Abstract
Context: During the last few decades, the prevalence of obesity in the western world has dramatically increased with epidemic proportions. Hand in hand with this statistic, the incidences of obesity-linked diseases such as diabetes are increasing with pandemic rate. The search for novel drugs and nutritional intervention approaches for obesity is now of significant importance.
Objective: The anti-obesity potential of eriodictyol (ERD) and its close structural analogue, sigmoidin A (SGN), were evaluated. SGN was isolated from Erythrina abyssinica Lam. ex DC. (Fabaceae).
Materials and methods: Concentrations between 300 and 0.1 µM of test samples and reference drugs made in three-fold dilutions were tested for enzyme inhibitory effects. The major obesity target, pancreatic lipase, was used to test the anti-obesity potential while the selective effects of the compounds were determined through assessments of effects on α-glucosidase.
Results: The inhibitory effect of SGN on pancreatic lipase (IC50, 4.5 ± 0.87 µM) was 30-times greater than that of ERD (IC50, 134 ± 19.39 µM) while their effect on α-glucosidase enzyme was comparable (IC50 value of 62.5 ± 9.47 and 57.5 ± 13.15 µM). The anti-obesity drug, orlistat, inhibited pancreatic lipase with an IC50 value of 0.3 ± 0.04 µM, while the anti-diabetic drug, acarbose, inhibited α-glucosidase with an IC50 value of 190.6 ± 16.05 µM.
Discussion: Although less active than the standard anti-obesity drug, orlistat, the observed activity indicated that prenylation of the flavonoid skeleton potently enhances anti-lipase activity.
Conclusion: Such groups of flavonoids need to be further investigated for their therapeutic and nutritional benefit in combating obesity problems.
Introduction
Obesity has become the most serious public health problem worldwide and its prevalence during the last few decades, especially in the western world, has dramatically increased with epidemic proportions. The UK is considered the ‘fattest’ country in Europe with the number of obese adults is forecasted to reach 26 million (a rise by 73%) over the next 20 years (CitationDiabetes UK, 2012). Current estimates further indicate that approximately one in every five adults in the UK is overweight, and one in every 15 is obese (CitationDiabetes UK, 2012). The USA figure parallels that of the UK with about one-third of adults (33.8%) and approximately 17% (or 12.5 million) of children and adolescents aged 2–19 years being obese [CDC (Centres for Disease Control and Prevention), 2012]. The figures for other western countries are also alarming. For example, one in four Canadian adults and 8.6% of children and youth aged 6–17 are obese (PHAC (Public Health Agency of Canada), 2012). The WHO’s (World Health Organisation’s) chilling report for European countries further indicate over 50% of both men and women are overweight, and roughly 23% of women and 20% of men are obese (CitationWHO, 2012). Obesity is now also becoming an emerging threat in many developing countries (CitationAbubakari & Bhopal, 2008; CitationYoon et al., 2006).
To date, a number of genetic and environmental factors that predispose individuals to weight gain have been proposed but the fundamental cause of obesity is still an imbalance between dietary intake and energy expenditure (CitationSchoeller, 2008). Hence, obesity results from excess calories being stored overtime as triglyceride in adipose tissue and ectopically in other tissues. Living conditions in the western world with the ever increasing readily available energy dense food and more sedentary lifestyles and increasing reliance on fast foods are all known to contribute for the increased prevalence of obesity. The economic burden of obesity to our society has become huge as it is linked to various disease conditions (CitationGolay & Ybarra, 2005; CitationHaslam, 2010). Hand in hand with obesity, the incidence of diabetes is increasing with pandemic rate and the link between these two conditions has been well established (CitationDiabetes UK, 2012; CitationGolay & Ybarra, 2005). Obesity has also shown to be associated with the risks for coronary heart disease, atrial fibrillation, heart failure, hypertension and various others cardiovascular diseases (CitationZalesin et al., 2011). The links between obesity and cancer (CitationPolednak, 2003) as well as many other diseases have also been established in recent years. All these facts and figures underscore the urgent need to control obesity through various measures; from lifestyle adjustment to nutritional and therapeutic interventions.
One of the most popular drug therapeutic approaches for obesity is through reducing body weight by inducing energy malabsorption. A prototype of drug reducing body weight through this mechanism is orlistat which inhibits pancreatic lipase and reduces weight by around 3 kg on average as well as decreasing the progression to diabetes in high-risk patients (CitationPadwal & Majumdar, 2007). A number of side effects, predominantly of gastrointestinal origin were, however, reported for such drugs (CitationBallinger & Peikin, 2002) and there remains a case that new anti-obesity drugs are in high demand.
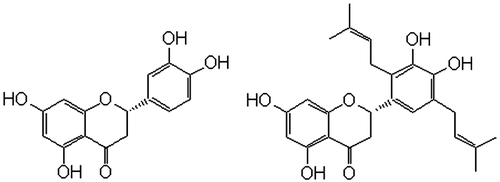
Sigmoidin A (SGN) is a prenelyated derivative of a flavanone compound, eriodictyol (ERD, ). SGN isolated from Erythrina sigmoidea Hua (Fabaceae) has been shown to display anti-inflammatory activity (CitationNjamen et al., 2004) while that obtained from the stem bark of E. abyssinica Lam. ex DC. displayed anti-malarial (CitationYenesew et al., 2004) and antioxidant/prooxidant-dependent cytotoxicity in cancer cells (CitationHabtemariam & Dagne, 2010). In the present communication, the potential of SGN as an anti-obesity agent was investigated through in vitro pancreatic lipase assay.
Materials and methods
Materials
Acarbose, 5,5-dithiobis(2-nitrobenzoic acid) (DTNB), 4-methylumbelliferyl oleate, p-nitrophenyl-α-d-glucopyranoside (pNPG), orlistat, pancreatic lipase (type II from porcine pancreas, 200–400 units/mg), sodium carbonate, yeast α-glucosidase (from Saccharomyces cerevisiae, 750 units), and Tris–HCl were obtained from Sigma-Aldrich Chemical Company, Dorset, UK. Eriodictyol (ERD) was purchased from AApin Chemicals Ltd (Abingdon, Oxon, UK).
Isolation of SGN from the stem bark of E. abyssinica
Sigmoidin A (SGN), isolated from the stem bark of E. abyssinica following the previously described procedure (CitationCui et al., 2007; CitationIchimaru et al., 1996), was a kind gift from Professor Ermias Dagne (Addis Ababa University, Ethiopia). Briefly, the dried powdered bark of E. abyssinica (1.4 kg) was extracted three times with CHCl3 to give 106 g of the extract residue. Some of the extract (36 g) was applied on flash silica gel, eluting with increasing amount of ethyl acetate in hexane. The fraction obtained from hexane:ethyl acetate (7:3) upon standing gave crystals (500 mg). Spectroscopic analysis including 2D-NMR study resulted in the identification of the compound as sigmodin A (CitationCui et al., 2007; CitationIchimaru et al., 1996).
Anti-lipase assay
The method described by CitationZhang et al. (2010) was used. The reaction mixture in 100 µL volume contained 4-methylumbelliferyl oleate (0.05 M), pancreatic lipase (0.5 mg/mL) and various concentrations of test agents. A blank and control groups in four replicates were set up for each concentration. After incubation of microtiter plates at 37°C for 30 min, the fluorescence associated with enzymatically released 4-methylumbelliferone product was measured using Fluoroskan Ascent FL Fluorometer (Altrincham, UK). All experiments were repeated at least four times.
α-Glucosidase inhibition assay
The published procedure of routine microtiter-based α-glucosidase inhibition assay was used (CitationHabtemariam, 2011). The reaction mixture (100 μL) in 250 mM phosphate buffer (pH 6.8) contained 2.5 mM pNPG, experimental drugs and 1.2 U/mL of α-glucosidase. After incubation of microtiter plates at 37°C for exactly 10 min, 25 µL of 0.2 mol/L sodium carbonate solution was added to each well to stop the reaction. The 4-nitrophenol absorption was measured at 405 nm using Multiskan plate reader (Thermo Labsystems, Altrincham, UK).
Statistical analysis
Dose-response curves were calculated using the Prism 4.03 software package (GraphPad, San Diego, CA, USA) and statistical significance were necessary was determined by unpaired t-test. All data are presented as mean and SEM values.
Results and discussion
To date, the antioxidant effects of flavonoids are by far the most studied biological activity (CitationHabtemariam & Dagne, 2010). Accordingly, the flavanone, ERD, has been demonstrated to show radical scavenging effect in a variety of assay models (CitationChen et al., 2008; CitationNarváez-Mastache et al., 2008; CitationTsimogiannis & Oreopoulou, 2004, Citation2006). ERD has also shown to display various other pharmacological effects including cytoprotection (CitationLee et al., 2011), in vitro anti-inflammatory (CitationLee, 2011), suppression of pain signalling receptors (CitationRossato et al., 2011) and cytotoxicity in leukaemic cells (CitationOgata et al., 2000). In comparison to ERD and other related flavonoid aglycones, data on the pharmacological activity of the prenylated ERD, SGN, is scarce. Some of the published literature on SGN reveals moderate antioxidant (CitationNjamen et al., 2004), antimicrobial (CitationFomum et al., 1983; CitationYenesew et al., 2004) and anti-inflammatory effects (CitationNjamen et al., 2004). The most prominent biological effect of SGN reported in recent years however is on its pronounced prooxidative effect than ERD (CitationGalati et al., 2002) that attributes to its potential anticancer properties (CitationHabtemariam & Dagne, 2010). The demonstration of such a level of differential biological activity for these close structural analogues () prompted us to further investigate their comparative biological effects including possible anti-obesity properties.
A number of flavonoid aglycones such as quercetin (CitationYou et al., 2012) have been demonstrated to display moderate activity against the key anti-obesity target, pancreatic lipase. On such grounds, the present study was designed to evaluate the comparative effect of ERD and SGN in this clinically validated anti-obesity target. As shown in , a concentration-dependent lipase inhibition was observed for both compounds. For comparitive purposes, the activity profile of the standard anti-obesity drug, orlistat, is also shown in and . It is evident that while the activity profile of ERD is moderate as reported for many flavonoid aglycones (CitationYou et al., 2012), a potent activity was observed for SGN. It is also apparent from that SGN was about 30 times more potent than ERD. Thus, the addition of the two prenyl groups in ring-B of the ERD flavanone skeleton appears to considerably enhance pancreatic lipase inhibition.
Table 1. Antilipase and anti-α-glucosidase inhibitory effects of test compounds.
Figure 2. Anti-lipase activity profile of eriodictyol (ERD), sigmoidin (SGN) and orlistat. Data from a representative result shows mean and SEM values (n = 4).
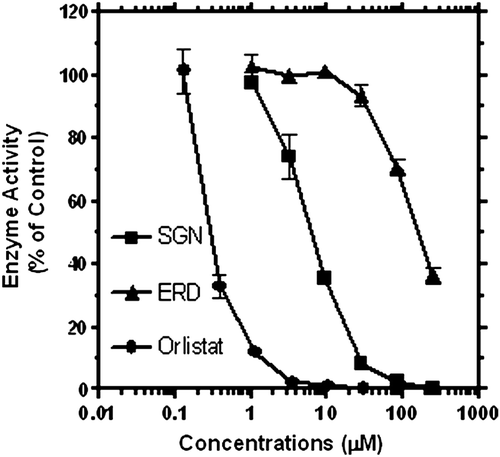
Recent research from several laboratories, including ours (CitationHabtemariam, 2011; CitationRoselli et al., 2011), have documented the anti-α-glucosidase effects of many flavonoids. In this in vitro diabetes assay model, both ERD and SGN displayed moderate activity (). The activity of SGN in α-glucosidase inhibition was about 14-times weaker than its lipase inhibition, while ERD (though moderate activity) appears to be more active against α-glucosidase than pancreatic lipase.
Conclusion
Although the observed effect for SGN was less than that of orlistat, this study for the first time documented a potent anti-lipase activity for SGN. This activity was selective to pancreatic lipase as a weaker effect was observed for α-glucosidase. Given that prenylation in ring-B of the ERD flavanone skeleton appears to increase the anti-lipase effect by an order of magnitude, future the anti-lipase work should be directed in the evaluation of various natural prenylated flavonoids.
Acknowledgement
The author wishes to thank Professor Ermias Dagne for the kind gift of sigmoidin A.
Declaration of interest
The author declares that there is no conflict of interest.
References
- Abubakari AR, Bhopal RS. (2008). Systematic review on the prevalence of diabetes, overweight/obesity and physical inactivity in Ghanaians and Nigerians. Public Health, 122, 173–182.
- Ballinger A, Peikin SR. (2002). Orlistat: Its current status as an anti-obesity drug. Eur J Pharmacol, 440, 109–117.
- CDC (Centres for disease control and prevention). (2012). U.S. Obesity Trends. http://www.cdc.gov/obesity/data/trends.HTML. Accessed on 6 April 2012.
- Chen Y-H, Chang F-R, Lin Y-J, Hsieh P-W, Wu M-J, Wu Y-C. (2008). Identification of antioxidants from rhizome of Davallia solida. Food Chem 107, 684–691.
- Cui L, Ndinteh DT, Na M, Thuong PT, Silike-Muruumu J, Njamen D, Mbafor JT, Fomum ZT, Ahn JS, Oh WK. (2007). Isoprenylated flavonoids from the stem bark of Erythrina abyssinica. J Nat Prod, 70, 1039–1042.
- Diabetes UK (2012). Diabetes and obesity. Available at: http://www.diabetes.co.uk/diabetes-and-obesity.html. Accessed on 6 April 2012.
- Fomum ZT, Ayafor JF, Mbafor JT. (1983). Erythrina studies: Part 1. Novel antibacterial flavanones from Erythina sigmoidea. Tet Lett, 24, 4127–4130.
- Galati G, Sabzevari O, Wilson JX, O’Brien PJ. (2002). Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology, 177, 91–104.
- Golay A, Ybarra J. (2005). Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab, 19, 649–663.
- Habtemariam S. (2011). A-glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat Prod Commun, 6, 201–203.
- Habtemariam S, Dagne E. (2010). Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med, 76, 589–594.
- Haslam D. (2010). Obesity and diabetes: The links and common approaches. Prim Care Diabetes, 4, 105–112.
- Ichimaru M, Moriyasu M, Nishiyama Y, Kato A. (1996). Structural elucidation of new flavanones isolated from Erythrina abyssinica. J Nat Prod, 59, 111–116.
- Lee ER, Kim JH, Choi HY, Jeon K, Cho SG. (2011). Cytoprotective effect of eriodictyol in UV-irradiated keratinocytes via phosphatase-dependent modulation of both the p38 MAPK and Akt signaling pathways. Cell Physiol Biochem, 27, 513–524.
- Lee JK. (2011). Anti-inflammatory effects of eriodictyol in lipopolysaccharide-stimulated raw 264.7 murine macrophages. Arch Pharm Res, 34, 671–679.
- Njamen D, Mbafor JT, Fomum ZT, Kamanyi A, Mbanya JC, Recio MC, Giner RM, Máñez S, Ríos JL. (2004). Anti-inflammatory activities of two flavanones, sigmoidin A and sigmoidin B, from Erythrina sigmoidea. Planta Med, 70, 104–107.
- Narváez-Mastache JM, Novillo F, Delgado G. (2008). Antioxidant aryl-prenylcoumarin, flavan-3-ols and flavonoids from Eysenhardtia subcoriacea. Phytochemistry, 69, 451–456.
- Ogata S, Miyake Y, Yamamoto K, Okumura K, Taguchi H. (2000). Apoptosis induced by the flavonoid from lemon fruit (Citrus limon BURM. f.) and its metabolites in HL-60 cells. Biosci Biotechnol Biochem, 64, 1075–1078.
- Padwal RS, Majumdar SR. (2007). Drug treatments for obesity: Orlistat, sibutramine, and rimonabant. Lancet, 369, 71–77.
- PHAC (Public Health Agency of Canada) (2012). Obesity in Canada. Available at: http://www.phac-aspc.gc.ca/hp-ps/hl-mvs/oic-oac/index-eng.php. Accessed on 6 April 2012.
- Polednak AP. (2003). Trends in incidence rates for obesity-associated cancers in the US. Cancer Detect Prev, 27, 415–421.
- Rossato MF, Trevisan G, Walker CI, Klafke JZ, de Oliveira AP, Villarinho JG, Zanon RB, Royes LF, Athayde ML, Gomez MV, Ferreira J. (2011). Eriodictyol: A flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem Pharmacol, 81, 544–551.
- Roselli M, Lentini G, Habtemariam S. (2011). Phytochemical, antioxidant and anti-α-glucosidase activity evaluations of Bergenia cordifolia. Phytother Res. DOI: 10.1002/ptr.3655.
- Schoeller DA. (2008). Insights into energy balance from doubly labeled water. Int J Obes (Lond), 32 Suppl 7, S72–S75.
- Tsimogiannis DI, Oreopoulou V. (2004). Free radical scavenging and antioxidant activity of 5,7,3′,4′-hydroxy-substituted flavonoids. Innov Food Sci Emerg Technol 5, 523–528.
- Tsimogiannis DI, Oreopoulou V. (2006). The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3′,4′-hydroxy substituted members. Innov Food Sci Emerg Technol 7, 140–146.
- Visscher TL, Seidell JC. (2001). The public health impact of obesity. Annu Rev Public Health, 22, 355–375.
- WHO (2012). Obesity: Facts and figures. Available at: http://www.euro.who.int/en/what-we-do/health-topics/noncommunicable-diseases/obesity/facts-and-figures. Accessed on 6 April 2012.
- Yenesew A, Induli M, Derese S, Midiwo JO, Heydenreich M, Peter MG, Akala H, Wangui J, Liyala P, Waters NC. (2004). Anti-plasmodial flavonoids from the stem bark of Erythrina abyssinica. Phytochemistry, 65, 3029–3032.
- Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. (2006). Epidemic obesity and type 2 diabetes in Asia. Lancet, 368, 1681–1688.
- You Q, Chen F, Wang X, Jiang Y, Lin S. (2012). Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT - Food Sci Technol, 46, 164–168.
- Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. (2011). Impact of obesity on cardiovascular disease. Med Clin North Am, 95, 919–937.
- Zhang L, Li J, Hogan S, Chung H, Welbaum GE, Zhou K. (2010).Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chem, 119, 592–599.