells. Five different concentrations of this fraction (0.25–1.5 mg/mL) were applied for 48 h. Values are presented as mean ± SE of three independent experiments, performed in triplicate. Bars marked with * are significantly different compared with the control. (p < 0.05).
Effect of chloroform fraction on cell cycle arrest
Cell cycle progression of MCF7 and BHY cells in the presence of chloroform fraction was monitored by flow cytometry. Cultured MCF7 and BHY cells were incubated with two concentrations of the chloroform fraction (0.25 and 0.5 mg/mL) for 24 h, stained with PI and analyzed by flow cytometry in order to determine the total population distribution in the different phases (G0/G1, S, and G2/M). Control cells treated only with DMSO using the highest amount into the experiments proceeded through a normal cell cycle. When MCF7 cells were treated with 0.5 mg/mL of chloroform fraction it was observed a statistically significant increase of cell population in phase sub-G1 from 8.25% for control to 53.81% after treatment. In addition, treatment of BHY cells with 0.5 mg/mL of chloroform fraction led to the enrichment of sub-G1 cell population going from 11.9% for control to 47.35% after treatment, while cell population in G0/G1 decreased significantly for MCF7 and BHY treated cells ().
Table 3. Effect of chloroform fraction on cell cycle progression with respect to control.a
These observations together with the cytotoxicity data imply that the growth inhibition of these cancer cells produced by chloroform fraction is probably due to a combination of apoptosis and cell cycle derangements in which G0/G1 arrest is a key event.
Discussion
Cancer is a multi-mechanistic disease that has many sides and requires a wide range approach for its treatment, control and prevention. Cancer is the second largest disease which has a sizable contribution in the total number of deaths (CitationMarimuthu, 2008). The World Cancer Report indicates that cancer rates are increasing at an alarming rate globally. Plants have been used for prevention and the treatment of human diseases from distant past, also of cancer diseases. J. regia has been reported as the anticancer plant used in folk medicine. J. regia species has been asserted to possess anticancer activities. Yang et al. demonstrated a strong antiproliferative effect of walnut seed extract against human HepG2 liver and Caco-2 colon cancer cells (CitationYang et al., 2009). The anticancer capacities of these nuts are usually assigned to their chemical composition. It was also well documented that walnut leaf methanol extract showed antiproliferative activity in both A-498, 769-P renal, and Caco-2 colon cancer cells with IC50 ranging from 0.226 to >0.5 mg/mL (CitationCarvalho et al., 2010). Reported anticancer activity of some walnut extracts offers additional advantage in its use (CitationKaur et al., 2003). Since differential cytotoxicity is also a useful feature for potential antitumor agents, the cytotoxicity activity of the walnut leaf extracts was evaluated in both cancer and normal cell lines by the MTT assay. In this study, we examined the inhibitory effects of various leaf extracts, including total extract, hexane, chloroform, ethyl acetate, and methanol extracts of J. regia collected from central parts of Tehran, Iran on BHY, MCF7, and HT-29 cell lines. All J. regia leaf extracts showed degrees of inhibitory effects on cultured cancer cell lines. For BHY, MCF7, and HT-29 cells, chloroform and ethyl acetate fractions revealed good cytotoxicity effects and chloroform fraction was the most active, especially on MCF7 and BHY cell lines. We also confirmed the antiproliferative activity of chloroform fraction on cell cycle progression of MCF7 and BHY cells using flow cytometry. Since plant phenolics constitute one of the major groups of compounds and have a protective role in carcinogenesis, it was reasonable to determine their total amounts in various leaf extracts to discover if there is any correlation between anticancer properties and the amount of phenolic compounds. It is interesting to know that there was not any significant relation and these properties seem more likely related to other chemicals such as steroids or tepenoids.
Conclusion
The results of this study demonstrated for the first time that walnut leaf chloroform fraction possess effective human oral and breast cancer antiproliferative activity. However, with a holistic approach, the most sensitive cell line was breast cancer. The cells exposed to the chloroform fraction exhibited the features of programmed cell death such as an increase in the percentage of cells with a sub-G1 DNA content and G0/G1 phase cycle arrest. Currently, the available data could find no correlation between these properties and the phenolic constituents. According to the strong antiproliferative effects of chloroform fraction, it is likely that anticancer effects attributed to this fraction may be based on synergistic or additive interactions of many compounds present in it. Therefore, further studies will be aimed at investigating the substances responsible for anticancer property of the chloroform fraction.
Acknowledgments
This work was supported by the Pasteur Institute of Iran. The authors gratefully thank staff at Quality Control and Viral Departments, especially Narges Miandehi, for their valuable assistance.
Declaration of interest
The authors declare no conflicts of interest.
References
- Block G, Patterson B, Subar A. (1992). Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer, 18, 1–29.
- Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, Silva BM. (2010). Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol, 48, 441–447.
- Chang CC, Yang MH, Wen HM, Chern JC. (2002). Estimation of total flavonoid content in Propolis by two complementary colorimetric methods. J Food Drug Anal, 10, 178–182.
- Cragg GM, Newman DJ. (2005). Plants as a source of anti-cancer agents. J Ethnopharmacol, 100, 72–79.
- Cragg GM, Boyd, MR, Khanna R, Kneller R, Mays TD, Mazan KD, Newman DJ, Sausville EA. (1999). International collaboration in drug discovery and development: The NCI experience. Pure Appl Chemistry, 71, 1619–1633.
- Hardman WE, Ion G. (2008). Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer, 60, 666–674.
- Heimendinger J, Van Duyn MA, Chapelsky D, Foerster S, Stables G. (1996). The national 5 A Day for Better Health Program: A large-scale nutrition intervention. J Public Health Manag Pract, 2, 27–35.
- Jenab M, Ferrari P, Slimani N, Norat T, Casagrande C, Overad K, Olsen A, Stripp C, Tjønneland A, Boutron-Ruault MC, Clavel-Chapelon F, Kesse E, Nieters A, Bergmann M, Boeing H, Naska A, Trichopoulou A, Palli D, Krogh V, Celentano E, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Ocké MC, Peeters PH, Engeset D, Quirós JR, González CA, Martínez C, Chirlaque MD, Ardanaz E, Dorronsoro M, Wallström P, Palmqvist R, Van Guelpen B, Bingham S, San Joaquin MA, Saracci R, Kaaks R, Riboli E. (2004). Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev, 13, 1595–1603.
- Kaur K, Michael H, Arora S, Härkönen PL, Kumar S. (2003). Studies on correlation of antimutagenic and antiproliferative activities of Juglans regia L. J Environ Pathol Toxicol Oncol, 22, 59–67.
- Ludwiczuk A, Saha A, Kuzuhara T, Asakawa Y. (2011). Bioactivity guided isolation of anticancer constituents from leaves of Alnus sieboldiana (Betulaceae). Phytomedicine, 18, 491–498.
- Makkar HPS, Blummel M, Borowy NK, Becker K. (1993). Gravimetric Determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric, 61, 161–165.
- Marimuthu P. (2008). Projection of cancer incidence in five cities and cancer mortality in India. Indian J Cancer, 45, 4–7.
- Mathew A, Peters U, Chatterjee N, Kulldorff M, Sinha R. (2004). Fat, fiber, fruits, vegetables, and risk of colorectal adenomas. Int J Cancer, 108, 287–292.
- Nahrstedt A, Vetter U, Hammerschmidt FJ. (1981). Composition of the steam distillation product from the leaves of juglans regia (author’s transl). Planta Med, 42, 313–332.
- Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L, Pereira JA. (2008). Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol, 46, 2326–2331.
- Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, Ferreres F, Bento A, Seabra R, Estevinho L. (2007). Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol, 45, 2287–2295.
- Porter LJ, Hrstich LN, Chan BG (1986). The conversion of procyanidin and prodelphinidins to cyaniding and delphindin. Phytochemistry, 25, 223–230.
- Reddy L, Odhav B, Bhoola KD. (2003). Natural products for cancer prevention: A global perspective. Pharmacol Ther, 99, 1–13.
- Salgado PR, Favarin JL, Leandro RA, de Lima Filho OFL. (2008).Total phenol concentration in coffee tree leaves during fruit development. Sci Agric, 65, 354–359.
- Verpoorte R. (1998). Exploration of nature’s chemodiversity: The role of secondary metabolites as leads in drug development. Drug Discover Today, 3, 232–238.
- Verpoorte R. (2000). Pharmacognosy in the new millennium: Lead finding and biotechnology. J Pharm Pharmacol, 52, 253–262.
- Wang YN, Shi GL, Zhao LL, Liu SQ, Yu TQ, Clarke SR, Sun JH. (2007). Acaricidal activity of Juglans regia leaf extracts on Tetranychus viennensis and Tetranychus cinnabarinus (Acari: Tetranychidae). J Econ Entomol, 100, 1298–1303.
- Yang J, Liu RH, Halim L. (2009). Antioxidant and antiproliferative activities of common edible nut seeds. Food Sci Technol, 42, 1–8.