Abstract
Context: Black mustard [Brassica nigra (L.) Koch] of the Brassicaceae (Cruciferae) family is commonly used as a spice and a cheap source of antimicrobial agents for bacterial infections.
Objectives: The present investigation was to demonstrate the protective effect of the methanol extract of B. nigra leaves against d-galactosamine (d-GalN)-induced hepatic and nephrotoxicity in Wistar rats.
Methods: Activity of the methanol extract of B. nigra at doses of 200 and 400 mg/kg b.wt. against d-GalN (500 mg/kg b.wt.) induced toxicity, with silymarin used as the standard. Histological damage, activities of serum marker enzyme, hematological changes, metabolites such as bilirubin, urea, uric acid, and creatinine levels, tissue thiobarbutric acid reactive substance, enzymic and non-enzymic antioxidants and inflammatory marker enzymes such as myeloperoxidase, cathepsin D, and acid phosphatase were assessed.
Results: The d-GalN-induced toxicity was evident from a significant increase (p < 0.001) in the serum and tissue inflammatory markers in toxic rats, when compared with the control (saline alone treated animals). The B. nigra pretreated groups (200 and 400 mg/kg b.wt.) showed significant (p < 0.001) reduction in the d-GalN-induced toxicity as obvious from biochemical parameters. Histopathological observations confirm the protective effect of B. nigra leaf extract by reduction in hepatic and renal tissue damage. Experimentals extract showed a similar effect as the standard.
Conclusions: The crude methanol extract of B. nigra leaf lacks inherent toxicity and exhibits hepatic and nephroprotective effects against d-GalN-induced toxicity in Wistar rats.
Introduction
The liver is an indispensible exocrine organ of the human body which performs detoxification of various xenobiotics such as drug metabolites and alcohol and helps in maintaining homeostasis (CitationRathi et al., 2008). During the detoxification process, liver cells frequently experience stress due to oxidative damage from free radicals. Progressive impairment of liver cells due to oxidative stress would lead to life threatening complications if left untreated (CitationWolf, 1999). CitationAnand et al. (2002) reported d-galactosamine-induced acute liver failure results in renal damage and is mediated by endothelin receptors. Recently, the term hepatorenal syndrome (HRS) has been introduced to define the development of renal failure related with hepatocellular dysfunction (CitationEpstein, 1996). Although several chemosynthetic products are recommended for liver therapy and to prevent renal damage most of them prove to be immunosuppressive. To solve this problem, studies on novel therapeutic strategies using plant product based drugs are in the process of vigorous testing (CitationLiu & Xiao, 1994; CitationLin et al., 1998; CitationVenkateswaran et al., 1998; CitationGerbes et al., 2006).
Brassica nigra (L.) Koch (Brassicaceae) is commonly known as black or brown mustard (CitationNair & Henry, 1983). The black mustard has a stronger pungent flavor than the white and brown species. B. nigra has been used in traditional herbal medicine for a long time, especially the seeds as rubiefacient poultice. B. nigra is cultivated worldwide. In addition to its significance as a food flavoring ingredient, the seeds of B. nigra have important medicinal uses in treatment of rheumatism and joint pains, indurations of the liver and spleen, throat tumors and as a laxative (CitationGerald & Williams, 1989; CitationObi et al., 2009). Recent reports revealed that B. nigra was found to have antioxidant and antimicrobial activities (CitationObi et al., 2009; CitationHussein et al., 2010). Although the therapeutic properties of B. nigra seeds have been clearly elucidated in earlier studies, there is little scientific information about the pharmacological activities of leaves of the plant. Recently, we studied in vitro antioxidant property and phytochemical constituents of the B. nigra leaf extract (CitationRajamurugan et al., 2011). The HPTLC analysis of B. nigra leaf extract quantitatively revealed the presence of phenolics compounds such as gallic acid (4.31 mg/g), quercetin (0.91 mg/g), ferulic acid (0.76 mg/g), caffeic acid (0.55 mg/g) and rutin (0.36 mg/g). The GC-MS analysis showed that B. nigra extract consists of a mixture of bioactive compounds such as α-amyritin, l-proline, 5-oxo-methyl ester, 1,2-benzenedicarboxylic acid, di-isooctyl ester, 1,4-dichloro-benzene, etc. (CitationRajamurugan et al., 2011). Hence, the present study evaluated hepatic and nephroprotective effects of the leaf extract against d-GalN-induced liver and nephrotoxicity in Wistar albino rats.
Materials and methods
Experimental animals
Adult albino Wistar rats of weight variation not exceeding ±20% of the mean weight (130–160 g) were selected for the present study. Animals were procured from Tamil nadu Veterinary and Animal Sciences University, Madhavaram milk colony, Chennai, India. All the animals were acclimatized to laboratory conditions for a week before the commencement of experiments. The animals were housed in polycarbonate cages (three rats in each cage) at controlled room temperature of 28–30°C and a relative humidity between 30 and 70% and a constant 12 h light:dark cycle. The rats had free access to water and dry rat pellets ad libitum. The experiments were carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India and the experimental protocol was approved by the institutional animal ethics committee (CPCSEA/CRIS/PHARMA/54/2008).
Plant material and extraction
Plant material (B. nigra leaves) was collected between October and November 2008, around the villages of Panruti, Postal code-607805, Tamil Nadu, India and authenticated by Prof. Dr. P. Jayaraman, Director, Plant Anatomy Research Centre, National Institute of Herbal Science, Chennai, South India (PARC/2008/560).
About 500 g of the shade-dried, powdered leaves of B. nigra was exhaustively extracted in a Soxhlet extractor by continuous hot percolation with methanol. The residue was filtered and concentrated under reduced pressure and the extract (yield = 11.2%) was used for evaluating hepatic and nephroprotective effect.
Chemicals
Phenazonium methosulphate, nitroblue tetrazolium (chloride), nicotine adenine dinucleotide hydride, thio barbituric acid, butylated hydroxyltoluene, sodium azide, d-GalN, reduced glutathione (GSH), were purchased from Sigma Chemical Co., St. Louis, USA. Tris-HCl buffer, 5,5′-dithiobis 2-nitrobenzoic acid, 2,4-dinitrophenylhydrazine, ascorbic acid, sodium acetate buffer, hexadecyl trimethyl ammonium bromide, O-dianizidine and all other chemicals and solvents were of analytical grade and obtained from S.D. Fine Chemicals, Mumbai, India. Kits were obtained from Accurex Biomed Pvt Ltd and BAYER Pvt Ltd, India.
Hepatoprotective activity study
Animals were divided in to six groups of six animals each (comprising three males and three females). Group I animals served as control and received only vehicle (saline) for 21 days. Group II animals served as toxic control received vehicle (saline) for 21 days. Groups III, IV and V were prophylactically treated with silymarin (100 mg/kg, p.o.), B. nigra extract 200 and 400 mg/kg (p.o.), respectively, for 21 days. Group VI received B. nigra extract (400 mg/kg, p.o.) alone for 21 days. Groups II, III, IV and V received d-GalN (500 mg/kg, i.p.) on the 22nd day. After 24 h of d-GalN administrations, blood was collected under mild anesthesia. Immediately after blood withdrawal, all the animals were sacrificed by cervical decapitation and tissue samples were collected. Hematological, biochemical, and histological parameters were observed in all groups.
Hematological parameters
Blood samples from the sacrificed animals were analyzed for hematological parameters such as total white blood cells (WBC) count, total lymphocytes (LYM), total monocytes (MONO), total granulocytes (GRAN), lymphocyte percentage (LYM%), monocyte percentage (MONO%), granulocyte percentage (GRAN%), total red blood cells (RBC), hemoglobin (HGB%), hematocrit (HCT%), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red blood cell distribution width (RDW), platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW%) and plateletctrit (PCT) using a BC-2800 Vet AGAPPE Auto hematology analyzer.
Serum biochemistry
The hemolysis free serum sample from the clotted blood was analyzed for the following parameters: blood glucose, cholesterol, total bilirubin, total protein, albumin, globulin, urea, uric acid and creatinine. The enzymes analyzed were serum glutamate oxaloacetate transaminase (SGOT), serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH) and γ-glutamyl transferase (γ-GT). The biochemical analysis of serum sample was performed using BAYER–RA-50 semi auto analyzer, BAYER kits and reagents.
Hepatic and renal antioxidant studies
The liver and renal tissues were excised from the rats and homogenized with 10% KCl to make a 10% (w/v) homogenate. The homogenate was centrifuged at 10,000g for 15 min in a refrigerated centrifuge at 4°C. The supernatant was used for antioxidant studies such as superoxide dismutase (SOD) (CitationKakkar et al., 1984), thiobarbutric reactive substances (TBARS) (CitationOhkawa et al., 1979), glutathione peroxidase (GPx) (CitationRotruck et al., 1973), reduced glutathione (GSH) (CitationMoren et al., 1979), vitamin C (CitationOyaizu, 1986), vitamin E (CitationPilar et al., 1999) and tissue inflammatory markers such as cathepsin D (CitationSapolsky et al., 1973) and myeloperoxidase (MPO) (CitationBradley et al., 1982).
Biochemical markers in liver and kidney
The enzyme parameters: ALP, ACP, AST, ALT, γ-GT, and LDH in liver and kidney tissues were measured using Accurex kits, Accurex Biomed Pvt Ltd, Mumbai. Liver and kidney tissue homogenate supernatant (50 μl each) were taken and the activity was measured in a semiautoanalyzer, STAR21plus, USA.
Histological examinations
Liver and kidney were removed from the sacrificed animals, flushed with saline for removal of blood clot and preserved in 10% buffered formalin for histological examination. The paraffin blocks of tissue were microtomed and paraffin sections of thickness 5 μm were obtained. The sections were deparaffinized with alcohol-xylene series and stained with hematoxylin and eosin for examination by light microscopy.
Statistical analysis
The differences among experimental and control groups were determined using SPSS15.0 statistical software. Comparisons were performed by ANOVA test. Resulting data were expressed as mean ± SD (n = 6). p < 0.05 was considered significant.
Results
Hematological parameters
shows the hematological changes in control and different experimental groups. Lymphocytes% and PLT were significantly (p < 0.001) lower in (group II) d-GalN intoxicated rats (p < 0.001) when compared with control (group I) rats. The levels of lymphocytes% and PLT were increased in group III (p < 0.001), group IV and group V (p < 0.05) when compared with group II animals. However, there were no statistical significance in group III, group IV and group V when compared with normal control (group I) rats. The granulocytes% was significantly increased (p < 0.001) in (group II) d-GalN treated toxic rats (p < 0.001) compared to the control rats (group I).The silymarin and B. nigra extract treated groups (group III, IV and V) showed values similar to control group but were significantly (p < 0.05) lower when compared to group II. Other hematological parameters were not significantly altered among the groups as compared with the normal control rats ().
Table 1. Effect of methanol extract of B. nigra on hemocomponents in control and different experimental groups.
Serum biochemistry
A significant increase in serum glucose (p < 0.001), cholesterol (p < 0.001), total bilirubin (p < 0.001), urea (p < 0.001), uric acid (p < 0.001) and creatinine (p < 0.001) were observed in animals treated with d-GalN (group II) as compared the with the normal control group (group I). B. nigra extract (200 and 400 mg/kg, p.o) and silymarin (100 mg/kg, p.o) pretreatment for 21 days decreased serum glucose (p < 0.001), cholesterol (p < 0.001), total bilirubin (p < 0.001), urea (p < 0.001), uric acid (p < 0.001) and creatinine (p < 0.001) in groups IV, V and III, respectively, when compared with toxic rats (group II). The activities of SGOT (p < 0.001), SGPT (p < 0.001), ALP (p < 0.001), LDH (p < 0.001) and γ-GT (p < 0.001) were found to be increased whereas, decreased levels of total protein (p < 0.001), and albumin (p < 0.001) [globulin-not significant] were observed in animals intoxicated with d-GalN (group II) when compared with normal control group (group I). Pretreatment with B. nigra extract (200 and 400 mg/kg, p.o) and silymarin (100 mg/kg, p.o) for 21 days decreased activities of SGOT (p < 0.001), SGPT (p < 0.001), ALP (p < 0.001), LDH (p < 0.001) and γ-GT (p < 0.001) whereas, the total protein (p < 0.001) and albumin (p < 0.001) were significantly increased in groups IV, V, and III when compared with group II rats. In B. nigra extract alone treated rats (group VI) levels of metabolites and activities of markers enzymes in serum were similar to control group ().
Table 2. Effect of methanol extract of B. nigra on serum biochemical components in control and different experimental groups.
Tissue enzymic and non-enzymic antioxidants
A significant increase in TBARS (p < 0.001) level was observed in liver and kidney tissues of d-GalN intoxicated rats (group II) when compared with control rats (group I), on the other hand the enzymic and non-enzymic antioxidants such as SOD (p < 0.001), GPx (p < 0.001), GSH (p < 0.001), vitamin C (p < 0.001) and vitamin E (p < 0.001) were decreased. Pretreatment with B. nigra extract (group IV and V) and silymarin (group III) significantly decreased TBARS and increased the activities of SOD (p < 0.05) and GPx (p < 0.05) in liver and kidney tissues when compared with group II rats, the non-enzymic antioxidant levels [GSH (p < 0.05), vitamin C (p < 0.05) and vitamin E (p < 0.05)] were also increased when compared with d-GalN intoxicated rats (group II). In B. nigra extract alone treated rats (group VI), levels of TBARS, GSH, vitamin C and vitamin E, the activities of SOD and GPx in liver and kidney were similar to that of control group ( and ).
Table 3. Effect of methanol extract of B. nigra on lipid peroxidation and antioxidant components in liver of control and experimental groups.
Table 4. Effect of methanol extract of B. nigra on lipid peroxidation and antioxidant components in kidney of d-galactosamine intoxicated rats.
Inflammatory markers
The changes in the activities of ACP, MPO and cathepsin D in liver and kidney of the various experimental groups are shown in and . A marked increase in activities of ACP (p < 0.001), MPO (p < 0.001) and cathepsin D (p < 0.001) in liver and kidney tissues were observed in d-GalN intoxicated rats (group II), whereas pretreatment with B. nigra (group IV and V) and silymarin (group III) decreased the activities significantly (p < 0.001). In B. nigra extract alone treated rats (group VI) activities of these enzymes were found to be similar as that of control rats ( and ).
Table 5. Effect of methanol extract of B. nigra on inflammatory markers in liver of different experimental groups.
Table 6. Effect of methanol extract of B. nigra on inflammatory markers in kidney of d-galactosamine intoxicated rats.
Tissue enzymes
In d-GalN intoxicated rats (group II), the activities of marker enzymes, viz., ALP (p < 0.001), AST (p < 0.001), ALT (p < 0.001), LDH (p < 0.001) and γ-GT ((p < 0.001) were found to be significantly decreased in liver and kidney tissues when compared with control (group I) rats ( and ). Whereas, in B. nigra extract (group IV and V) and silymarin (group III) pretreated rats, activities of these enzymes, in liver and kidney, were found to be significantly (p < 0.001) improved when compared with group II rats. In B. nigra extract alone treated rats (group VI), the activities of marker enzymes were found to be similar with that of control rats.
Table 7. Activities of some marker enzymes in liver of different experimental groups.
Table 8. Effect of B. nigra on activities of some marker enzymes in kidney of different experimental groups.
Histopathological observations of liver and kidney
Histology of the liver and kidney sections of normal control animals treated with saline vehicle alone (group I) shows normal architecture ( and ). Animals treated with d-GalN alone (group II) show severe toxicity characterized by scattered inflammation across liver parenchyma, sinusoidal congestion, vacuolar degeneration and necrosis of hepatocytes in liver sections () tubular epithelial cell degeneration, necrosis and perivascular mononuclear cell infiltration in kidney sections (). B. nigra extract pretreatment (group IV and V) appeared to alleviate d-GalN toxicity as revealed by reduction in degeneration of hepatocytes of liver sections and tubular epithelial cells of kidney sections when compared with group II rats ( and and ). Rats pretreated with silymarin (group III) exhibited protection against d-GalN induced degenerative changes in the liver and kidney ( and ). Histology of the liver and kidney of rats pretreated with B. nigra extract alone (group VI) shows normal architecture as control animals (group I) ( and ). We did not observe any differences in liver or kidney histopathological (biochemical analysis also) sections obtained in male and female of different experimental rats.
Figure 1. Histological observation in liver sections (Hematoxylin and Eosin staining) of different experimental groups. (A) control, (B) toxic control (d-galactosamine, 500 mg/kg, i.p.), (C) standard (silymarin, 100 mg/kg), (D) B. nigra extract (200 mg/kg, p.o.) + d-galactosamine treated, (E) B. nigra extract (400 mg/kg, p.o.) + d-galactosamine treated, and (F) B. nigra extract (400 mg/kg, p.o.) alone treated.
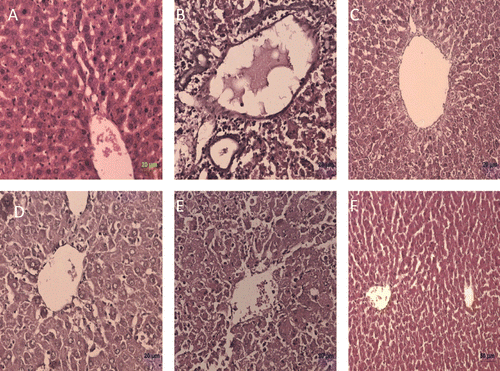
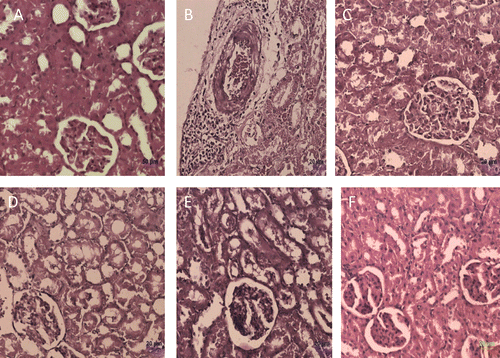
Figure 2. Histological observation in renal sections (Hematoxylin and Eosin staining) of different experimental groups. (A) control, (B) toxic control (d-galactosamine, 500 mg/kg, i.p.), (C) standard (Silymarin, 100mg/kg), (D) B. nigra extract (200 mg/kg, p.o.) + d-galactosamine treated, (E) B. nigra extract (400 mg/kg, p.o.) + d-galactosamine treated and (F) B. nigra extract (400 mg/kg, p.o.) alone treated.
Discussion
Study of herbal drugs is gaining more attention due to their ameliorating effect on acute and chronic disease conditions. The plant extracts have been used in traditional medicines for centuries, since they act as a source of antioxidants and efficient pharmacophores. In the present investigation, protective effects of the B. nigra methanol extract were studied in d-GalN induced liver and kidney damages. d-GalN induced liver damage in rats is a model system which is recognized to be much similar to viral hepatitis in humans from both morphological and physiological point of view (CitationKeppler et al., 1968). d-GalN causes hepatic injury with spotty necrosis of hepatic parenchyma and marked portal and parenchymal infiltration (CitationKeppler & Decker, 1969). d-GalN also causes depletion of uridine diphosphate (UDP) by increasing the formation of UDP-sugar derivatives, which results in inhibition of RNA and protein synthesis leading to cell membrane deterioration (CitationDecker et al., 1973; CitationEI-Mofty et al., 1975).
Javle et al. (1998) reported that d-GalN-induced liver injury is associated with alteration in renal hemodynamics thus altering its function. Recently the term hepatorenal syndrome (HRS) has been introduced to define the development of renal failure in the absence of its clinical, anatomical and pathological causes. Classically, HRS is associated with the end-stage liver cirrhosis and it has been observed that renal failure occurs with this liver disease in about 50% of patients. CitationAnand et al. (2002) reported that d-GalN intoxication causes liver injury followed by renal impairment which is manifested by the decrease in renal blood flow and creatinine clearance. Renal dysfunction due to d-GalN administration was also manifested by the increase in serum urea and creatinine levels and kidney TBARS and MPO activity together with degenerative changes in kidney histology (CitationTunc et al., 2010).
d-GalN administration in rats disrupts the membrane permeability of the plasma membrane causing leakage of the enzymes and metabolites from the cell, which leads to elevation in activities of serum enzyme and metabolites (CitationMitra et al., 2000). Elevated serum enzymes and metabolites are indicative of cellular leakage and loss of functional integrity of the cell membrane in liver (CitationDrotman & Lawhorn, 1978). As a result of the cellular leakage, activities of these enzymes in liver tissue were also decreased. In our study, significant increase in the activities of serum enzymes, viz., SGOT, SGPT, ALP, LDH and γ-GT, the levels of metabolites such as total bilirubin, urea, uric acid and creatinine were also increased in d-GalN intoxicated rats. We also observed decreased levels of the total protein and albumin in d-GalN intoxicated rats with concomitant decrease in activities of ALP, AST, ALT, γ-GT and LDH in liver and kidney. Pretreatment with B. nigra extract and silymarin reversed these biochemical alterations, indicating that B. nigra extract has protective effects against d-GalN-induced liver and kidney injury.
A detailed mechanism of the hepato-and nephrotoxic effects induced by d-GalN has not been found, but the toxicity might be mediated through the immune system or oxidative stress. Vitamin E and vitamin C are naturally occurring free radical scavengers (CitationYu, 1994). Both vitamin E and vitamin C are known to be decreased in liver diseases maybe due their increased utilization for scavenging oxygen-derived radicals (CitationJohnson et al., 1987). Glutathione is an important endogenous antioxidant system that is found in particularly high concentration in liver and is known to have key functions in protective processes. The GSH becomes readily oxidized (GSSG) upon interacting with free radicals. Excessive production of radicals results in oxidative stress, which leads to disruption of the macromolecules, e.g. lipids and can induce lipid peroxidation (CitationSinclair et al., 1991). In present investigation, there was a significant decrease in the activities of tissue enzymic antioxidants such as SOD and GPx and increase in the level of TBARS with a concomitant decrease in levels of nonenzymic antioxidants such as vitamin E, vitamin C and GSH in d-GalN intoxicated rats (group II) which indicate severity of oxidative stress induced as a result of administration of d-GalN. Considerable increase in the activities of antioxidant enzymes, decrease in the level of TBARS, and increased levels of GSH, vitamin C, and vitamin E in the B. nigra extract pretreated rats (group IV and V) clearly indicate that B. nigra extract possesses in vivo antioxidant effect. We have shown that B. nigra extract possesses in vitro antioxidant activity (CitationRajamurugan et al., 2011).
Lysosomal enzyme activities in inflammatory exudates serve as a good marker to assess the intensity of inflammation in experimental groups. Hydrolytic enzymes are released by the rupture of the lysosomal membrane, which in turn initiates the synthesis of inflammatory mediators. Drugs capable of stabilizing the lysosomal membrane can reduce the inflammation (CitationAzza & Mohamed, 1995). An important mechanism of anti-inflammatory activity is due to the membrane stability–modulating effect (CitationDe et al., 1994). Cathepsin D has been found to play a role in the intracellular degradation of exogenous and endogenous proteins (CitationIgdoura et al., 1995). The proteolytic activity of cathepsin D is increased during various pathogenic processes leading to injury of lysosomes (CitationEiki et al., 1991). Extensive infiltration of leukocytes leads to an increase in lysosomal hydrolases (CitationAnderson, 1970). The marked decrease in liver and kidney tissue ACP, cathepsin D and MPO activities in extract treated groups (groups IV and V) indicates that the B. nigra may have membrane stabilization effect. Many sesquiterpenes are found to possess anti-inflammatory activity (CitationHall et al., 1979). Thus the anti-inflammatory activity may be due to the terpenoids that may be present in the B. nigra extract. B. nigra seed extract has shown, one of the excellent sources of terpenoids (CitationJakupovic et al., 1986; CitationKakali et al., 1997). Our previous findings show that B. nigra leaf also contains terpenoids (CitationRajamurugan et al., 2011). The present study suggests that methanol extract of B. nigra may also possess anti-inflammatory activity.
d-GalN is reported to produce intensive inflammatory infiltration in the liver and kidney parenchyma and peripheral area (CitationKeppler et al., 1968; CitationTunc et al., 2010). In our study, d-GalN administration shows severe hepato-and nephrotoxic effects with heavy infiltration of inflammatory cells around the parenchyma of liver and kidney. Silymarin is a well known hepatoprotective drug in wide usage; hence, the hepatoprotective effect of B. nigra was compared with that of silymarin (CitationPradhan & Girish, 2006). Pretreatment with B. nigra leaf extract and silymarin protected the rat liver and kidney from d-GalN- induced histopathological changes. The hepatoprotective effect might have been contributed by hepatoprotectants such as (Z, Z)- 9,12-octadecadienoic acid (linoleic acid) and α-amyrin (triterpenoid) and/or also due to presence of various antioxidant present in the extract, which was reported from our recent investigation (CitationRajamurugan et al., 2011).
Conclusions
On the basis of the results obtained in the present study, it is evident that the methanol extract of B. nigra leaves exhibit protective effect against d-GalN-induced hepatic and renal injury. Biochemical observations were supported by histological examinations of liver and kidney. The group treated with B. nigra extract alone proved that the extract is non-toxic and is safe. Based on the antioxidant and anti-inflammatory effects of extract from B. nigra leaves it may be suggested as a remedy in treatment of hepatic and renal injury.
Acknowledgments
One of the authors (R.R) is very grateful to the Director, additional director, research officers, staff in department of pharmacology and biochemistry, Siddah Central Research Institute, Chennai, India and Prof. S. P. Thyagarajan, Pro-chancellor (Research), Sri Ramachandra University, Chennai, India. He also acknowledges Head and the department of veterinary pathology, Madras Veterinary College, Chennai.
Declaration of interest
The authors declare no conflicts of interest.
References
- Anand R, Harry D, Holt S, Milner P, Dashwood M, Goodier D, Jarmulowicz M, Moore K. (2002). Endothelin is an important determinant of renal function in a rat model of acute liver and renal failure. Gut, 50, 111–117.
- Anderson AJ. (1970). Lysosomal enzyme activity in rats with adjuvant-induced arthritis. Ann Rheum Dis, 29, 307–313.
- Azza MA, MohamedZG. (1995). Lipid peroxidation and lysosomal integrity in different inflammatory models in rats: The effects of indomethacin and naftazone. Pharmacol Res, 32, 279–285.
- Bradley PB, Pribat DA, Christensen RO, Rothstein G. (1982). Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol, 78, 206–209.
- Decker K, Keppler D, Pausch J. (1973). The regulation of pyrimidine nucleotide level and its role in experimental hepatitis. Adv Enzyme Regul, 11, 205–230.
- Drotman RB, Lawhorn GT. (1978). Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol, 1, 163–171.
- Eiki K, Takashi U, Daisaku M, Nobuhiko K. (1991). The selective role of cathepsins B and D in the lysosomal degradation of endogenous and exogenous proteins. FEBS Letters, 287, 189–192.
- EI-Mofty SK, Scrutton MC, Serroni A, Nicoloni C, Farber JL. (1975). Early reversible plasma membrane injury in galactosamine induced liver cell death. Am J Pathol, 79, 579–596.
- Epstein M. (1996). The kidney in liver disease. 4th ed. Edited by Epstein M. Hepatorenal syndrome. Philadelphia: Hanley and Belfus Inc. 75–108.
- Gerald ET, Williams C. (1989). Oil Crops of the World. 2nd ed. Oklahoma: Royal Botanic Gardens Inc. pp 341, 355–356.
- Gerbes AL, Avila MA, Caselmann WH. (2006). Liver injury and liver protection: Mechanisms and novel treatment strategies. Liver Int, 26, 902–903.
- Hall IH, Lee KH, Starnes CO, Sumida Y, Wu RY, Waddell TG, Cochran JW, Gerhart KG. (1979). Anti-inflammatory activity of sesquiterpene lactones and related compounds. J Pharm Sci, 68, 537–542.
- Hussein EA, Taj-Eldeen AM, Alzubairi AS, Elhakimi AS, Al-Dubaie AR. (2010). Phytochemical screening, total phenolics and antioxidant and antibacterial activities of callus from Brassica nigra L. hypocotyl explants. Int J Pharmacol, 6, 464–471.
- Igdoura SA, Morales CR, Hermo L. (1995). Differential expression of cathepsins B and D in testis and epididymis of adult rats. J Histochem Cytochem, 43, 545–557.
- Jakupovic J, Banerjee S, Castro V, Bohlmann F, Schuster A, Msonthi JD, Keeley S. (1986). Poskeanolide, a seco-germacranolide and other sesquiterpene lactones from Vernonia species. Phytochemistry, 25, 1359–1364.
- Javlé P, Yates J, Kynaston HG, Parsons KF, Jenkins SA. (1998). Hepatosplanchnic haemodynamics and renal blood flow and function in rats with liver failure. Gut, 43, 272–279.
- Johnson J, Bjorneboe A Morland J, Drevon CA. (1987). Effect of heavy alcohol consumption on serum concentration of fat-soluble vitamins and selenium. Alcohol Alcohol Suppl 1, 533–537.
- Kakali S, Pulok K, Mukherjee JD, Pal M, Saha BP. (1997). Wound healing activity of Leucas lavandulaefolia Rees. J Ethnopharmacol, 56, 139–144.
- Kakkar P, Das B, Viswanathan PN. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys, 21, 130–132.
- Keppler D, Lesch R, Reutter W, Decker K. (1968). Experimental hepatitis induced by D-galactosamine. Exp Mol Pathol, 9, 279–290.
- Keppler D, Decker K. (1969). Studies on the mechanism of galactosamine-1-phosphate and its inhibition of UDP-glucose pyrophosphorylase. Eur J Biochem, 10, 219–225.
- Lin CC, Yen MH, Lo TS, Lin JM. (1998). Evaluation of the hepatoprotective and antioxidant activity of Boehmeria nivea var. nivea and B. nivea var. tenacissima. J Ethnopharmacol, 60, 9–17.
- Liu J, Xiao PG. (1994). Recent advances in the study of antioxidative effects of Chinese medicinal plants. Phytother Res, 8, 445–451.
- Mitra SK, Seshadri SJ, Venkataranganna MV, Gopumadhavan S, Udupa UV, Sarma DN. (2000). Effect of HD-03–a herbal formulation in galactosamine-induced hepatopathy in rats. Indian J Physiol Pharmacol, 44, 82–86.
- Moren MS, Desplerra JW, Mannervik B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activity in rat liver and lung. Biochem Biophys Acta, 585, 67–71.
- Nair NC, Henry AN. (1983). Flora of Tamil Nadu, Series–I, Vol. I. Coimbatore, India: Botanical Survey of India, southern - Circle, 159–169
- Obi RK, Nwanebu FC, Ndubuisi UU, Oriji NM. (2009). Antibacterial qualities and phytochemical screening of the oils of Circuit pepo and Brassica nigra. J Med Plants Res, 3, 429–432.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Oyaizu M. (1986). Studies on products of the browning reaction. Antioxidative activities of browning reaction products prepared from glucosamine. Jp J Nutr, 44, 307–315.
- Pilar P, Manuel P, Miguel A. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem, 269, 337–341.
- Pradhan SC, Girish C. (2006). Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res, 124, 491–504.
- Rajamurugan R, Selvaganabathy N, Kumaravel S, Ramamurthy C, Sujatha V, Thirunavukkarasu C. (2011). Polyphenol contents and antioxidant activity of Brassica nigra (L.) Koch. leaf extract. Nat Prod Res.
- Rathi A, Srivastava AK, Shirwaikar A, Singh Rawat AK, Mehrotra S. (2008). Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine, 15, 470–477.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.
- Sapolsky AI, Altman RD, Howell DS. (1973). Cathepsin D activity in normal and osteoarthritic human cartilage. Fed Proc, 32, 1489–1493.
- Sinclair AJ, Barnett AH, Lunie J. (1991). Free radical and antioxidant systems in health and disease. J App Med, 17, 409–412.
- De S, Ravishankar B, Bhavsar GC. (1994). Investigation of the anti-inflammatory effects of Paederia foetida. J Ethnopharmacol, 43, 31–38.
- Tunc C, Ozlem S, Refiye Y, Sehnaz B . (2010). Protective effects of antioxidant combination against d-galactosamine–induced kidney injury in rats. Cell Biochem Funct, 28, 107–113.
- Venkateswaran S, Pari L, Viswanathan P. (1998). Antiperoxidation effect of liver, and herbal formulation against erythromycin estolate induced lipid peroxidation in rats. Phytother Res, 12, 465–471.
- Wolf PL. (1999). Biochemical diagnosis of liver diseases. Indian J Clin Biochem, 14, 59–60.
- Yu BP. (1994). Cellular defenses against damage from reactive oxygen species. Physiol Rev, 74, 139–162.