Abstract
Context: Pentacyclic triterpenes, mainly, asiatic acid, madecassic acid, asiaticoside, and madecassoside are the active constituents of Centella asitica (L.) Urban. (Apiaceae). These compounds possess various pharmacological activities that have been shown to assist with wound healing and brain enrichment.
Objective: Determination of these active pentacyclic triterpenes in extracts from the various parts of C. asiatica plants harvested at different times of the year and grown in different environments.
Materials and methods: The separate plant parts selected were leaves, stolons, petioles, flowers, fruits, and nodes with roots. Dried powder from each part was extracted with ethanol by microwave-assisted extraction and subjected to determination of their content of the four pentacyclic triterpenes using a HPLC method. The effects of the places of cultivation as well as harvesting periods on the content of the four pentacyclic triterpenes in the extracts were also determined.
Results and discussion: Among the various parts of C. asiatica, the leaves contained the highest amount of pentacyclic triterpenes with a total content of pentacyclic triterpenes of 19.5 mg/g dry powder. However, the contents of the pentacyclic triterpenes in C. asiatica varied according to the place of cultivation and the harvesting period. C. asiatica collected from Trang, Thailand gave the highest content of total pentacyclic triterpenes (37.2 mg/g dry powder) when harvested in March, while those collected from Songkhla, Thailand gave the highest value (37.4 mg/g dry powder) when collected in December. C. asiatica collected from Nakornsrithammarat and Ratchaburi, Thailand gave the lowest content of total pentacyclic triterpenes in all experimental harvesting periods.
Introduction
Centella asiatica (L.) Urban. (Apiaceae) is a well known medicinal plant in Asian countries. The aerial part of this plant possesses a wide range of pharmacological properties used for treating skin diseases and wounds (CitationFarnsworth & Bunyapraphatsara, 1992) and for brain enrichment (promoting brain growth and improving learning and memory) (CitationSubathra et al., 2005; CitationShinomol & Muralidhara, 2008; CitationZheng & Qin, 2007). Pentacyclic triterpenes, mainly, asiatic acid (1), madecassic acid (2), asiaticoside (3), and madecassoside (4) () are the active constituents that possess this variety of pharmacological activities, including stimulation of fibroblast proliferation and collagen synthesis (CitationBonte et al., 1994; CitationMaquart et al., 1990; CitationLu et al., 2004), anti-inflammatory (CitationYun et al., 2008; CitationWon et al., 2010), antiulcer (CitationCheng et al., 2004), antibacterial (CitationDjoukeng et al., 2005) and anticancer (CitationPark et al., 2005).
The quality of herbal medicines is a major challenge because the content of active constituents in plants varies according to a large number of factors, and often the plants used in phytomedicine have a complex and variable chemical composition. These challenges include the choice of the highest yielding plant species/variety, genetic composition of the plant, growth conditions, climatic variation, age or harvesting period, and the specific parts of the plants harvested for processing (CitationPanichayupakaranant, 2011). The effects of different plant parts, and harvesting period on the content of active constituents in Senna alata (L.) Roxb. (Leguminosae) and Rhinacanthus nasutus (L.) Kurz (Acanthaceae) have been reported (CitationPanichayupakaranant & Intaraksa, 2003; CitationPanichayupakaranant et al., 2006). Using the correct plant raw materials (high level of active ingredients) for production of herbal medicines might be a way to ensure quality, but how do we identify, which plant, which part and proper time for harvest? Information of the many factors that might affect the level of the active constituent of each medicinal plant is still very rare.
As part of our interest in the effects of plant parts, place of cultivation and harvesting period on the quality of C. asiatica raw material, we have determined the distribution of pentacyclic triterpenes accumulated in various parts of C. asiatica. The effects of five different places for cultivation and three different harvesting periods have been studied. This work will contribute to our knowledge of potentially good harvesting practices required for the production of herbal medicines from C. asiatica.
Materials and methods
Chemicals
Standard asiatic acid was from Sigma-Aldrich (Missouri, MO, USA). Standard madecassic acid, asiaticoside, and madecassoside were from Chengdu Biopurify Phytomedicals (Sichaun, China). Acetonitrile (HPLC grade) and ethanol (analytical grade) were from Labscan Asia (Bangkok, Thailand). Water was purified in a Milli-Q system (Millipore, Bedford, MA, USA).
Plant material
Centella asiatica plants were collected from five provinces of Thailand (Nakornsrithammarat, Phatthalung, Ratchaburi, Songkhla and Trang) in different periods (March, July and December) in 2010. The voucher specimens (specimens no. SKP 199 03 01 01) were identified by Associate Professor Pharkphoom Panichayupakaranant and deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand.
Plant harvesting
The aerial parts of the plants (2-month-old) were harvested and washed. The plants were packed in plastic bags and sent to the laboratory immediately. The plants were dried at 50°C for 24 h in a hot air oven, and ground to powder using a grinder and a sieve no. 45.
Preparation of Centella asiatica extracts
The dried C. asiatica powders (0.5 g) were extracted with ethanol (20 mL) by microwave-assisted extraction (MAE). MAE was carried out in a laboratory scale microwave extraction apparatus (MS2127CW; LG Electronics Inc., Bangkok, Thailand). Ethanol was added into a 125 mL Erlenmeyer flask containing C. asiatica dried powder. The flask was then placed in a microwave resonance cavity (2450 MHz, 600 W). The conditions of MAE were: irradiation power 600 W, 75°C, four irradiation cycles (1 cycle: 15 s power on and 30 s power off), and four times of extraction. After the extraction, the filtered solutions were concentrated to dryness under reduced pressure at 45°C. The residue was reconstituted and volume was adjusted to 5 mL with methanol. These samples were filtered through a 0.45 μm membrane filter and subjected to HPLC analysis.
Determination of sites of accumulation of pentacyclic triterpenes
The plant materials were collected from Nakornsrithammarat and divided into the six groups according to plant parts; leaves, stolons, petioles, flowers, fruits, and nodes with roots. The dried plant powders were separately extracted by MAE. The extracts were then subjected to HPLC analysis. The experiment was performed in triplicate.
Effect of places for cultivation and harvesting periods
The aerial parts of C. asiatica were collected from five provinces of Thailand (Nakornsrithammarat, Phatthalung, Ratchaburi, Songkhla and Trang) in three different periods (March, July, and December). The dried plant powders were separately extracted by MAE. The extracts were then subjected to HPLC analysis. Each experiment was performed in triplicate.
HPLC quantitative analysis
HPLC analysis of pentacyclic triterpene content was modified from previous reports (CitationInamdar et al., 1996; CitationRafamantanana et al., 2009) and validated. The method was carried out using an Agilent 1100 series equipped with a photodiode array detector and auto sampler (Palo Alto, CA, USA). Separation was achieved at 25°C on a 150 mm × 4.6 mm. TSK-gel ODS-80Tm column (ToshoBioscience, Tokyo, Japan). The mobile phase was a gradient of acetonitrile/water (0–5 min, 20:80; 5–10 min, 30:70; 10–20 min, 65:35; 20–30 min, 70:30). The mobile phase flow rate was 1 mL/min. Sample injection volumes were 20 µL, and detection was by UV at wavelength 210 nm. The calibration curves were established from the standards of asiaticoside, madecassoside, asiatic acid and madecassic acid at the concentration of 0.03–0.50 mg/mL. The linear equations of Y = 3230.4X−1.82 (r2 = 0.9999), Y = 3588.2X + 34.37 (r2 = 0.9999), Y = 6655.9X−35.14 (r2 = 0.9999) and Y = 6672.7X + 40.93 (r2 = 0.9998) correspond to asiaticoside, madecassoside, asiatic acid, and madecassic acid, respectively.
Statistical analysis
Values were expressed as a mean ± S.D. The Statistic Package for Social Science (SPSS for windows; SPSS Inc., Chicago, IL, USA) was used for data analysis. The statistical significance was calculated by one-way analysis of variance (ANOVA), followed by Tukey’s test (p ≤ 0.05).
Results and discussion
Sites of accumulation of pentacyclic triterpenes
Although, the aerial part of C. asiatica is the recommended part for use in herbal medicine treatment, there was a very big difference in the content of pentacyclic triterpenes accumulated in various parts of the plant. All four pentacyclic triterpenes were present in good quantities in the leaves of this plant followed by flowers, fruits, stolon, nodes with roots, and petioles, respectively (). The total content of pentacyclic triterpenes in the leaves was 19.5 ± 0.90 mg/g dry powder. The stolons, petioles, and nodes with roots of this plant contained very low amounts of the pentacyclic triterpenes. In addition, petacyclic triterpenes that accumulated in C. asiatica were in a glycoside form rather than an aglycone form.
Table 1. Pentacyclic triterpene content of Centella asiatica extracts obtained from various plant parts.
Effect of places for cultivation and harvesting periods
Determination of pentacyclic triterpene content in C. asiatica extracts, which were collected from five places for cultivation in three different harvesting periods, revealed that the content of pentacyclic triterpenes in the extracts varied according with the place of cultivation () and harvesting periods. The plants collected from Trang and Songkhla gave extracts with the highest content of total pentacyclic triterpenes (). However, C. asiatica collected from Trang gave the highest one when collected in March (37.2 ± 0.48 mg/g dry powder), while that from Songkhla was in December (37.4 ± 0.27 mg/g dry powder). The plant collected from Phatthalung gave a high content of total pentacyclic triterpenes when collected in both March (32.9 ± 1.38 mg/g dry powder) and December (31.0 ± 0.35 27 mg/g dry powder), but the content were significantly lower than those of Trang and Songkhla. In contrast, the plants collected from Nakornsrithammarat and Ratchaburi gave a very low amount of total pentacyclic triterpenes in all experimental harvesting periods. This may be due to the different conditions of plant cultivation. The plants collected from Trang, Songkhla and Phatthalung were wild, while those from Nakornsrithammarat and Ratchaburi were cultivated in farms. In addition, the genetic composition of the plants may also affect the content of total pentacyclic triterpenes in C. asiatica.
Table 2. Total pentacyclic triterpene content of Centella asiatica extracts obtained from different places for cultivation and harvesting periods.
Figure 2. HPLC chromatograms of C. asiatica extracts obtained from different places for cultivation: (A) Trang; (B) Songkhla; (C) Phattalung; (D) Nakornsrithammarat; (E) Ratchaburi; (1) Asiatic acid; (2) Madecassic acid; (3) Asiaticoside; (4) Madecassoside.
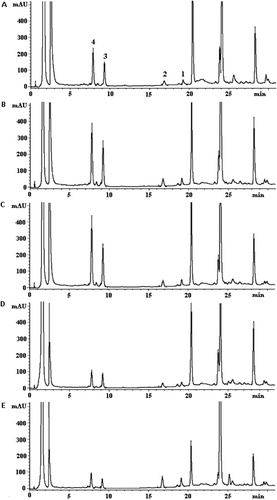
The compositions of the four pentacyclic triterpenes from C. asiatica collected from different places and period were also different. In all harvesting periods the plants collected from Trang, Songkhla and Phatthalung contained a higher content of the glycoside form (asiaticoside and madecassoside), while those from Nakornsrithammarat and Ratchaburi contained a higher content of the aglycone form (asiatic acid and madecassic acid) when harvested in March and December (). In July, the composition of pentacyclic triterpenes in the plants collected from all provinces contained a higher content of the glycoside form. The ratio of the content of aglycone to glycoside may affect the efficacy of C. asiatica extracts. Because asiatic acid and madecassic acid possessed a higher anti-inflammatory activity than asiaticoside and madecassoside (CitationYun et al., 2008; CitationWon et al., 2010), while asiaticoside and madecassoside possessed a good wound healing activity (CitationLu et al., 2004; CitationKimura et al., 2008; Rougier A, 2008).
Table 3. Content of four pentacyclic triterpene in Centella asiatica extracts obtained from different places for cultivation and harvesting periods.
Conclusions
These results indicate that the leaves of C. asiatica contained the highest amount of the active compounds. C. asiatica collected from Trang and Songkhla gave the highest content of total pentacyclic triterpenes. However, C. asiatica collected from Trang gave the highest one when collected in March, while that from Songkhla was in December. In contrast, C. asiatica collected from Nakornsrithammarat and Ratchaburi gave a very low content of total pentacyclic triterpenes in all harvesting periods. C. asiatica raw materials harvested from different places or from different conditions of cultivation as well as different periods will provide a different quality of the plant extracts, and this may affect the efficacy of the herbal medicines from C. asiatica.
Declaration of interest
The authors wish to thank Prince of Songkla University and the Royal Golden Jubilee PhD program (Grant No. PHD/0271/2551). The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bonte F, Dumas M, Chaudagne C, Meybeck A. (1994). Influence of asiatic acid, madecassic acid, and asiaticoside on human collagen I synthesis. Planta Med, 60, 133–135.
- Cheng CL, Guo JS, Luk J, Koo MW. (2004). The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci, 74, 2237–2249.
- Djoukeng JD, Abou-Mansour E, Tabacchi R, Tapondjou AL, Bouda H, Lontsi D. (2005). Antibacterial triterpenes from Syzygium guineense (Myrtaceae). J Ethnopharmacol, 101, 283–286.
- Farnsworth RN, Bunyapraphatsara N. (1992). Thai Medicinal Plants Recommended for Primary Health Care System. Bangkok: Prachachon.
- Inamdar EK, Yeole RD, Ghogare AB, Souza NJ. (1996). Determination of biologically active constituents in Centella asiatica. J Chromatogr A, 742, 127–130.
- Kimura Y, Sumiyoshi M, Samukawa K, Satake N, Sakanaka M. (2008). Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur J Pharmacol, 584, 415–423.
- Lu L, Ying K, Wei S, Fang Y, Liu Y, Lin H, Ma L, Mao Y. (2004). Asiaticoside induction for cell-cycle progression, proliferation and collagen synthesis in human dermal fibroblasts. Int J Dermatol, 43, 801–807.
- Maquart FX, Bellon G, Gillery P, Wegrowski Y, Borel JP. (1990). Stimulation of collagen synthesis in fibroblast cultures by a triterpene extracted from Centella asiatica. Connect Tissue Res, 24, 107–120.
- Panichayupakaranant P. (2011). Quality Control and Standardization of Herbal Medicine. Songkhla: Neopoint.
- Panichayupakaranant P, Chatkrapunt U, Supavita T. (2006). Pharmacognostic and chemical studies on the leaves of Rhinacanthus nasutus. Nigerian J Nat Prod Med, 10, 1–5.
- Panichayupakaranant P, Intaraksa N. (2003). Distribution of hydroxyanthracene derivatives in Cassia alata and the factors affecting the quality of the raw material. Songklanakarin J Sci Technol, 25, 497–502.
- Park BC, Bosire KO, Lee ES, Lee YS, Kim JA. (2005). Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells. Cancer Lett, 218, 81–90.
- Rafamantanana MH, Rozet E, Raoelison GE, Cheuk K, Ratsimamanga SU, Hubert P, Quetin-Leclercq J. (2009). An improved HPLC-UV method for the simultaneous quantification of triterpenic glycosides and aglycones in leaves of Centella asiatica (L.) Urb (Apiaceae). J Chromatogr B Analyt Technol Biomed Life Sci, 877, 2396–2402.
- Rougier A. (2008). Clinical efficacy on epidermal wound healing of topically applied madecassoside associated with copper/zinc/manganese salts. J Am Acad Dermatol, 58, AB144.
- Shinomol GK, Muralidhara. (2008). Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and its in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine, 15, 971–984.
- Subathra M, Shila S, Devi MA, Panneerselvam C. (2005). Emerging role of Centella asiatica in improving age-related neurological antioxidant status. Exp Gerontol, 40, 707–715.
- Won JH, Shin JS, Park HJ, Jung HJ, Koh DJ, Jo BG, Lee JY, Yun K, Lee KT. (2010). Anti-inflammatory effects of madecassic acid via the suppression of NF-kappaB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med, 76, 251–257.
- Yun KJ, Kim JY, Kim JB, Lee KW, Jeong SY, Park HJ, Jung HJ, Cho YW, Yun K, Lee KT. (2008). Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int Immunopharmacol, 8, 431–441.
- Zheng C, Qin L. (2007). Chemical components of Centella asiatica and their bioactivities. J Chin Integr Med, 5, 348–351.