Abstract
Context: Maillard reaction is implicated in the development of pathophysiology in age-related diseases. The search for newer Maillard reaction inhibitors is a priority among strategies to combat diabetes complications.
Objective: To evaluate the inhibitory potential of hesperidin, its derivatives and their stereoisomers against advanced glycation end-products (AGEs) formation.
Materials and methods: Hesperidin and hesperetin were chirally separated and the inhibitory effects of 1:1 mixture of (2S)- and (2R)-hesperidin (1), (2S)-hesperidin (2), (2R)-hesperidin (3), 1:1 mixture of (S)- and (R)-hesperetin (4), (S)-hesperetin (5), (R)-hesperetin (6), and monoglucosyl hesperidin (7) [1:1 mixture of (2S)-glucosyl hesperidin (8) and (2R)-glucosyl hesperidin (9)] at a concentration of 1 mM on protein glycation reaction have been revealed using the newly constructed RNase A-methylglyoxal (MGO) assay for the early stage and the bovine serum albumin (BSA)-glucose assay for the late stage of Maillard reaction.
Results: This study has demonstrated that hesperidin and its derivatives possessed relatively strong activity against the formation of AGEs. (S)-Hesperetin (5) possessed the highest inhibitory rate up to 57.4% in BSA-glucose assay, 38.2% in RNase A-MGO assay.
Discussion and conclusion: The new RNase A-MGO assay system could be used for the screening of AGEs inhibitors and hesperidin, and its derivatives could be promising candidate adjuvants for the treatment of diabetes complication, and age-related chronic diseases.
Introduction
The Maillard reaction, also referred to as non-enzymatic glycation, was first studied by Louis Camille Maillard in 1912. The Maillard reaction is a complex series of reactions that involve the reaction between carbonyl groups of reducing-sugars and amino groups of proteins, enzymes, nucleic acids or phospholipids. Reactive carbonyl species, such as 3-deoxyglucosone, glyoxal (GO) and methylglyoxal (MGO), are critical intermediates formed during glycation of proteins by glucose (CitationThornalley et al., 1999). In the late stage, these highly reactive carbonyl compounds like GO and MGO can react very rapidly in contrast to glucose with proteins leading to the formation of a group of chemically stable substances known as advanced glycation end-products (AGEs) (CitationThornalley, 2005), which are often colored, fluorescent, and prone to produce crosslinks in proteins (CitationHorvat & Jakas, 2004). AGEs, contributing to the accumulation of random damage in extracellular proteins, are known to have deleterious effects on biological function, and are associated with aging and diabetic complications, such as cataract, nephropathy, vasculopathy, proliferative retinopathy, and atherosclerosis complication in chronic diseases (CitationVinson & Howard, 1996).
Flavonoids are found ubiquitously in plants and are also the most common group of polyphenolic compounds in the human diet (CitationSpencer, 2008). Flavonoids, including genistein (CitationLv et al., 2011), have been discovered possessing the inhibitory activity against AGEs formation over the past decade (CitationUrios et al., 2007). Their relatively low toxicity compared to other active plant compounds (for instance alkaloids) mean they could be promising drug candidates.
Within the large family of flavonoids, flavanones present a unique structural feature known as chirality, and therefore exist in an S- and R-configuration. Hesperidin, as a flavanone found abundantly in citrus fruits, has many pharmacological activities such as anti-inflammatory, antioxidant, anticancer, immunomodulation, radioprotective effects, etc. During the course of our previous screening program, hesperidin (1) was found possessing Maillard reaction inhibitory activity, which was characterized to be a 1:1 mixture of (2S)-hesperidin (2) and (2R)-hesperidin (3). Although (2S)-hesperidin (2) and subsequently the (2S)-hesperetin (5) are naturally predominant in citrus fruits, hesperidin (1) and hesperetin (4) are commercially available as a mixture of both stereoisomers. As a result, most of the biological studies on hesperidin (1) and hesperetin (4) generally do not take the chirality into account, whereas in theories the two diasteromers or enantiomers may display distinct kinetic and dynamic properties (CitationAriëns, 1984) ().
Figure 1. Structures of hesperidin and its derivatives. Hesperidin (1): a 1:1 mixture of 2 and 3; hesperetin (4): a 1:1 mixture of 5 and 6; glucosyl hesperidin (7): a 1:1 mixture of 8 and 9.
To date, no study regarding hesperidin and its derivatives had been reported concerning the inhibitory activity against AGEs formation. In the present study, we explored the effects of hesperidin and its derivatives on the formation of AGEs for the first time, and separated stereoisomers of hesperidin and hesperetin using a chiral HPLC method and examined their activities respectively by glucose and methylglyoxal mediated modeling systems.
Materials and methods
Materials
Bovine serum albumin (BSA) (fraction V), bovine pancreatic RNase A, yeast transfer ribonucleic acid and hesperidin were purchased from Sigma Chemical Co. (St. Louis, MO, USA); hesperetin was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan); monoglucosyl hesperidin (G-hesperidin) was kindly provided by Hayashibara Biochemical Laboratories, Inc (Okayama, Japan).
Isolation of samples
All the samples were prepared by HPLC or recrystallization. A Hitachi Series consisting of a L-7100 pumps, a L-7000 column oven and a L-7455 diode array detector operating at 282 nm were used for HPLC analyses (Hitachi High-Technologies Corporation, Tokyo, Japan).
Separation of hesperidin C-2 diastereomers (CitationBelboukhari et al., 2010) were performed on a Chiralpak IA (amylose tris-3,5-dimethylphenylcarbamate) column (20 × 250 mm) coated on 5 µm silica gel (Daicel Chemical Industries, Ltd. Tokyo, Japan). The mobile phase was n-hexane/ethanol doped with 0.13% TFA (60/40). Flow rate was 5 mL·min−1, and the column temperature was 40°C.
Separation of hesperetin C-2 enantiomers (CitationCaccamese et al., 2005) were performed on a Chiralpak AS-H (cellulose tris-(S)-1-phenylethylcarbamate) column (4.6 × 250 mm) coated on 5 µm silica gel (Daicel Chemical Industries, Ltd. Tokyo, Japan). The mobile phase was n-hexane/2-propanol (80/20). Flow rates were 1 mL·min−1, and the column temperature was 40°C.
Bovine serum albumin (BSA)-glucose assay: BSA-glucose glycation and fluorescence detection of protein adducts in vitro
Advanced glycation end-products (AGEs) have a characteristic fluorescence with an excitation maximum at 370 nm and an emission maximum at 445 nm. The evaluation of inhibitory activity against the Maillard reaction in vitro was performed according to the previously described method (CitationMatsuura et al., 2002) with slight modification. The modeling of AGEs formation was between BSA and glucose. In brief, 200 µL of reaction mixture was prepared containing 0.8 mg·mL−1 BSA, 100 mM glucose with or without 2 µL of the 100 mM compound in 50 mM phosphate buffer (pH 7.4), and the reaction mixture was allowed to proceed at 60°C for 24 h. The inhibitory activity, described by fluorescence intensity (ex. 360 nm, em. 465 nm) based on AGEs formation, was measured by GENios FL Fluorescent Microplate Reader (Tecan, Männedorf, Switzerland). The inhibitory rate was calculated by the following equation:
Where FC, fluorescence intensity of BSA (control); FA, fluorescence intensity of AGEs without inhibitor; FA+I, fluorescence intensity of AGEs with inhibitor.
RNase A-MGO assay: RNase A-MGO glycation and detection of enzyme activity in vitro
Detection of the enzyme activity of RNase A was achieved by employing the method described in the literatures (CitationBergmeyer, 1984; CitationFatima et al., 2008). The modeling for cross-linking of proteins was constructed using 1 mg·mL−1 RNase A with 100 mM MGO according to previously described method (CitationSasaki et al., 2009) with modification. The 200 µL reaction mixture was prepared containing 1 mg·mL−1 RNase A, 100 mM MGO with or without 2 µL of the 100 mM compound in 20 mM phosphate buffer (pH 7.2), and the reaction mixture was allowed to proceed at 37°C on a heat block thermo-alumi-bath for 24 h. After dilution with extra-pure water by 200-fold, this reaction mixture were transferred into a 1.5 mL Eppendorf tube and incubated with 25 µg of transfer RNA in 60 mM phosphate buffer (pH 8.0) in a total volume of 250 µL.
The reaction was arrested after incubation for 20 min at 37°C on Eppendorf Thermomixer (Eppendorf AG, Hamburg, Germany) by using stopping reagent (22 mM lanthanum chloride in 1.2 M perchloric acid). Subsequently 0.2 mL of the solution was diluted with distilled water and the absorption of acid-soluble transfer ribonucleotides was measured at 260 nm by GeneQuant pro Spectrophotometer (GE healthcare, CT, USA). The protection activity of compound was calculated by absorbance of degraded tRNA by RNase A, which survived in the MGO mediated inactivation. The inhibitory rate was calculated by the following equation:
Where AB, absorbance of MGO and tRNA (blank); AC, absorbance of RNase A and tRNA (control); AA, absorbance of MGO, RNase A, and tRNA without inhibitor; AA+I, absorbance of MGO, RNase A, and tRNA with inhibitor.
Results and discussion
To clarify the structure–activity relationship of hesperidin, its derivatives and their corresponding stereoisomers against AGEs formation, the inhibitory effects of 1–7 were examined using the model systems of a newly constructed RNase A-MGO assay by detecting activity of survived RNase A for the early stage of Maillard reaction, and BSA-glucose assay for the late stage of Maillard reaction.
Hesperidin and its derivatives against AGEs formation
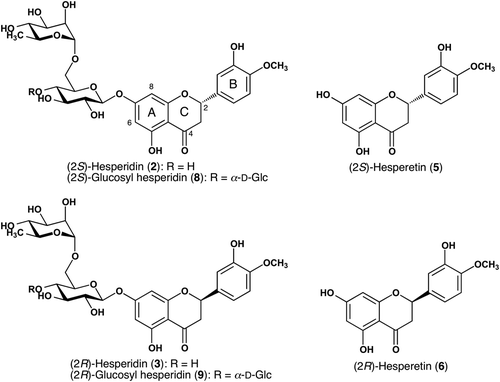
The calculated inhibitory rate of 1, 4 and 7 at a concentration 1 mM are 45.8, 56.7, and 43.5%, respectively, in the BSA-glucose assay; 33.6, 37.0, and 27.7% in the RNase A-MGO assay. The results demonstrated that hesperidin and its derivatives possess relatively strong inhibitory activity against AGEs formation in both of the assays. Especially in the early stage of Maillard reaction, protective effects of 1, 4 and 7 on RNase A targeted by MGO attack bear comparison with that of aminoguanidine (AG), an investigational drug for the treatment of diabetic nephropathy (CitationAbdel-Rahman & Bolton, 2002). The inhibitory mechanism could be the trapping of reactive dicarbonyl species by the two unsubstituted carbons (C-6 and C-8) at the A ring of flavonoids via electrophilic aromatic substitution as elucidated by CitationLv et al., (2011). In addition, their studies indicated that C ring may not play an important role in the trapping of reactive dicarbonyl species (CitationLv et al., 2011).
Both in the RNase A-MGO assay for the early stage and the BSA-glucose assay for the late stage of Maillard reaction, the inhibitory activities of hesperetin (4) were the strongest among these three flavanone derivatives 1, 4 and 7, while glucosyl hesperidin (7) were the weakest as shown in . Comparing to 1 and 7, the loss of the sugar chain at 7-position in 4 lead to the enhanced inhibitory activities (CitationMatsuda et al., 2003). In addition, the conjugated rhamnosyl-glucose in 1 also causes decrease in the inhibitory activity (CitationUrios et al., 2007). Comparing to 1, the additional glucoside residue with increased number of hydroxyl groups in 7 reduced the inhibitory activity (CitationMatsuda et al., 2003), though 7 has higher solubility than 1.
Figure 2. Protective effects of hesperidin (1), hesperetin (4), and glucosyl hesperidin (7) on (a) RNase A targeted by methyglyoxal (MGO) attack. (b) Advanced glycation end-products (AGEs) formation between bovine serum albumin (BSA) and glucose. The concentrations of tested samples and aminoguanidine (AG) were 1 mM. Each value was expressed as the mean ± SD, n = 3.
Hesperidin epimers and hesperetin enantiomers against AGEs formation
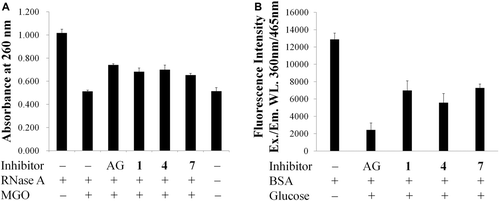
The relationship of configuration and activity of hesperidin epimers 2 and 3, and hesperetin enantiomers 5 and 6 against AGEs formation were also examined using the two model assay systems. According to the results given in , , R-configuration and S-configuration showed relatively similar inhibitory activity against the formation of AGEs at a concentration of 1 mM in vitro.
Figure 3. Protective effects of hesperidin (1), (2S)-hesperidin (2), and (2R)-hesperidin (3) on (a) RNase A targeted by methyglyoxal (MGO) attack. (b) Advanced glycation end-products (AGEs) formation between bovine serum albumin (BSA) and glucose. The concentrations of tested samples and aminoguanidine (AG) were 1 mM. Each value was expressed as the mean ± SD, n = 3.
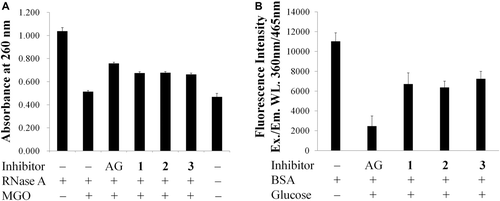
Figure 4. Protective effects of hesperetin (4), (2S)-hesperetin (5), and (2R)-hesperetin (6) on (a) RNase A targeted by methyglyoxal (MGO) attack. (b) Advanced glycation end-product (AGE) formation between bovine serum albumin (BSA) and glucose. The concentrations of tested samples and aminoguanidine (AG) were 1 mM. Each value was expressed as the mean ± SD, n = 3.
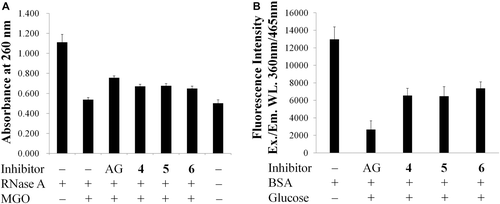
These results indicate both S- and R-configuration of hesperidin and hesperetin possess AGEs inhibitory activities, and moreover, the activities are not affected by differences in configuration. This was consistent with the finding of CitationBrand et al. (2010), that hesperetin enantiomers showed no notable differences in activation of EpRE mediated gene expression in vitro. Whether those epimers and enantiomers can show different inhibitory activity in vivo against formation of AGEs is a subject for future study.
Conclusions
In conclusion, the newly constructed modeling assay systems could be used for screening of AGEs inhibitors. Both in the RNase A-MGO assay for the early stage and the BSA-glucose assay for the late stage of Maillard reaction, all the samples being examined exhibited inhibitory activities against AGEs formation. The inhibitory activities of hesperetin (4) were the strongest among the three flavanones 1, 4, and 7. Between the two hesperetin enantiomers, (S)-hesperetin (5) possessed higher inhibitory rate up to 57.4% in BSA-glucose assay, 38.2% in RNase A-MGO assay. Protective effects of hesperidin derivatives and their stereoisomers in RNase A-MGO assay bear comparison with that of aminoguanidine. In present study, hesperidin and its derivatives showed in vitro activity against AGEs formation. Combined with the previous report in which hesperidin has been in vivo proved to be successful in reducing oxidative stress in diabetic rats (CitationMiyake et al., 1998), hesperidin and its derivatives could have potential therapeutic possibilities as a promising candidate adjuvants for treatment of diabetes complication, and age-related chronic diseases.
Acknowledgements
The authors thank Hayashibara Biochemical Laboratories, Inc. for supply of monoglucosyl hesperidin and Akita Konno Co., Ltd. for supply of part of experimental materials.
Declaration of interest
This work was financially supported in part by a fund from the Institute for Fermentation, Osaka (IFO), Japan.
References
- Abdel-Rahman E, Bolton WK. (2002). Pimagedine: a novel therapy for diabetic nephropathy. Expert Opin Investig Drugs, 11, 565–574.
- Ariëns EJ. (1984). Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology. Eur J Clin Pharmacol, 26, 663–668.
- Belboukhari N, Cheriti A, Roussel C, Vanthuyne N. (2010). Chiral separation of hesperidin and naringin and its analysis in a butanol extract of Launeae arborescens. Nat Prod Res, 24, 669–681.
- Bergmeyer HU. (1984). Methods of Enzymatic Analysis (Third ed.), New York, USA: Academic Press, 136–139.
- Brand W, Shao J, Hoek-van den Hil EF, van Elk KN, Spenkelink B, de Haan LH, Rein MJ, Dionisi F, Williamson G, van Bladeren PJ, Rietjens IM. (2010). Stereoselective conjugation, transport and bioactivity of S- and R-hesperetin enantiomers in vitro. J Agric Food Chem, 58, 6119–6125.
- Caccamese S, Caruso C, Parrinello N, Savarino A. (2005). High-performance liquid chromatographic separation and chiroptical properties of the enantiomers of naringenin and other flavanones. J Chromatogr A, 1076, 155–162.
- Fatima S, Jairajpuri DS, Saleemuddin M. (2008). A procedure for the rapid screening of Maillard reaction inhibitors. J Biochem Biophys Methods, 70, 958–965.
- Horvat S, Jakas A. (2004). Peptide and amino acid glycation: New insights into the Maillard reaction. J Pept Sci, 10, 119–137.
- Lv L, Shao X, Chen H, Ho CT, Sang S. (2011). Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem Res Toxicol, 24, 579–586.
- Matsuda H, Wang T, Managi H, Yoshikawa M. (2003). Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg Med Chem, 11, 5317–5323.
- Matsuura N, Aradate T, Sasaki C, Kojima H, Ohara M, Hasegawa J, Ubukata M. (2002). Screening system for the Maillard reaction inhibitor from natural product extracts. J Health Sci, 48, 520–526.
- Miyake Y, Yamamoto K, Tsujihara N, Osawa T. (1998). Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids, 33, 689–695.
- Sasaki NA, Garcia-Alvarez MC, Wang Q, Ermolenko L, Franck G, Nhiri N, Martin MT, Audic N, Potier P. (2009). N-Terminal 2,3-diaminopropionic acid (Dap) peptides as efficient methylglyoxal scavengers to inhibit advanced glycation endproduct (AGE) formation. Bioorg Med Chem, 17, 2310–2320.
- Spencer JPE. (2008). Flavonoids: Modulators of brain function? Br J Nutr, 99, ES60–ES77.
- Thornalley PJ. (2005). Dicarbonyl intermediates in the Maillard reaction. Ann N Y Acad Sci, 1043, 111–117.
- Thornalley PJ, Langborg A, Minhas HS. (1999). Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J, 344 Pt 1, 109–116.
- Urios P, Grigorova-Borsos AM, Sternberg M. (2007). Flavonoids inhibit the formation of the cross-linking AGE pentosidine in collagen incubated with glucose, according to their structure. Eur J Nutr, 46, 139–146.
- Vinson J, Howard T. (1996). Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J Nutr Biochem, 7, 659–663.