Abstract
Context: Bauhinia purpurea L. (Fabaceae) is a native plant species of many Asian countries, including Malaysia and India. In India, the root, stem, bark, and leaf of B. purpurea are used to treat various ailments, including ulcers and stomach cancer.
Objective: In an attempt to establish its pharmacological potential, we studied the antiulcer activity of lipid-soluble extract of B. purpurea obtained via extraction of air-dried leaves using chloroform.
Materials and methods: The rats were administered the chloroform extract (dose range of 100–1000 mg/kg) orally after 24 h fasting. They were subjected to the absolute ethanol- and indomethacin-induced gastric ulcer, and pyloric ligation assays after 30 min. The acute toxicity study was conducted using a single oral dose of 5000 mg/kg extract and the rats were observed for the period of 14 days. omeprazole (30 mg/kg) was used as the standard control.
Results: At 5000 mg/kg, the extract produced no sign of toxicity in rats. The extract exhibited significant (p < 0.05) dose-dependent antiulcer activity for the ethanol-induced model. The extract also significantly (p < 0.05) increased the gastric wall mucus production and pH of gastric content, while significantly (p < 0.05) reducing the total volume and total acidity of the gastric content in the pylorus ligation assay.
Discussion and conclusion: The extract possesses antiulcer, antisecretory and cytoprotective activities, which could be attributed to its flavonoid and tannin content. These findings provide new information regarding the potential of lipid-soluble compounds of B. purpurea for the prevention and treatment of gastric ulcers.
Introduction
Gastric ulcer affects a large portion of the human population and is caused by several factors such as ingestion of nonsteroidal antiinflammatory drugs (NSAIDs), stress, smoking, and nutritional deficiencies (CitationNash et al., 1994). The pathophysiology of this disorder has focused on the imbalance between aggressive and protective factors in the stomach including acid–pepsin secretion, mucosal barrier, mucus secretion, blood flow, cellular regeneration, prostaglandins, and epidermal growth factors (CitationLima et al., 2006). The current approach to control gastric ulceration is to promote gastroprotection, to inhibit gastric acid secretion, to block apoptosis, and to stimulate epithelial cell proliferation for healing (CitationBandyopadhyay et al., 2002). Proton pump inhibitors (e.g. omeprazole and lansoprazole) and the histamine H2-receptor blockers (e.g. ranitidine and famotidine) are widely used to treat this disease. However, there are reports of adverse effects related to the long term usage of these drugs (CitationMartelli et al., 1998; CitationWolfe & Sachs, 2000).
This has increased the need to find new antiulcer agents with less or possibly no side effects from other sources. Plant-based extracts have been one of the attractive sources of new drugs with some of them demonstrated to possess promising gastroprotective activity (CitationHiruma-Lima et al., 2001). One of the plants traditionally used to treat gastric ulcer is Bauhinia purpurea L. (Fabaceae), which is native of southern Asia, southeast Asia, Taiwan, and China. In Malaysia, the tree is known as ‘tapak kerbau’ or ‘tapak kuda’, while in India, it is known as ‘kachnar’ or ‘khairwal’ (CitationZakaria et al., 2007). The root, stem, bark, and leaf of B. purpurea are also reported to be used in the treatment of jaundice, leprosy, cough, pain, fever, ulcers, stomach cancer, rheumatism, convulsions, delirium, and septicaemia (CitationChopra et al., 1956; CitationAsolker et al., 2000; CitationParrota, 2001; CitationKirthikar & Basu, 2001; CitationJanardhanan et al., 2003). In India, the root of B. purpurea is used for the treatment of diarrhea, ulcer, boils, and abscesses (CitationKirthikar & Basu, 2001), while in Pakistan, the fresh and dried flower buds of B. purpurea are used as a food material while the leaves, stems, and roots are also widely used to treat infections, pain, diabetes, jaundice, leprosy, and cough (CitationMorais et al., 2005). Scientifically, the plant has been reported to possess several pharmacological activities. The leaves of B. purpurea exerted antidiarrheal (CitationMukherjee et al., 1998), antispasmodic, and antimicrobial activities (Citationda Silva et al., 2000), antinociceptive, antiinflammatory, and antipyretic (Citationda Silva et al., 2000; CitationZakaria et al., 2007a), and antioxidant (CitationZakaria, 2007b) activities. Despite the traditional use of B. purpurea in the treatment of ulcer, no scientific study has been carried out to confirm this. Thus, in the present study the potential antiulcer activity of chloroform extract of the leaf of B. purpurea was investigated using various animal models.
Materials and methods
Chemicals and drugs
Absolute ethanol was purchased from Fischer Scientific (Pittsburgh, PA, USA) while indomethacin (≥99%) and omeprazole (99%) were purchased from Sigma Chemical Co. (St. Louis, MI, USA). Other reagents used for the experiments were all of analytical grade. All drugs and reagents were prepared immediately before use.
Plant material and extract preparation
The leaves of B. purpurea were collected from its natural habitat in Shah Alam, Selangor, Malaysia between June and September 2009. The plant has been re-identified by Dr. Shamsul Khamis, a botanist at the Institute of Bioscience (IBS), Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia, based on the voucher specimen (SK 1095/05) deposited earlier at the Herbarium of the Laboratory of Natural Products, Institute of Bioscience, UPM, Serdang, Selangor, Malaysia. The dried leaves were ground into small pieces and soaked in chloroform in the ratio of 1:20 (w/v) for 72 h. After that, the supernatant was filtered using cotton wool followed by Whatman No. 1 filter paper. Then, the supernatant was evaporation using a rotary evaporator at 40°C under reduced pressure. This extraction process was repeated three times using the same residues. This method was carried out according to CitationZakaria et al. (2007c). The crude dried chloroform extract of B. purpurea leaf (CEBP) was prepared at doses of 100, 500 and 1000 mg/kg prior to use following acute toxicity study.
Phytochemical screening and HPLC profiling of the chloroform extract of B. purpurea leaf (CEBP)
Phytochemical screening of the CEBP
The phytochemical screening of CEBP was carried out according to the methods described by CitationIkhiri et al. (1992).
Preparation of sample for HPLC analysis
Crude dried CEBP (10 mg) was dissolved in 1 mL methanol and filtered through a membrane filter with pore size of 0.45 µm prior to analysis.
HPLC analysis
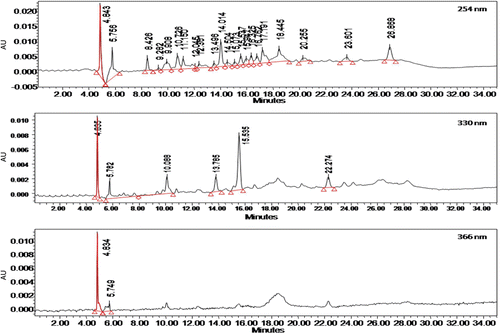
HPLC profile of CEBP was analysed by means of a HPLC system (Waters Delta 600 with 600 Controller) with photodiode array detector (Waters 996) (Milford, MA, USA). A Phenomenex Luna (5 µm) (Torrance, CA, USA) column was used (4.6 mm i.d. × 250 mm) and for elution of the constituents, two solvents denoted as A and B was employed. A was 0.1% aqueous formic acid, B was 0.1% formic acid in acetonitrile. Initial conditions were 85% A and 15% B with a linear gradient reaching 25% B at t = 12 min. This was maintained for 10 min after which the programme return to the initial solvent composition at t = 25 min and continued for 10 min. The flow rate used was 0.1 mL/min and the injection volume was 10 µL. The column oven was set at 27°C and the eluant was monitored at 254, 300 and 366 nm. The retention times and UV spectra of major peaks were analysed. The HPLC analysis was carried out in the Laboratory of Phytomedicine, Medicinal Plants Division, Forest Research Institute of Malaysia (FRIM), Kepong, Malaysia.
Animals
Male Sprague–Dawley rats (180–220 g) were used in the present study. The animals were obtained from the Veterinary Animal Unit, Universiti Putra Malaysia (UPM) and housed at the Animal House, Faculty of Pharmacy, Universiti Teknologi MARA (UiTM). They were kept in polypropylene cages with wood shaving, fed with standard pellet with free access to water and kept in standard conditions of 12 h dark/light cycle at 27 ± 2°C. The rats were fasted prior to all assays, standard drugs and CEBP were administered orally (p.o.) by gavage with 8% Tween 80 (10 mL/kg) as the vehicle. At all times, the rats were cared for in accordance with current UPM principles and guidelines for the care of laboratory animals and UPM ethical guidelines for investigations of experimental pain in conscious animals (Ref. No: UPM/FPSK/PADS/BR-UUH/00451) as adopted from CitationZimmermann (1983).
Acute toxicity studies
The acute toxicity study for chloroform extract of B. purpurea (CEBP) was performed using a single dose of 5000 mg/kg (p.o.) given in the volume of 10 mL/kg of body weight (CitationMohamed et al., 2011). Rats were fasted for 24 h before the administration of CEBP. Observation period was set for 14 days. The group that received 8% Tween 80 represents the control group. The toxicity signs and symptoms or any abnormalities were observed at 0, 30, 60, 120, 180 and 240 min after CEBP administration. Observation was conducted once a day for the next 14 days. The number of rats that survived was recorded at the end of the study period. The rats that survived were sacrificed and major organs were collected for histological examination.
Antiulcerogenic activity
Ethanol-induced gastric ulcer
The experiment was carried out according to CitationNoor et al. (2006) with slight modifications. CEBP (100, 500, and 1000 mg/kg), 8% Tween 80 (10 mL/kg) or omeprazole (30 mg/kg) was administered orally to rats after 48 h fasting. After 30 min, ulcer was induced in rats by administrating absolute ethanol (1 mL/200 g). The rats were sacrificed by exposure to diethyl ether followed by the exposure to ethanol after 15 min. Stomachs were removed and opened along the greater curvature. All the stomachs were gently rinsed with water to remove the gastric contents and blood clots. After the identification of ulcer areas, the length of ulcer was measured and the number of ulcer spots were considered equivalent to 1 mm2 of ulcer (CitationCho & Ogle, 1979). The ulcer area (UA) in mm2 was determined as the total sum of gastric lesions for each stomach in the group. The protection percentage was calculated according to the following formula:
Indomethacin-induced gastric ulcer
The experiment was carried out according to CitationNwafor et al. (2000) with slight modifications. CEBP (100, 500 and 1000 mg/kg), 8% Tween 80 (10 mL/kg) or omeprazole (30 mg/kg) were administered orally to rats after 48 h fasting. After 30 min, ulcer was induced in rats by administrating 100 mg/kg indomethacin. The rats were sacrificed by exposure to diethyl ether after 4 h of exposure to indomethacin. Stomachs were removed and opened along the greater curvature. All the stomachs were gently rinsed with water to remove the gastric contents and blood clots. The macroscopic analysis was done as described earlier.
Histopathology analysis
Samples of gastric tissue from each group were fixed in 10% formalin. Then, the formalin fixed specimens were embedded in paraffin and sectioned (3–5 µm), and further stained with haematoxylin and eosin dyes. The sections were evaluated by light microscopy and photographed.
Pylorus-ligation
Pylorus ligation was carried out according to the method by CitationShay et al. (1945) with slight modifications. Thirty minutes after oral administration of CEBP (100, 500 and 1000 mg/kg), 8% Tween 80 (10 mL/kg) or 30 mg/kg omeprazole as positive control, pylorus ligation was performed. The rats were lightly anesthetized with diethyl ether and their abdomen was opened without damaging any blood supply followed by the ligation of the pylorus. The abdomen was closed by suturing and the rats were allowed to recover for 4 h. After 4 h, the animals were sacrificed by exposure to overdose of diethyl ether. The abdomen was opened and a ligature was placed around the esophagus junction. The stomachs were removed and the content was measured before drained into a centrifuge tube and subjected to centrifugation at 3000 rpm for 10 min. The pH of the gastric secretion was recorded with a pH meter. The total acidity of the gastric secretion was determined by titration with 0.01 N NaOH and phenolphthalein was used as indicator. The total acidity is expressed as meq/L according to the following formula:
where n is the volume of NaOH quantified, 36.45 is the molecular weight of NaOH, 0.01 is normality of NaOH and 1000 is the factor represented in litre. Gastric lesions were also examined as described earlier.
Determination of mucus content of the gastric wall
Mucus content was determined by the method described by CitationCorne et al. (1974) with slight modifications. The stomach was opened along the greater curvature, weighed and immersed in 10 mL of 0.1% Alcian blue in 0.16 M sucrose/0.05 M sodium acetate, pH 5.8 for 2 h. Then, the excessive dye was removed by two successive rinses of 0.25 M sucrose solution for 15 and 45 min. The remaining dye that complexed with the gastric mucus were then extracted with 0.5 M MgCl2 over 2 h and shaken intermittently every 30 min. The blue extract was then shaken vigorously and the optical density was measured using a spectrophotometer at 580 nm. The quantity of Alcian blue extract per gram of wet stomach was calculated from the standard curve.
Statistical analysis
The results are expressed as mean ± SEM. One-way analysis of variance (ANOVA) was applied. Statistical significance was calculated at the level of p < 0.05 using Tukey post hoc test.
Results
Phytochemical constituents and HPLC profile of the chloroform extract of B. purpurea leaf (CEBP)
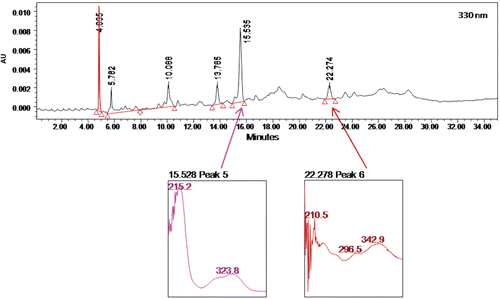
The phytochemical screening of CEBP demonstrated the strong presence of flavonoids and steroids, and weak occurrence of condensed tannins, but no alkaloids, triterpenes, or saponins. The HPLC profile of CEBP was analyzed at three different wavelengths, namely 254, 330 and 366 nm (). The extract was best separated at 330 nm with six separate peaks recorded at the retention time (RT) of 4.885, 5.782, 10.088, 13.785, 15.535 and 22.274 min ().
Percentage yield of CEBP
Dried leaves of B. purpurea (875 g) were soaked in chloroform to yield approximately 16.1 g (approximately 1.84%) CEBP.
Acute toxicity study on CEBP
A single oral administration of 5000 mg/kg CEBP did not produce any symptom or sign of toxicity in the treated animals. Observations after treatment were made over 14 days. All animals survived until the end of the study.
Gross studies of the ulcer-induced stomach
Effect of CEBP on ethanol-induced gastric ulcer
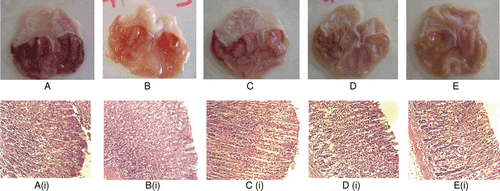
Gross pathological investigation of the ethanol-treated stomachs removed from animals that were pre-treated with 8% Tween 80 (negative control group) showed complete ulceration (). Pretreatment with CEBP, at the doses of 100, 500 and 1000 mg/kg, exhibited significant (p < 0.05) and dose-dependent reduction of gastric lesion. The percentage of protection recorded for the 100 and 500 mg/kg CEBP was approximately 50 and 86%, respectively. One hundred percent (100%) protection of the stomach against ulcer formation was observed in rats treated with the highest dose (1000 mg/kg) of CEBP when compared to the control group (). On the other hand, 30 mg/kg of omeprazole was found to produce approximately 67% protection. The overall gross histopathological evaluation is summarized in .
Table 1. Effect of CEBP and omeprazole on ethanol-induced gastric lesion in rats.
Figure 3. Histological evaluation of antiulcer activity of chloroform extract of B. purpurea (CEBP) against absolute ethanol-induced gastric lesions in rats (A) Stomach of an ulcer control rat; (B) Stomach of a rat pre-treated with omeprazole; (C) Stomach of a rat pre- treated with 100 mg/kg CEBP; (D) Stomach of a rat pre-treated with 500 mg/kg CEBP; (E) Stomach of a rat pre- treated with 1000 mg/kg CEBP; The respective histopathological sections are shown below; (Ai) Stomach of the ulcer control animal showing mild effect on mucosa and moderate hemorrhagic erosion, severe edema, mild infiltrate leukocytes and presence of mild inflammatory exudates; (Bi) Stomach of omeprazole treated animals show moderate effect on mucosa with moderate hemorrhage and edema; (Ci) Stomach of 100 mg/kg CEBP treated animals showing mild effect on mucosa with moderate effect on edema and presence of inflammatory exudates; (Di)Stomach of 500 mg/kg CEBP treated animals showing almost normal mucosa with moderate effect of edema and mild presence of inflammatory exudates and leukocytes infiltrate; (Ei) Stomach of 1000 mg/kg CEBP treated animals showing almost normal mucosa with moderate effect edema and presence of inflammatory exudates
Effect of CEBP on indomethacin-induced gastric ulcer
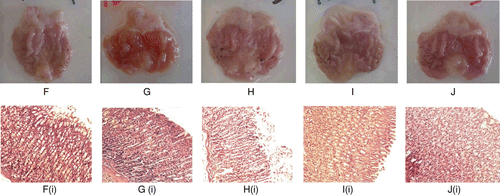
Indomethacin, at the dose of 100 mg/kg, caused severe gastric lesions in comparison to 8% Tween 80-pretreated group (negative control group) (). Pretreatment with 100, 500 and 1000 mg/kg CEBP demonstrated significant (p < 0.05) reduction in gastric lesion with the percentage of protection recorded at approximately 31, 24 and 59%, respectively. This effect of CEBP was less effective than 30 mg/kg omepraxole, which caused 100% protection from gastric ulcer formation ().
Table 2. Effect of CEBP and omeprazole on indomethacin-induced gastric lesion in rats.
Figure 4. Histological evaluation of antiulcer activity of CEBP against indomethacin-induced gastric lesions in rats (F) Stomach of an ulcer control rat; (G) Stomach of a rat pre-treated with omeprazole; (H) Stomach of a rat pre- treated with 100 mg/kg CEBP; (I) Stomach of a rat treated with 500 mg/kg CEBP; (J) Stomach of a rat treated with 1000 mg/kg CEBP. The respective histopathological section is shown below; (Fi) Stomach of the ulcer control animal showed normal on mucosa but with mild hemorrhagic and mild edema; (Gi) Stomach of omeprazole pre-treated animals showed normal mucosa; (Hi) Stomach of CEBP 100 mg/kg pre-treated animals showed mild hemorrhage and edema; (Ii) Stomach of CEBP 500 mg/kg pre-treated animals showed mild edema; (Ji): Stomach of CEBP 1000 mg/kg pre-treated animals showed mild effect on mucosa and mild hemorrhagic erosion but with moderate effect of edema
Histopathological studies of the ulcer-induced stomach
CEBP effect against ethanol-Induced gastric ulcer
Gross pathological studies of the stomachs removed from animals that were pretreated orally with 8% Tween 80 followed by oral administration of absolute ethanol showed complete ulceration indicated by the presence of marked lesions and severed damage to the gastric mucosa, hemorrhagic erosion, edema and leukocytes infiltration of the submucosal layer ( and ). Pre-treatment of ulcer-induced rats with CEBP at 100 ( and ), 500 ( and ) and 1000 mg/kg ( and ) exerted significant (p < 0.05) reduction of gastric lesion indicated by the presence of severe lesions, mild lesions to almost normal mucosa architecture, respectively. This finding is supported by reduction of hemorrhage, edema, inflammatory exudates formation, and leukocytes infiltration. Omeprazole (30 mg/kg) caused reduction in edema and inflammatory exudates formation, and leukocytes infiltration( and ). The histopathological evaluation is summarized in . Generally, tissues pretreated with CEBP demonstrated comparatively better protection of gastric mucosa in a dose-dependent manner.
Table 3. Histopathological evaluation of antiulcer activity of CEBP and omeprazole on ethanol-induced gastric lesion in rats.
CEBP effect against indomethacin-induced gastric ulcer
Indomethacin, at the dose of 100 mg/kg, caused gastric lesions in the 8% Tween 80-treated group (negative control; ) which was indicated by the presence of mild hemorrhage and edema ( and ). Pretreatment with 100 ( and ), 500 ( and ) and 1000 mg/kg ( and ) of CEBP also demonstrated significant (p < 0.05) reduction of the gastric lesion, which were supported by a decrease in the hemorrhage, edema, inflammatory exudates formation, and leukocytes infiltration. However, the effect was not dependent on dose with 30 mg/kg omeprazole exhibiting almost normal stomach architecture ( and ). The histopathological evaluation is summarized in . Overall, pretreatment with CEBP demonstrated significant antiulcer activity in a dose-independent manner.
Table 4. Histopathological evaluation of antiulcer activity of CEBP and omeprazole on indomethacin-induced gastric lesion in rats.
Pylorus-ligation
The CEBP also demonstrated dose-independent activity in the pylorus ligation model as seen in the percentage of protection. CEBP at 100, 500 and 1000 mg/kg caused approximately 49, 81 and 54% protection, respectively (). The effect of 500 mg/kg CEBP was not significantly different (p > 0.05) when compared to the 300 mg/kg omeprazole indicating their equal strength or effectiveness. In term of the gastric content, all doses of CEBP caused significant (p < 0.05) reduction of gastric content in a non-dose-dependent manner when compared to the negative control group (). However, the CEBP was less effective in reducing the gastric content when compared to the omeprazole. In term of the pH of gastric content, only omeprazole, but not all doses of CEBP, significantly (p < 0.05) reversed the extremely acidic pH of gastric content when compared to the negative control group (). In terms of the total acidity of gastric content, the CEBP, at all doses tested, caused a significant (p < 0.05) and non-dose-dependent decrease in the total acidity of gastric content. However, it was less effective than omeprazole. In terms of the gastric wall mucus production, all doses of CEBP significantly (p < 0.05) increased the gastric mucus production and were more effective than omeprazole in a dose-dependent manner ().
Table 5. Effect of CEBP and omeprazole on gastric juice parameters assessed using the pylorus ligation model in rats.
Table 6. Effect of CEBP and omeprazole on gastric wall mucus.
Discussion
In the present study, we have demonstrated the antiulcer activity of CEBP using different standard experimental models of induced gastric ulcers. Administration of CEBP significantly reduced the ulcer index in all models with a dose-dependent effect observed only in the ethanol-induced gastric ulcer models. In addition, CEBP also significantly increased the production of gastric wall mucus and the pH of gastric content while, at the same time, reducing the total volume and total acidity of gastric content. These findings were further supported by the histopathological study of stomach tissues and were comparable to the standard antiulcer agent, omeprazole. Overall, these findings indicated the potential antiulcerogenic, antisecretory and cytoprotective actions of B. purpurea.
Despite unclear etiology associated with the development of ulcer, the imbalances between aggressive and defensive factors have been suggested to be the contributing factor (CitationOkoli et al., 2009). Under normal physiological conditions, local production of prostaglandins helps stimulate and maintain the mucus and bicarbonate synthesis that protect the gastric mucosa. Disruption in these defense mechanisms will lead to formation of gastric or duodenal ulcers. According to CitationGoswami et al. (2011), the treatment and prevention of these acid-related disorders can be accomplished by using antiulcer agents that work either to enhance mucosal production or to decrease the acidity level.
Ethanol and indomethacin induce ulcers via different but related mechanisms that principally weaken gastric mucosal reliability and consequently cytoprotection. The ethanol induced gastric ulcer has been proven to involve a combination of direct toxic action of ethanol, decrease in the presence of gastric wall mucus and reduce in the secretion of bicarbonate (see CitationOkoli et al., 2009). In addition, ethanol has also been reported to reduce endogenous glutathione and prostaglandin levels, to increase release of histamine, influx of Ca2+, stimulation of leukotriene C4 (LTC4) and oxygen free radicals synthesis, which, in turn, lead to increase lipid peroxidation. Increase in lipid peroxidation will cause alterations in membrane permeability of cells that contributes to cells damage (see CitationOkoli et al., 2009). This damage is attributed to the release of reactive oxygen derived species (ROS) (e.g. hydroperoxy and superoxide anion free radicals) resulting from the metabolism of ethanol and these ROS will take part in the mechanism of acute and chronic ulceration in the gastric mucosa. Several studies have reported that ROS and lipid peroxidation will initially attack and damage many biological molecules (e.g., prostaglandins) followed by a continuing chain of reactions that will lead to cell injury and ultimately cell death (CitationLondonkar & Poddar, 2009). The ability of CEBP to reduce ethanol-induced gastric ulcer in the present study suggests the involvement of its cytoprotective effect, which could be associated with the antioxidant and free radical scavenging effects of the extract. This suggestion is supported by our previous report on the antioxidant and free radical scavenging, and cytoprotective activities of CEBP as well as aqueous and methanol extracts of B. purpurea (CitationZakaria et al., 2011). Furthermore, based on the ability of the ethanol to stimulate LTC4, antiulcer activity of CEBP against ethanol-induced gastric ulcer could due to suppression of lipoxygenase pathway (CitationNwafor et al., 2000). It is also claimed that agents that improve mucosal defensive factors could be used to inhibit ethanol-induced gastric ulcer (CitationOkoli et al., 2009). This claim is in agreement with report by CitationGoswami et al. (2011) as described above and is consistent with our finding on the ability of CEBP to increase gastric wall mucus production while reducing the total acidity of the gastric content. Furthermore, the extract could increase gastric wall mucus secretion by enhancing prostaglandins synthesis.
On the other hand, indomethacin induces ulcer particularly in an empty stomach and mainly on the glandular part of the stomach via inhibitory action on prostaglandin synthetase within the cyclooxygenase pathway (CitationOkokon & Nwafor, 2009). In addition, the gastric mucosal damage induced by indomethacin (and other related NSAIDs) results from the inhibition of arachidonic-acid-induced prostaglandin synthesis (CitationOkoli et al., 2009). Prostaglandins play a protective role in the stomach by maintaining the mucosal blood flow, regulating mucosal turn over and repair, and promoting the secretion of mucus and bicarbonate within the gastric compartment (CitationHiruma-Lima et al., 2006; CitationOkoli et al., 2009). Suppression of prostaglandins synthesis by indomethacin enhances stomach susceptibility to mucosal injury and gastroduodenal ulceration. Hence, the ability of CEBP to inhibit ulcer formation induced using indomethacin suggests the extract may have cytoprotective activity possibly by increasing prostaglandins synthesis. Moreover, findings by CitationMuralidharan and Srikanth (2009) that leukotriene antagonists and 5-lipoxygenase inhibitors also inhibited NSAIDs-induced gastric ulceration in rats leads us to suggest that antiulcer activity of CEBP against indomethacin-induced gastric ulcer involved in part inhibition of 5-lipoxygenase pathway or leukotriene’s antagonistic activity.
The pylorus ligated ulcer model is widely used to study the effect of extract/compound on gastric secretion whereby pylorus ligation directly caused the accumulation of gastric secretion. The increase in gastric secretion will lead to the upper gastrointestinal damage (e.g., development of lesions, ulcers and life threatening perforation and hemorrhage), which will progress to form gastric ulcer as a result of auto digestion of gastric mucosa and breakdown of the gastric mucosal barrier. It is well known that prostaglandin E2 and I2 are mainly synthesized by the gastric mucosa and, their functions include inhibition of gastric acid secretion, stimulation of mucus and bicarbonate secretion, as well as the hydrophobic surfactant-like phospholipids secretion in the gastric epithelial cells. Thus, damage to the gastric mucosa induced via pylorus ligation procedures will inhibit the formation of prostaglandin E2 and I2 leading to increase in total acidity, free acidity, and ulcer index of gastric secretions. In addition, CitationGoswami et al. (2011) also suggested the involvement of histamine in the formation of pylorus ligated ulcers since it mediates the gastric secretions stimulated by gastric vagal excitation. The H2-receptor antagonists mainly block the basal acid secretion, which is attributed to their efficiency in suppressing nocturnal acid secretion, via reversible competition with histamine for binding to H2 receptors found on the basolateral membrane of parietal cells. Thus, CEBP is partly suggested to act as an antiulcer agent by inhibiting H2-receptors.
Phtyochemical screening of CEBP demonstrated the presence of flavonoids, condensed tannins and steroids in the extract. The presences of flavonoids and dimeric flavonoids in the leaf of B. purpurea have been reported (CitationYadav & Bhadoria, 2005). Our HPLC profiling of CEBP also supported the presence of flavonoid-types of compounds wherein two of the major peaks (peak 5 and peak 6) detected at 330 nm in the chromatogram were found to have UV spectra of 324 nm and 296–343 nm, respectively. According to CitationTsimogiannis et al. (2007) flavonoids can be divided into five major subgroups (e.g., flavonols, flavones, dihydroflavonols, flavanonols and flavanones), whereby the UV-Vis spectra of flavonoids falls within two absorbance bands, labelled as Band A and Band B. Band A, which represents flavones or flavonols, lies in the range of 310–350 nm or 350–385, respectively, while Band B, which falls between 250 and 290 nm, is much the same in all the aforementioned flavonoid subgroups. In the case of flavanones and dihydroflavonols, Band A falls in the range of 300–330 nm while Band B was detected in the range of 277–295 nm. CitationRaj Narayana et al. (2001) have earlier discussed on the potential of flavonoids as antiulcer agents and their report could be used to justify the role of flavonoids in the leaf of B. purpurea and, particularly, in the CEBP, for antiulcer activity.
Other than that, flavonoids have been shown to scavenge free radicals (CitationLondonkar & Poddar, 2009) and could be responsible for the recent report on the antioxidant activity of CEBP (CitationZakaria et al., 2011). This is further supported by CitationShetty et al. (2008) who discussed that flavonoids could possibly inhibit the gastric mucosal injury by scavenging the ethanol- and indomethacin-generated oxygen metabolites. Furthermore, severe damage to the gastric mucosa will increase infiltration of neutrophils, which are the major source of inflammatory mediators, into the damage areas. These neutrophils mediate lipid peroxidation by releasing the highly cytotoxic and tissue damaging ROS leading to the prevention of gastric ulcer healing. It has been reported that gastric ulcer can be attenuated by suppressing the neutrophil infiltration during inflammatory processes (CitationCheng & Koo, 2000). Thus, the anti-inflammatory activity exhibited by CEBP (CitationZakaria et al., 2009) is believed to take part in suppressing the neutrophil infiltration into the gastric ulcer area leading to enhancement in gastric ulcer healing. The flavonoids present in CEBP was suggested to, partly, modulate arachidonic acid metabolism via the inhibition of cyclo-oxygenase and lipooxygenase activities (CitationSandhar et al., 2011).
Overall, the present data demonstrate the presence of antiulcer activity and improve ulcer healing properties of CEBP. These data also confirm the traditional claim on the use of B. purpurea for the treatment of gastric ulcers in the Indian subcontinent. Although it is difficult to explain the actual antiulcer mechanism involved with the CEBP, the effects obtained using various gastric ulcer models suggest a multifactorial mechanism, involving free-radical scavenging properties, antioxidant and anti-inflammatory activities of the CEBP. In conclusion, CEBP exerts antiulcer activity, which is attributed partly to its flavonoids’ antioxidant and anti-inflammatory activities.
Acknowledgements
The authors thank the Faculty of Pharmacy, Universiti Teknologi MARA and Faculty of Medicine and Health Sciences, Universiti Putra Malaysia for providing the facilities to carry out this study.
Declaration of interest
This study was supported by the Science Fund Research Grant (Reference no. 06-01-04-SF1127) from the Ministry of Science Technology and Innovation, Malaysia and the Research University Grant Scheme (Reference no. 04-01-11–1169RU) from the Universiti Putra Malaysia, Malaysia.
References
- Asolker LV, Kakkar KK, Chakre OJ. (2000). Supplement to Glossary of Indian Medicinal Plants, Part I (A-K). New Delhi, India: National Institute of Science Communication.
- Bandyopadhyay U, Biswas K, Chatterjee R, Bandyopadhyay D, Chattopadhyay I, Ganguly CK, Chakraborty T, Bhattacharya K, Banerjee RK. (2002). Gastroprotective effect of neem (Azadirachta indica) bark extract: Possible involvement of H(+)-K(+)-ATPase inhibition and scavenging of hydroxyl radical. Life Sci, 71, 2845–2865.
- Cheng CL, Koo MW. (2000). Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci, 67, 2647–2653.
- Cho CH, Ogle CW. (1979). Cholinergic-mediated gastric mast cell degranulation with subsequent histamine H1-and H2-receptor activation in stress ulceration in rats. Eur J Pharmacol, 55, 23–33.
- Chopra RN, Nayar SL, Chopra IC. (1956).Glossary of Indian Medicinal Plants. New Delhi, India: Publication and Information Directorate Hill Side Road.
- Corne SJ, Morrissey SM, Woods RJ. (1974). Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol (Lond), 242, 116P–117P.
- Goswami M, Kulshreshtha M, Rao CV, Yadav S, Yadav S. (2011). Anti-ulcer potential of Lawsonia inermis l. leaves against gastric ulcers in rats. J Appl Pharmaceut Sci, 1, 69–72.
- Hiruma-Lima CA, Gracioso JS, Toma W, Almeida AB, Paula AC, Brasil DS, Muller AH, Souza Brito AR. (2001). Gastroprotective effect of aparisthman, a diterpene isolated from Aparisthmium cordatum, on experimental gastric ulcer models in rats and mice. Phytomedicine, 8, 94–100.
- Hiruma-Lima CA, Santos LC, Kushima H, Pellizzon CH, Silveira GG, Vasconcelos PC, Vilegas W, Brito AR. (2006). Qualea grandiflora, a Brazilian “Cerrado” medicinal plant presents an important antiulcer activity. J Ethnopharmacol, 104, 207–214.
- Ikhiri K, Boureima D, Dan-Kouloudo D. (1992). Chemical screening of medicinal plants used in the traditional pharmacopoeia of Niger. Int J Pharmacog, 30, 251–262.
- Janardhanan K, Vadivel V, Pugalenthi M. (2003). Biodiversity in Indian Underexploited/Tribal Pulses. In: Jaiwal KP, Singh RP (Eds), Improvement Strategies for Leguminosae Biotechnology. Dordrecht, The Netherlands: Kluwer Academic Publishers.
- Kirthikar KR, Basu DB. (2001). Indian Medicinal Plants. 3rd Ed. Vol. 4. Dehradun, India: Oriental Enterprises.
- Lima ZP, Severi JA, Pellizzon CH, Brito AR, Solis PN, Cáceres A, Girón LM, Vilegas W, Hiruma-Lima CA. (2006). Can the aqueous decoction of mango flowers be used as an antiulcer agent? J Ethnopharmacol, 106, 29–37.
- Londonkar RL, Poddar PV. (2009). Studies on activity of various extracts of Mentha arvensis Linn against drug induced gastric ulcer in mammals. World J Gastrointest Oncol, 1, 82–88.
- Martelli A, Mattlioli F, Mereto E, Brambilla CG, Sini D, Bergamaschi R, Brabilla G. (1998). Evaluation of omeprazole genotoxicity in a battery of in vitro and in vivo assays. Toxicol, 30, 19–41.
- Mohamed EA, Lim CP, Ebrika OS, Asmawi MZ, Sadikun A, Yam MF. (2011). Toxicity evaluation of a standardised 50% ethanol extract of Orthosiphon stamineus. J Ethnopharmacol, 133, 358–363.
- Morais SM, Dantas JDP, Silva ARA, Magalhães EF. (2005). Plantas medicinais usadas pelos índios Tapebas do Ceará. Rev Bras Farmacogn, 15, 169–177.
- Mukherjee PK, Gopal TK, Subburaju T, Dhanbal SB, Duraiswamy B, Elango K, Suresh B. (1998). Studies on the anti-diarrheal profiles of Bauhinia purpurea L. leaves (Caesalpiniaceae) extract. Nat Prod Sci, 4, 234–237.
- Muralidharan P, Srikanth J. (2009). Antiulcer activity of Morinda citrifolia Linn fruit extract. J Sci Res, 1, 345–352.
- Nash J, Lambert L, Deakin M. (1994). Histamine H2-receptor antagonists in peptic ulcer disease. Evidence for a prophylactic use. Drugs, 47, 862–871.
- Noor SM, Mahmood AA, Salmah I, Philip K. (2006). Prevention of acute gastric mucosal lesions by R. hasseltii in rats. J Anim Vet Adv, 5, 161–164.
- Nwafor PA, Okwuasaba FK, Binda LG. (2000). Antidiarrhoeal and antiulcerogenic effects of methanolic extract of Asparagus pubescens root in rats. J Ethnopharmacol, 72, 421–427.
- Okokon JE, Nwafor PA. (2009). Antiulcer and anticonvulsant activity of Croton zambesicus. Pak J Pharm Sci, 22, 384–390.
- Okoli CO, Ezike AC, Akah PA, Udegbunam SO, Okoye TC, Mbanu TP, Ugwu E. (2009). Studies on wound healing and antiulcer activities of extract of aerial parts of Phyllanthus niruri L. (Euphorbiaceae). Am J Pharmacol Toxicol, 4, 118–126.
- Parrota JA. (2001). Healing Plants of Peninsular India. Oxford, UK: CABI Publishing.
- Raj Narayana K, Sripal Reddy M, Chaluvadi MR, Krishna DR. (2001). Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol, 33, 2–16.
- Sandhar HK, Kumar B, Prasher S, Tiwari P, Salhan M, Sharma P. (2011). A review of phytochemistry and pharmacology of flavonoids. Int Pharmaceut Sci, 1 (Online). Available at: http://www.ipharmsciencia.com/Dacuments/1/4.pdf. Accessed on 29 July 2011.
- Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, Siplet H. (1945). A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology, 5, 43–61.
- Shetty BV, Arjuman A, Jorapur A, Samanth R, Yadav SK, Valliammai N, Tharian AD, Sudha K, Rao GM. (2008). Effect of extract of Benincasa hispida on oxidative stress in rats with indomethacin induced gastric ulcers. Indian J Physiol Pharmacol, 52, 178–182.
- da Silva KL, Biavatti MW, Leite SN, Yunes RA, Delle Monache F, Cechinel Filho V. (2000). Phytochemical and pharmacognositc investigation of Bauhinia forficata Link (Leguminosae). Z Naturforsch, C, J Biosci, 55, 478–480.
- Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. (2007). Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules, 12, 593–606.
- Wolfe MM, Sachs G. (2000). Acid suppression: Optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology, 118, S9–31.
- Yadav S, Bhadoria BK. (2005). Two dimeric flavonoids from Bauhinia purpurea. Indian J Chem, 44B, 2604–2607.
- Zakaria ZA. (2007b). Free radical scavenging activity of some plants available in Malaysia. Iranian J Pharmacol Ther, 6, 87–91.
- Zakaria ZA, Abdul Rahman NI, Loo YW, Abdul Ayub AH, Sulaiman MR, Mat Jais AM, Gopalan HK, Fatimah CA. (2009). Antinociceptive and anti-inflammatory activities of the chloroform extract of Bauhinia purpurea (Leguminosae) leaves. Int J Trop Med, 4, 140–145.
- Zakaria ZA, Wen LY, Abdul Rahman NI, Abdul Ayub AH, Sulaiman MR, Gopalan HK. (2007). Antinociceptive, anti-inflammatory and antipyretic properties of the aqueous extract of Bauhinia purpurea leaves in experimental animals. Med Princ Pract, 16, 443–449.
- Zakaria ZA, Mat Jais AM, Mastura M, Mat Jusoh SH, Mohamed AM, Mohd Jamil NS, Rofiee MS, Sulaiman MR. (2007c). In vitro antistaphylococcal activity of the extracts of several neglected plants in Malaysia. Int J Pharmacol, 3, 428–431.
- Zakaria ZA, Rofiee MS, Teh LK, Salleh MZ, Sulaiman MR, Somchit MN. (2011). Bauhinia purpurea leaves extracts exhibited in vitro antiproliferative and antioxidant activities. Afr J Biotechnol, 10, 65–74.
- Zimmermann M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16, 109–110.