Abstract
Context: Several studies have reported the antioxidant activity and potential therapeutic properties of Punica granatum L. (Lythraceae) fruit. Medicinal properties have also been attributed to other parts of P. granatum tree, which are rich in bioactive phytochemicals.
Objective: To explore the phytochemical characteristics, in vitro and in vivo antioxidant and in vivo antigenotoxic potential of P. granatum leaf extract (PLE).
Materials and methods: The in vitro antioxidant potential of PLE was assessed by DPPH (1,1-diphenyl-2-picrylhydrazyl), ferric reducing antioxidant power (FRAP). Inhibition of lipid peroxidation (LPO) and the total phenolic content of the samples were also determined. Thirty-six male Swiss albino mice were divided into six groups (six animals each). Group 1 (control) and group 2 mice received vehicle and genotoxin alone, respectively. Groups 3, 4 and 5 were pretreated with PLE (400, 600 and 800 mg/kg body weight, respectively) prior to the administration of genotoxin. Group 6 received highest test dose of PLE. DNA damage in the bone marrow cells, hepatic LPO and antioxidants were recorded.
Results: Phytochemical analysis of PLE showed the presence of flavonoids, phenols, phytosterols, tannins and carbohydrates. Aqueous PLE demonstrated free radical scavenging activity, reducing power and inhibition of LPO with the EC50 values of 10.25, 59.88 and 20.05, respectively. A significant protective effect was observed against cyclophosphamide induced DNA damage and inhibition of hepatic LPO with concomitant increase in reduced glutathione (GSH) glutathione S-transferase (GST), superoxide dismutase (SOD) and catalase (CAT) in mice pretreated with PLE.
Discussion and conclusion: PLE demonstrated a significant antioxidant and antigenotoxic potential and hence can be a potential natural source in health and medicine.
Introduction
Punica granatum L. (Lythraceae), commonly known as pomegranate, has been described as an ancient, mystical and unique plant which is used in several systems of medicine for curing a variety of ailments (CitationLansky & Newman, 2007; CitationJurenka, 2008). It has been held as sacred by many of the world’s major religions (CitationLanglely, 2000; CitationJurenka, 2008). In Ayurvedic medicine, pomegranate is considered as “a pharmacy unto itself”. During the past few years, a large number of scientific papers have appeared on the antioxidant activity and potential therapeutic properties of pomegranate which includes treatment and prevention of cancer, cardiovascular disease and diabetes. Some of these studies have elucidated the molecular mechanisms associated with the beneficial health effects of pomegranate (CitationAviram & Dornfeld, 2001; CitationAfaq et al., 2005; CitationMalik et al., 2005; CitationAdams et al., 2006; CitationLansky & Newman, 2007). The long list of chemical constituents of P. granatum tree and fruit suggest that it is “loaded” with chemopreventive phytochemicals like ascorbic acid, ellagic acid, gallic acid, caffeic acid, chlorogenic acid, catechin, epicatechin, epigallocatechin 3-gallate, quercetin, kaempferol, rutin, apigenin, naringin, delphinidin, cyanidin, pelargonidin, punicalin, punicalagin and melatonin (CitationLansky & Newman, 2007). This clearly indicates that the medicinal properties of pomegranate can be attributed to the combined effects of many constituents.
Interesting pharmacological activity has been reported for almost every part of pomegranate tree/fruit (CitationLansky & Newman, 2007; CitationJurenka, 2008). From the list of chemical constituents of the seed, juice, peel, leaf, flower, bark and root, it is evident that there is variation in the type of chemopreventive phytochemicals isolated from each part (CitationLansky & Newman, 2007). Currently, the main focus of research is on the anticarcinogenic effects of pomegranate juice, seed oil, fruit peel and whole fruit extract. Comparatively less work has been carried out on the bioactivity of pomegranate leaf, which is also rich in antioxidants (CitationZhang et al., 2008). In China, pomegranate leaf tea is consumed to increase cerebral blood flow, treat skin ulcers, traumatic injuries and disorders in the digestive system (CitationXiang et al., 2008). A recent study has shown the anti-obesity effects of pomegranate leaf extract in high fat obese mice and this beneficial effect is mediated by inhibition of pancreatic lipase (PL) activity and suppression of energy intake (CitationLei et al., 2007).Since obesity is a major health problem in many countries, this finding on the anti-obesity effects of pomegranate leaf extract is likely to enhance its consumption.
The recent proliferation of scientific information on the health benefits of pomegranate has resulted in considerable increase in the consumption of this fruit and its products (CitationLansky & Newman, 2007). Pomegranate is generally considered as safe, owing to the fact that it has been consumed by humans for several centuries, largely without adverse health effects (CitationMeerts et al., 2009). However, a recent investigation employing both in vitro and in vivo assay systems has shown that pomegranate fruit extract can exert a weak genotoxic effect (CitationSanchez-Lamar et al., 2008). On the contrary, there are other reports based on in vitro assays, which have demonstrated the absence of genotoxic effects (CitationNegi et al., 2003; CitationMeerts et al., 2009). Therefore, it would be of interest from the point of human health if more information is obtained on the in vivo genotoxicity of other parts of pomegranate tree which are often used for preparation of phytomedicines. Furthermore, there is need for investigations to ascertain whether or not the different naturally occurring combinations of antioxidants and chemopreventive compounds present in different parts of the tree can exert protective effects against genomic damage induced by environmental chemicals.
In view of the presence of antioxidant phytochemicals in PL and the therapeutic properties attributed to PL tea, we initiated the present work with the main objective of evaluating the chemoprotective activity of PLE. In vitro experiments were carried out to assess antioxidant effects. Subsequent in vivo assays using mice, evaluated the protective effects of PLE pretreatment on oxidative stress and genomic damage induced by the genotoxin cyclophosphamide.
Materials and methods
Chemicals
Gallic acid, quercetin, 1,1-diphenyl-2-picrylhydrazyl (DPPH), cyclophosphamide, fetal calf serum, May-Grünwald’s stain, Giemsa stain and thiobarbituric acid reactive substances (TBARS) were purchased from Sigma (St. Louis, MO, USA). Folin–Ciocalteu reagent was obtained from Merck India Pvt. Ltd., Bangalore, India. Pyrogallol, 1-chloro-2,4-dinitrobenzene (CDNB), 5,5′-dithiobisnitro benzoic (DTNB) acid, reduced glutathione (GSH) and bovine serum albumin (BSA) were purchased from Himedia Laboratories Pvt. Ltd., India. All other reagents and solvents were of analytical grade from Qualigens Chemicals Pvt. Ltd., India.
Animals
All the experiments were carried out with 10–12 weeks old male Swiss albino mice weighing 28 ± 2 g. These animals were obtained from Tamil Nadu Veterinary and Animal Sciences University, Chennai, India and maintained in the University animal house on the standard mouse diet (Sai Durga Feed and Foods, Bangalore, India) and water ad libitum. The animals used in the present study were maintained in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India and approved by the University ethical committee.
Plant materials
P. granatum leaves were collected fresh during September to November 2010 from a location in Tiruchirapalli (Tamil Nadu, India). The leaves were washed with double distilled water to remove dust. The clean leaves were shade dried till it became brown and crispy. These shade dried leaves were coarse powdered in an electrical grinder and stored in air tight glass container in dark condition until further use. The plant has been identified by Dr. S. John Britto SJ, Director, RAPINET Herbarium & Centre for Molecular Systematics, St. Joseph College campus, Tiruchirapalli and a voucher specimen No. RHT. 63874 has been deposited in the herbarium.
Preparation of extract
Powdered leaves (10 g) were extracted with 100 mL of water at 45°C for 1 h. Thereafter the extract was filtered using Whatman No. 42 filter paper. The residue was again extracted with the same solvent twice. Extracts were filtered and concentrated using rotary evaporator. The concentrates were dried in vacuum desiccators and the dried extract was stored at −20°C.
Qualitative phytochemical screening of extracts
Chemical tests were carried out using the different extracts from the powdered specimens, using standard procedures to identify the constituents as described by CitationSofowora (1993), CitationTrease and Evans (1989) and CitationHarborne (1973).
Estimation of total phenolic content of extract
Total phenolic content of P. granatum extracts (PLEs) was determined by Folin–Ciocalteu reagent (CitationMcDonald et al., 2001). PLEs (0.5 mL of 1:10 g/mL) or gallic acid (standard phenolic compound) was mixed with 5 mL of Folin–Ciocalteu reagent (1:10 diluted with distilled water) and 4 mL of aqueous Na2CO3 (1 M). The mixtures were allowed to stand for 15 min and the total phenolic content was determined by colorimetry at 765 nm. The standard solution was prepared using gallic acid (50–250 mg/L) in methanol: water (50:50, v/v). Total phenol values are expressed in terms of gallic acid equivalent (mg/g of dry mass), which is a common reference compound.
Estimation of total flavonoid content of extract
The total flavonoid content was determined by aluminum chloride colorimetric method described by CitationChang et al. (2002). Briefly, aliquots containing PLE (0.5 mL of 1:10 g/mL) was mixed with 1.5 mL of 95% alcohol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of deionized water. After incubation at room temperature for 40 min, the reaction mixture was measured at 415 nm against deionized water blank. Quercetin was chosen as a standard using series of concentrations (12.5–100 µg/mL). Total flavonoids contents were calculated as quercetin equivalent (mg/g of dry mass).
Determination of free radical scavenging activity of PLE
The stable DPPH was used for determination of free radical scavenging activity of the extracts (CitationKoleva et al., 2002). Different concentration of PLE was added, at an equal volume, to methanol solution of DPPH (100 µM). After 15 min at room temperature, the absorbance was recorded at 517 nm. The experiment was repeated three times. Quercetin was used as standard control.
Determination of reducing power of PLE
The reducing power of the PLE was determined according to the method of CitationOyaizu (1986). PLE (10–100 µg) in 1 mL of distilled water was mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide (K3Fe(CN)6). The mixture was incubated at 50°C for 20 min. To this mixture, 2.5 mL of 10% tri-chloroacetic acid (TCA) was added, and mixture was centrifuged at 1500g for 10 min. Then, 2.5 mL of the supernatant was mixed with 2.5 mL distilled water and 0.5 mL, 0.1% FeCl3. Absorbance was measured at 700 nm using spectrophotometer.
Estimation of lipid peroxidation
In thiobarbituric acid assay (TBA), TBA reacts with malondialdehyde (MDA) to form a di-adduct, a pink chromogen, which can be detected spectrophotometrically at 532 nm (CitationHalliwell & Guttridge, 1989). Briefly, normal male mice (25–30 g) were used for the preparation of liver homogenate. The perfused liver was isolated, and 10% (w/v) homogenate was prepared with 0.15 M KCl. Different concentrations (5–100 µg/mL in methanol) of PL extract, 1 mL of 0.15 M KCl and 0.5 mL of mice liver homogenate was added to the test tubes. Peroxidation was initiated by adding 100 µL of 0.2 mM FeCl3. After incubation at 37°C for 30 min, the reaction was stopped by adding 2 mL of ice-cold HCl (0.25 N) containing 15% TCA, 0.38% TBA, and 0.5% butylated hydroxytoluene (BHT). The reaction mixtures were heated at 80°C for 60 min. The samples were cooled and centrifuged, and the absorbance of the supernatants was measured at 532 nm. The percentage inhibition of lipid peroxidation (LPO) is calculated by the formula:
Mouse bone marrow micronucleus test for evaluating in vivo genomic damage
Three test doses (400, 600 and 800 mg/kg body weight) of freshly prepared P. granatum leaf extract (PLE) were administered by gavage (10 mL/kg body weight) to the experimental animals for 7 consecutive days. The control animals received the same volume of distilled water. The genotoxin cyclophosphamide (CPH) was dissolved in saline and injected intraperitoneally (40 mg/kg) 2 h after the final pretreatment with PLE. All the experimental mice were killed 24 h after CPH treatment. Each pretreatment group consisted of six mice. Doses were selected based on our preliminary studies in our laboratory. Genotoxic effects were evaluated by the mouse bone marrow micronucleus test, which was carried out according to CitationSchmid (1975). The bone marrow cells from both femurs were flushed in the form of a fine suspension into a centrifuge tube containing human FB serum. This cell suspension was centrifuged at 700 g for 10 min, and the pellet was resuspended in a drop of serum before being used for preparing slides. Air-dried slides were stained with May Grünwald and Giemsa as described by CitationSchmid (1975). For each experimental point, six mice were used and 2500 polychromatic erythrocytes (PCEs) were scored per animal per slide to determine the frequency of micronucleated polychromatic erythrocytes (Mn PCEs). In addition, the percentage of PCEs was calculated on the basis of the ratio of PCEs to NCEs (normochromatic erythrocutes) in different regions of the slides prepared from control and treated animals. All the slides were scored by the same observer.
Biochemical assays
After the experimental period, the animals were fasted overnight and were sacrificed by cervical decapitation. Then the animals were quickly dissected out and the liver was excised and perfused with ice-cold saline (0.9% sodium chloride). A 10% (w/v) homogenate in appropriate buffer (pH 7.4) was prepared and centrifuged at 8000 g for 10 min at 4°C and further processed for the estimating LPO and antioxidants enzymes.
Estimation of lipid peroxidation
LPO in liver was estimated colorimetrically by TBARS by the method of CitationOhkawa et al. (1979). In brief, 0.2 mL of tissue homogenate (Tris–Hcl buffer, pH 7.4) was treated with 1.5 mL of TBA and placed in water bath for 20 min and cooled. The absorbance of clear supernatant was measured against reference blank at 532 nm.
Assay of catalase
Catalase (CAT) was assayed colorimetrically at 620 nm and expressed as µmoles of H2O2 consumed/min/mg protein as described by CitationSinha (1971). The reaction mixture (1.5 mL) contained 1.0 mL of 0.01 M pH 7.0 phosphate buffer, 0.1 mL of tissue homogenate (supernatant) and 0.4 mL of 2 M H2O2. The reaction was stopped by the addition of 2.0 mL of dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio).
Assay of superoxide dismutase
Superoxide dismutase (SOD) was assayed utilizing the technique of CitationMarklund and Marklund (1974) based on inhibition of the pyrogallol auto-oxidation. The supernatant (0.5 mL), 2 mL of 0.1 M Tris buffer and 1.5 mL water were mixed. The reaction was initiated by adding 0.5 mL of pyrogallol. Change in OD (due to auto-oxidation) was noted at an interval of one min over a period of three min at 480 nm. A single unit of enzyme was expressed as 50% inhibition of pyrogallol auto-oxidation/min/mg protein.
Assay of glutathione S-transferase
The glutathione S-transferase (GST) activity was determined spectrophotometrically by the method of CitationHabig et al. (1974). The reaction mixture (3 mL) contained 1.0 mL of 0.3 mM phosphate buffer (pH 6.5), 0.1 mL of 30 mM CDNB and 1.7 mL of double distilled water. After preincubating the reaction mixture at 37°C for 5 min, the reaction was started by the addition of 0.1 mL of tissue homogenate and 0.1 mL of GSH as substrate. The absorbance was followed for 5 min at 340 nm. Reaction mixture without the enzyme was used as blank. The activity of GST is expressed as µmoles of GSH–CDNB conjugate formed/min/mg protein using an extinction coefficient of 9.6 mM−1·cm−1.
Estimation of reduced glutathione
GSH was determined by the method of CitationMoron et al., (1979) 1.0 mL of supernatant was treated with 1 mL of 10% TCA and 0.5 mL of DTNB then 2.0 mL of phosphate solution. The absorbance was read at 420 nm. GSH peroxidase activity was expressed in µg/mg protein
Estimation of protein
Protein was determined by the method of CitationBradford et al. (1976) using BSA as standard, at 595 nm.
Statistical analysis
For the experiments of the protective effects of PLE against genotoxicity induced by CPH and on anti-oxidative enzyme activities, One-way ANOVA followed by Tukey’s test was used to evaluate the interaction between PLE treatment and CP with GraphPad Prism 4.0 (GraphPad Software, San Diego; CA; USA). p < 0.05 was considered statistically significant. Values were expressed as mean ± standard deviation.
Results and discussion
Phytochemical screening of P. granatum leaf extract
Qualitative analysis of phytochemicals in aqueous PLE showed positive results for carbohydrates, phytosterol, phenols, tannins and flavonoids and negative results for alkaloids, terpenoids, phlobatannins. The total phenol and flavonoid contents were found to be 70.00 ± 5.00 mg/g and 50.43 ± 5.05 mg/g, respectively.
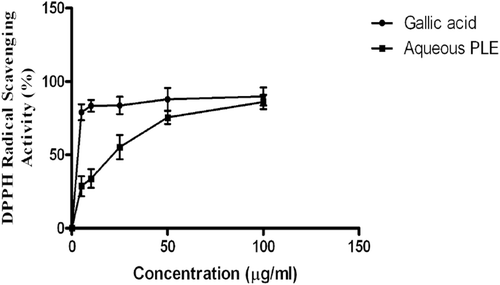
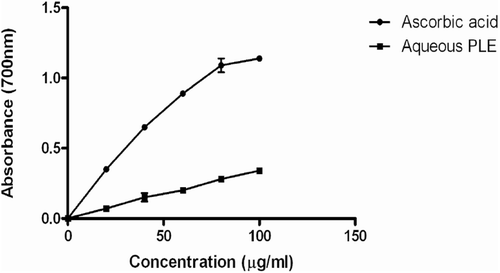
Evaluation of various leaf extracts of P. granatum for DPPH radical scavenging activity and reductive potential
illustrates the result of the DPPH radical scavenging assay. The percentage antioxidant activity of 100 µg/mL of the aqueous PLE was not significantly different from the positive control gallic acid. However, the antioxidant activity of 5–25 µg/mL of PLE was lower when compared to gallic acid. As shown in , the reductive potential of the aqueous PLE was <50% of that observed for ascorbic acid.
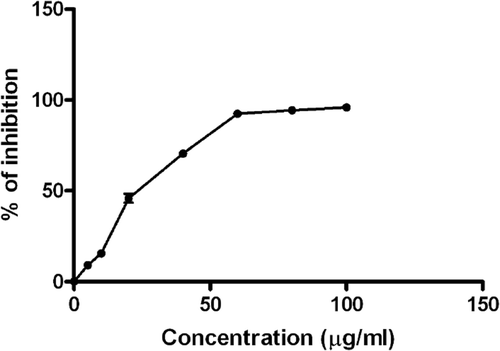
Inhibition of lipid peroxidation activity
showed that the aqueous PLE inhibited FeSO4 induced LPO on mouse liver homogenate in a dose-dependent manner, at concentrations 5, 10, 20, 40, 60, 80 and 100 µg/mL. The in vitro experiments () were performed under cell free conditions.
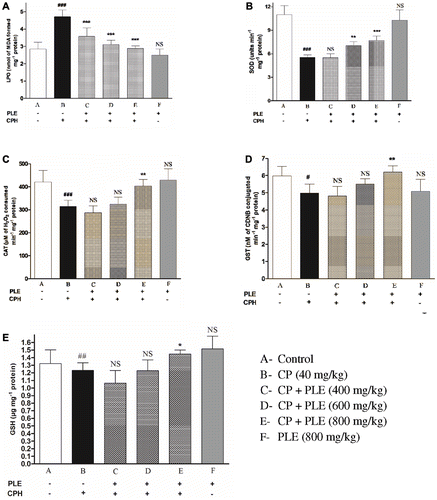
Effects of aqueous PLE on hepatic LPO, GSH levels and GST, SOD, CAT activities induced by cyclophosphamide
shows the extent of hepatic LPO, level of GSH and activity of GST, SOD and CAT in the control and treated animals. An increase in LPO level and a concomitant decrease in GSH, GST SOD and CAT were observed in mice treated with the genotoxin alone. The animals pretreated with three doses of aqueous PLE before exposure to the genotoxin showed a reduction in LPO and a dose-related increase in GSH, GST, SOD and CAT. Pretreatment with PLE exerted significant protection against genotoxin induced adverse changes and maintained the animals at near normal status.
Figure 4. Effects of aqueous PLE on cyclophosphamide induced oxidative stress in liver. (a) Effects of aqueous PLE on lipid peroxidation (LPO) expressed as nmol of MDA formed mg−1 protein. (b) Effects of aqueous PLE on superoxide dismutase (SOD) activity expressed as 50% inhibition of pyrogallol auto-oxidation min−1·mg−1 protein. (c) Effects of aqueous PLE on catalase (CAT) activity expressed as µmoles of H2O2 consumed min−1·mg−1 protein. (d) Effects of aqueous PLE on glutathione S-transferase (GST) activity expressed as nM of CDNB conjugated min−1·mg−1 protein (e) Effects of aqueous PLE on reduced glutathione (GSH) levels expressed as µg of GSH formed mg−1 protein. All values are expressed in mean ± SD with six mice in each group. ###p < 0.001; ##p < 0.01; #p < 0.05 compared with normal control group; ***p < 0.001; **p < 0.01; *p < 0.05 compared with cyclophosphamide treated group.
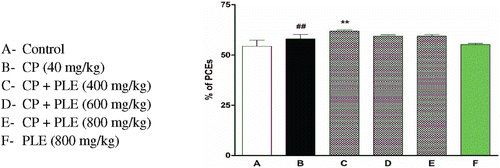
Effects of aqueous PLE on cyclophosphamide induced micronucleated polychromatic erythrocytes and total percentage of PCEs in bone marrow cells of mice
shows the frequencies of Mn PCEs and PCEs in bone marrow cells of mice which received aqueous PLE for 7 days before exposure to the genotoxin CPH. All the three doses of PLE were effective in exerting significant antigenotoxic effects against CPH. This effect was not always dose-dependent. The maximum reduction was observed in mice pretreated with a dose of 800 mg/kg PLE. Furthermore, there was no significant increase in genotoxicity following 7-day pretreatment with 800 mg/kg of aqueous PLE. There was no reduction in the percentage of PCEs following treatment with PLE and CPH ().
Figure 5. Effects of aqueous PLE on cyclophosphamide induced genotoxicity in bone marrow cells of mice. 2500 PCEs with or without micronuclei were randomly counted for each animal. All bars are expressed in mean ± SD of total number of MnPCEs per 2500 PCEs with six mice in each group. ###p < 0.001 compared with normal control group; ***p < 0.001 compared with cyclophosphamide treated group.
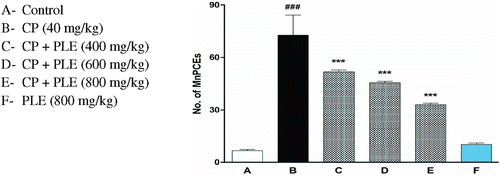
Figure 6. Effect of aqueous PLE on total percentage of PCEs. 2500 PCEs and corresponding NCEs were counted per each animal. The percentage of polychromatic erythrocytes was expressed as (PCEs/PCEs + NCEs) × 100. All bars are expressed in mean ± SD of total percentage of PCEs with six mice in each group. ##p < 0.01 compared with normal control group; **p < 0.01 compared with cyclophosphamide treated group.
The micronuclei in young erythrocytes (PCEs) arise mainly from chromosomal fragments that are not incorporated into the daughter nuclei at the time of cell division in the erythropoietic blast cells and changes in the incidence of MnPCEs are considered to reflect chromosomal damage. Hence, 2500 polychromatic erythrocytes were scored for the presence or absence of micronuclei. From the results of our in vitro and in vivo investigations, there is evidence for the antioxidant properties of PLE and the major role it can play as a chemopreventive agent against DNA damage. For the in vivo studies, we used the aqueous PLE in order to simulate the intake of PL tea, which is consumed in China.
Our in vitro tests have demonstrated antioxidant activity and protective effects against hydroxyl radical induced DNA damage. These results from in vitro assays would raise the question about its relationship with effectiveness under in vivo conditions wherein factors like bioavailability and metabolism have to be taken into consideration. The results we obtained from in vivo assays have shown that pretreatment of mice with PLE can significantly enhance the hepatic antioxidant status and reduce CPH induced genomic damage in the bone marrow erythrocytes, thereby establishing the positive relationship between in vitro and in vivo results.
It is most likely that traditional phytomedicines and the ingredients used for their preparation have not been evaluated for genotoxic effects. A recent investigation has reported a weak genotoxic effect for pomegranate whole fruit hydroalcoholic extract in in vitro and in vivo assays (CitationSanchez-Lamar et al., 2008). From our present in vivo micronucleus test, there is no evidence for any significant increase in genotoxicity following treatment of mice for seven days with 800 mg/kg b.w. aqueous PLE. From the point of chemoprevention, the important phytochemicals present in pomegranate leaf are apigenin, luteolin, gallitanins and ellagitanins such as punicalin, punicalagin, corilagin and punicafolin (CitationTanaka et al., 1985; CitationNawwar et al., 1994; CitationHussein et al., 1997). An evaluation of the ellagic acid content of leaves in eleven varieties of pomegranate from the Zaozhuang region in China showed values ranging from 1.30 to 6.46 mg·g−1 of dry weight during five consecutive seasons from June to October (CitationXiang et al., 2008). Some of the ellagitanins are not absorbed in vivo but reach the colon and release ellagic acid, which is well known for its antioxidant, anti-inflammatory, antimutagenic and anticarcinogenic effects (CitationLarrosa et al., 2006).
The presence of multiple chemopreventive phytochemicals in PLE suggests that the observed antioxidant effects and protection against genomic damage may be the result of additive or synergistic interaction of several chemical constituents of the extract. It has been stated that the benefits of phytomedicines often result from additive or synergistic actions of multiple active chemicals (CitationBriskin, 2000; CitationLiu, 2004). This is in contrast to synthetic pharmaceuticals which are based upon single chemicals (CitationBriskin, 2000). Furthermore, the problematic side effects associated with the predominance of a single xenobiotic can be eliminated by the synergistic or additive pharmacological effect of several phytochemicals (CitationTyler, 1999). Now it is widely believed that the activity of a single antioxidant nutrient does not explain the observed health benefits associated with dietary intake of fruits and vegetables (CitationLiu, 2004).
Conclusion
Our present work combining in vitro and in vivo assays has demonstrated the importance of PLE as a chemoprotective agent against oxidative stress and in vivo genomic damage. PLE alone has not shown any genotoxic effect.
Declaration of interest
We thank the University Grant Commission [No. F.37–109/2009 (SR)], and Department of Science and Technology [SR/FT/LS 004/2009], New Delhi, Government of India for the Financial Support. The authors report no conflicts of interest.
References
- Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. (2006). Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem, 54, 980–985.
- Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. (2005). Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer, 113, 423–433.
- Aviram M, Dornfeld L. (2001). Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis, 158, 195–198.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248–254.
- Briskin DP. (2000). Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol, 124, 507–514.
- Chang CC, Yang MH, Wen HM, Chern, JC. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal, 10, 178–182.
- Habig WH, Pabst MJ, Jakoby WB. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem, 249, 7130–7139.
- Halliwell B, Gutteridge JMC. (1989). In Free Radicals in Biology and Medicine. Tokyo, Japan: Japan Scientific Societies Press.
- Harborne JB. (1973). Phytochemical methods. A Guide to Modern Technique of Plant Analysis. London: Chapman and Hall Ltd., 49–188.
- Hussein SAM, Barakat HH, Merfort I, Nawwar MAM. (1997). Tannins from the leaves of Punica granatum. Phytochemistry, 45, 819–823.
- Jurenka JS. (2008). Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern Med Rev, 13, 128–144.
- Koleva II, van Beek TA, Linssen JP, de Groot A, Evstatieva LN. (2002). Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem Anal, 13, 8–17.
- Langlely P. (2000). Why a pomegranate? Br Med J 321, 1153–1154.
- Lansky EP, Newman RA. (2007). Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol, 109, 177–206.
- Larrosa M, Tomás-Barberán FA, Espín JC. (2006). The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem, 17, 611–625.
- Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H, Du LJ. (2007). Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond), 31, 1023–1029.
- Liu RH. (2004). Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J Nutr, 134, 3479S–3485S.
- Malik A, Afaq F, Sarfaraz S, Adhami VM, Syed DN, Mukhtar H. (2005). Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc Natl Acad Sci USA, 102, 14813–14818.
- Marklund S, Marklund G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem, 47, 469–474.
- McDonald S, Prenzler PD, Autolovich M, Robards K. (2001). Phenolic content and antioxidant activity of olive extracts. Food Chem, 73, 73–84.
- Meerts IA, Verspeek-Rip CM, Buskens CA, Keizer HG, Bassaganya-Riera J, Jouni ZE, van Huygevoort AH, van Otterdijk FM, van de Waart EJ. (2009). Toxicological evaluation of pomegranate seed oil. Food Chem Toxicol, 47, 1085–1092.
- Moron MS, Depierre JW, Mannervik B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta, 582, 67–78.
- Nawwar MAM, Hussein SAM, Merfort I. (1994). Leaf phenolics of Punica granatum. Phytochemistry, 37, 1175–1177.
- Negi PS, Jayaprakasha GK, Jena BS. (2003). Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem, 80, 393–397.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Oyaizu M. (1986). Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr, 44, 307–315.
- Sánchez-Lamar A, Fonseca G, Fuentes JL, Cozzi R, Cundari E, Fiore M, Ricordy R, Perticone P, Degrassi F, De Salvia R. (2008). Assessment of the genotoxic risk of Punica granatum L. (Punicaceae) whole fruit extracts. J Ethnopharmacol, 115, 416–422.
- Schmid W. (1975). The micronucleus test. Mutat Res, 31, 9–15.
- Sinha K. (1971). Colorimetric assay of catalase. Ann Biochem, 47, 389–394.
- Sofowora LA. (1993). Medicinal Plants and Traditional Medicine in Africa. Ibaban. Harborne: Spectrum Books Ltd., 55–71.
- Tanaka T, Nonaka GI, Nishioka I. (1985). Punicafolin, an ellagitannin from the leaves of Punica granatum. Phytochemistry, 24, 2075–2078.
- Trease GE, Evans WC. (1989). Trease and Evans’ Pharmacognosy: A Physician’s Guide to Herbal Medicine. 13th Edition. Bailliere Tindall London. Tsinghua Sci Technol, 13, 460–465.
- Tyler VE. (1999). Phytomedicines: Back to the future. J Nat Prod, 62, 1589–1592.
- Xiang L, Xing D, Lei F, Wang W, Xu L, Nie L, Du L. (2008). Effects of season, variety, and processing method on ellagic acid content in pomegranate leaves. Tsinghua Sci Technol, 13, 460–465.
- Zhang LH, Li LL, Li YX, Zhang YH. (2008). In vitro antioxidant activities of fruits and leaves of pomegranate. Acta Horticulturae, 765, 31–34.