Abstract
Context: Gymnema sylvestre (GS) R. Br. (Gymnema) (Asclepiadaceae) has been used from ancient times as a folk medicine for the treatment of diabetes, obesity, urinary disorder, and stomach stimulation.
Objective: The present study was designed to investigate the effects of G. sylvestre leaves ethanol extract on gastric mucosal injury in rats.
Materials and methods: Gastric mucosal damage was induced by 80% ethanol in 36 h fasted rats. The effect of G. sylvestre on gastric secretions induced in Shay rats was estimated. In stomach, wall mucus, non-protein sulfhydryl groups (NP-SH), malondialdehyde (MDA), total proteins and nucleic acids levels were estimated. Histopathological changes were observed.
Results: G. sylvestre pretreatment at doses of 100, 200 and 400 mg/kg provided 27, 49, and 63% protection against the ulcerogenic effect of ethanol, respectively. Pylorus ligation accumulated 10.24 mL gastric secretions with 66.56 mEq of acidity in control rats. Pretreatment with G. sylvestre significantly inhibited the secretions volume and acidity in dose-dependent manner. Ethanol caused significant depletion in stomach-wall mucus (p < 0.001), total proteins (p < 0.01), nucleic acids (p < 0.001), and NP-SH (p < 0.001) levels. Pretreatment with G. sylvestre showed protection against these depleted levels in dose-dependent manner. The MDA levels increased from 19.02 to 29.22 nmol/g by ethanol ingestion and decreased with G. sylvestre pretreatments in dose-dependent manner.
Conclusion: The protective effect of G. sylvestre observed in the present study is attributed to its effect on mucus production, increase in nucleic acid and NP-SH levels, which appears to be mediated through its free radical scavenging ability and/or possible cytoprotective properties.
Gastric hyperacidity and gastroduodenal ulcer are common global problems and are caused by a lack of equilibrium between the gastric aggressive and the mucosal defensive factors (CitationRao et al., 2000). The etiology of gastroduodenal ulcers is influenced by various aggressive and defensive factors such as acid-pepsin secretion, parietal cell activation, reduction in mucous secretion, mucosal blood flow, cellular regeneration process and endogenous protective agents (CitationRepetto & Llesuy, 2002). Ethanol consumption is one of the many factors that increase the risk of gastric ulcer (Stermer, 2002). Experimentally, 80% ethanol is widely used to induce gastric lesions in animals as an animal model for gastric ulcer (CitationSheeba & Asha, 2006). It was reported that the pathogenesis of acute experimental gastric lesion may involve generation of oxygen derived free radicals, primarily superoxide anions, hydroxyl radicals, and lipid peroxides (CitationAl-Shabanah et al., 2000). Furthermore, the imbalance between gastrotoxic agents and protective mechanisms may results in an acute inflammation accompanied by neutrophils infiltration of gastric mucosa (CitationKonturek et al. 2000).
Gymnema sylvestre (GS) R. Br. (Gymnema) is classified as part of the Asclepiadaceae family and is widely distributed in Southern India, tropical Africa, and Australia (CitationKanetkar et al., 2007). From ancient times, G. sylvestre has been used in Indian traditional medicine as a potent anti-diabetic plant and is considered effective in improving urination, eye complaints, asthma, inflammations, snakebite and stomach stimulation (CitationDaisy et al., 2009; CitationRamkumar et al., 2009; CitationGrover et al., 2002). In addition, it possesses antimicrobial, antihypercholesterolemic, hepatoprotective and sweet-suppressing activities (CitationShigematsu et al., 2001a,b). Literature survey revealed that the major bioactive constituents of G. sylvestre leaves are a group of oleanane type triterpenoid saponins known as gymnemic acids (CitationKanetkar et al., 2007; CitationYe et al., 2000), alkaloids, acidic glycosides and anthroquinones and their derivatives (CitationSurveswaran et al., 2010). All of these phytoconstituents are known to offer protection against experimentally induced gastric damage in animal models (CitationJainu & Devi, 2006; CitationNavarrete et al., 2002; CitationSairam et al., 2002). Moreover, saponins and their triterpene derivatives have been found to promote ulcer healing by forming a protective mucus barrier on the gastric mucosa (CitationOyagi et al., 2010; CitationZakaria et al., 2011). One formulated product of gymnemic acid was found to have useful effects against obesity (CitationPorchezhian & Dobriyal, 2003). This may be due to the ability of gymnemic acids to delay the glucose absorption in blood through the small intestine, the exact action being unknown. Several reports indicated that diabetes mellitus increased the mucosal susceptibility to ulcerogenic stimuli and a predisposition to gastric ulceration (CitationTakeuchi et al., 1994; CitationTashima et al., 1998). In such conditions of co-occurring diabetes and gastric ulcers, it would be better to manage with drugs that have both anti-diabetic and antiulcer activities. However, market products coming from different reputed natural product companies claim that the gymnemic acids in G. sylvestre are responsible for its health positive effects (Mother Herbs & Agro Products, Amway Natural Products).
Presently, there are no reports pertaining to the antiulcer activity of G. sylvestre against experimental gastric ulcers. Thus, the present study was designed to determine the effect of G. sylvestre on gastric acid secretion and chemically-induced ulcers in Wistar albino rats.
Materials and methods
Animals
Male Wistar albino rats (home bred), all roughly the same age (9–10 weeks old), weighing 200–220 g, were used in the present study. The animals were maintained under controlled conditions of temperature (22 ± 1°C), humidity (55%), and light (12 h dark/light). They were provided free access to Purina rat chow (Manufactured by Grain Silos & Flour Mills Organization, Riyadh, Saudi Arabia) and drinking water. All procedures, including euthanasia, were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (1996) as well as the Ethical Guidelines of the Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Six rats in each group were taken for every set of experiments and fasted for 36 h. The number of animals used per group is usually the minimum necessary to test reliability for statistical significance for these types of experiments (CitationFesting & Altman, 2002).
Plant material
Dried, ethanol extracted G. sylvestre leaves filled in capsules mentioning “Gymnema extract 200 mg” with the brand name “Diaglu” manufactured by MEPACO, Egypt was used in the present study. For the purpose of recoding, the voucher specimen of the G. sylvestre was kept in the herbarium, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. As mentioned in the product voucher, the extract was standardized as 25% gymnemic acids in 200 mg weight of Gymnema extract. Further we confirmed the presence of these gymneric acids in our phytochemical laboratory.
Phytochemical screening
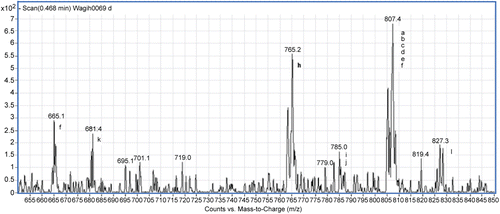
An Agilent 6410 Triple Quadrupole Mass Spectrometer (Agilent Technologies, Santa Clora, CA, USA) equipped with an electrospray ionization interface (ESI) coupled to an Agilent 1200 HPLC (Agilent Technologies, Santa Clora, CA, USA) was used. A connector was used instead of the column to allow direct injection of samples. Mobile phase composed of two solvents: [A] is HPLC grade water, and [B] is acetonitrile (ACN) mixed in the ratio 1:1. The content of whole capsule was screened for its phytochemical constituents. Test solutions for mass spectrometry (MS) were prepared by diluting the stock solutions with ACN/H2O mixture (1:1). Flow rate is 0.4 mL/min, run time was 3 min. Each sample (10 µL) was injected into the LC-MS/MS. MS parameters were optimized for scan mode. For screening of mass signals of the different compounds and to search for parent ions, MS2 scans were performed in the mass range of m/z 650–850. The results of the scan spectra of the capsule content are shown in and .
Table 1. List of compounds found in the negative MS scan spectra of the Gymnema sylvestre extract in capsule.
Estimation of gastric secretion in pylorus ligated (Shay) rats
Shay rat ulcer method (CitationShay et al., 1945) was followed in this study. The rats were fasted for 36 h with free access to water before the pylorus was ligated under ether anesthesia. Rats’ abdomens were opened by small midline incision below the xiphoid process. Then, pylorus portion of the stomach was slightly lifted out and ligated. Care has been taken not to cause bleeding or to occlude blood vessels (CitationShay et al. 1945). Each stomach was placed carefully in the abdomens and the wounds were sutured. G. sylvestre leaves extract suspensions (100, 200, and 400 mg/kg body weight) was administered by gavage immediately after pylorus ligation. The animals were sacrificed 6 h after pylorus ligation. Stomachs were removed, content collected, volume measured, centrifuged and analysis done for titratable acidity against 0.01 N NaOH to pH 7 using a pH meter and total acid output was calculated.
Gastric lesions induced by 80% ethanol
Fasted animals were administered with G. sylvestre dried leaves extract in the doses of 100, 200, and 400 mg/kg body weight, respectively. Cimetidine (50 mg/kg body weight) was used as reference drug. In the control group (vehicle), 0.25% CMC solution was administered. Sixty minutes after the drug and vehicle treatment, each animal was received 1 mL of 80% ethanol by gavage, as a necrotizing agent, which was known to produce gastric lesions (CitationAl-Shabanah et al., 2000; Citational-Bekairi et al., 1992). The animals were killed by decapitation 1 hour after the 80% ethanol treatment. The stomach was excised and opened along the greater curvature. After washing with normal saline, the gastric lesions were quantified using a binocular magnifier. The ulcers were scored according to the method of CitationValcavi et al. (1982) and assessed on the basis of their dimensions: Deep circular ulcers more than 8 mm = 10; 7–8 mm = 7; 5–6 mm = 6; 4–5 mm = 5; 3–4 mm = 4; 2–3 mm = 3; 1–2 mm = 2; 0–1 mm = 1. Furthermore, the deep linear ulcer more than 10 mm in length = 6 and linear ulcer less than 10 mm in length = 3. The score for each single lesion were then summed up for the determination of ulcer index.
Among the various biochemical parameters that may be studied in the impairment of gastric mucosal integrity and protection, we focused the attention on the alteration in endogenous levels of proteins, nucleic acids, MDA and NP-SH. Histopathological observation was then used to confirm the G. sylvestre and ethanol interaction in the gastric mucosa. The tissue samples, whether for biochemical analysis or histopathological screening, were coded and kept them as blind.
Determination of gastric wall mucus
The modified procedure of CitationCorne et al. (1974) was used to determine gastric mucus. The glandular segments from the stomach were removed and weighed then transferred immediately to 1% Alcian blue solution (in sucrose solution buffered with sodium acetate, pH 5). The excess dye was removed by rinsing with sucrose solution. The dye complexed with the gastric wall mucus was extracted with 10 mL of 0.5 M magnesium chloride solution. A 4 mL aliquot of blue extract was then shaken with an equal volume of diethyl ether. The resulting emulsion was centrifuged and the absorbance of the aqueous layer was recorded at 580 nm. The quantity of Alcian blue extracted (net) per grams of wet glandular tissue was then calculated.
Estimation of NP-SH in stomach
Gastric mucosal NP-SH was measured according to the method described by CitationSedlak and Lindsay (1968). The glandular stomach (400 mg) was removed and homogenized in 8.0 mL of ice-cold 0.02 M ethylenediaminetetraacetic acid (EDTA). The homogenate (5.0 mL) was mixed with distilled water (4 mL) and 1 mL (50% w/v) aqueous TCA and centrifuged. The supernatants (2 mL aliquots) were then mixed with 4 mL of Tris buffer (pH 8.9), 0.1 mL of 0.4% 5,5′-dithiobis (2-nitrobezoic acid) (DTNB) was added and the sample was shaken. The absorbance was read within 5 min of addition of DTNB, at 412 nm, against a reagent blank with no homogenate.
Estimation of MDA concentrations in stomach
The method described by CitationOhkawa et al. (1979) was followed with little modifications to estimate the MDA levels in stomach tissue. The stomach tissues (200 mg) were homogenized in aqueous 0.15 M KCl to give 10% homogenate. One milliliter of homogenate was mixed with 1 mL of 10% TCA and centrifuged at 3,000 rpm for 15 min. Then, 1 mL supernatant was mixed with 1 mL of 0.67% 2-thiobarbutaric acid. The test tubes were closed by glass stoppers and placed in a boiling water bath for 15 min. Tubes were allowed to cool down at room temperature. Optical density of the clear pink supernatants was recorded at 532 nm. Malondialdehyde bis (dimethyl acetal) was used as standard.
Estimation of protein and nucleic acids in stomach
The levels of proteins and nucleic acids in the stomach were determined according to the following procedures: the stomachs were rapidly dissected from the animals, frozen in liquid nitrogen, and stored at −70°C until they were analyzed for total proteins and nucleic acids (DNA and RNA). Total protein was determined by the method of CitationLowry et al. (1951). The method described by CitationBregman (1983) was used to determine levels of nucleic acids. Each stomach tissues were homogenized in ice-cold distilled water. The homogenates were extracted in different concentrations of cold and hot trichloroacetic acid (TCA) and 95% ethanol. DNA was determined by treating the nucleic acid extract with diphenylamine reagent and measuring the intensity of the blue color at 600 nm. For quantification of RNA, the nucleic acid extract was treated with orcinol reagent and the green color was recorded at 660 nm.
Histopathological assessment
The gastric tissue samples were preserved in 10% natural buffered formalin and processed for routine paraffin block preparation. Using an American optical rotary microtome, sections of thickness about 3 μm were cut and stained with hematoxyline and eosin (CitationCulling, 1974). The tissues were kept blind with the histopathologist. The slides were then examined under a microscope for pathomorphological changes as congestion, hemorrhage, edema and erosions using an arbitrary scale for the assessment of severity of these changes. The tissues were kept blind for the histopathologist. Microscopic reactive, reparative or degenerative changes seen in the mucosal layer were scored as follows: (1) mucosal congestion, (2) mucosal hemorrhage, (3) glandular disarray/degeneration, (4) mucosal erosions, (5) ulcer formation, (6) active inflammation, (7) submucosal edema, and (8) muscularis layer degeneration. The severity of histopathological changes was expressed according to scale of CitationRejaie (2009).
Statistical analysis
The data were expressed as mean ± standard error of means (S.E.M.). The difference between treatment groups was compared statistically via analysis of variance (One-way ANOVA) using graph-Pad prism 5 software and post hoc Student-Newman-Keuls multiple comparisons tests were used to analyze the different studies undertaken. p values of less than 0.05 were considered statistically significant.
Results
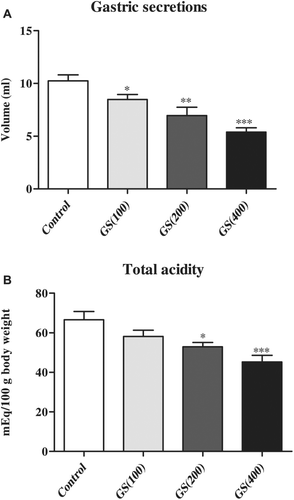
In the control group of rats, pylorus ligation for 6 h resulted in accumulation of 10.24 ± 0.56 mL of gastric secretions and a total acidity 66.56 ± 4.22 mEq (). Treatment of the pylorus ligatured rats with G. sylvestre (100, 200, and 400 mg/kg) reduced the volume of the gastric content secreted during a period of 6 h in 17, 32, and 47%, respectively (p < 0.05, p < 0.01, and p < 0.001, respectively). A significant decrease in total acid output was also observed in the rats treated with 200 mg/kg (48.75 ± 2.24; p < 0.05) and 400 mg/kg (41.57 ± 3.17; p < 0.001) of G. sylvestre, respectively ().
Figure 2. Effect of Gymnema sylvestre (GS) (100, 200 400 mg/kg) on the (a) gastric secretions and (b) acidity in pylorus ligated rats. All treated groups were statistically compared to control (vehicle) group. Six rats were used in each group. Data were expressed as Mean±S.E.M and analyzed using one-way ANOVA and post hoc Student-Newman-Keuls multiple comparisons test. Statistical significane at *p < 0.05, **p < 0.01 and ***p < 0.001.
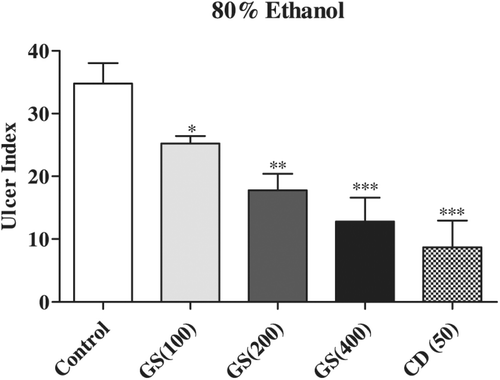
The treatment of rats with 80% ethanol produced extensive gastric lesions in the glandular mucosa in control (vehicle) animals. These lesions were characterized by multiple hemorrhagic like red patches of different sizes. The ulcer index was found to be 34.80 ± 3.23. Pretreatment of rats with G. sylvestre in three doses (100, 200 and 400 mg/kg; p < 0.05, p < 0.01 and p < 0.001, respectively) was found to provide the gastric mucosa with statistically significant protection against the ulceration caused by the 80% ethanol in a dose-dependent manner ().
Figure 3. Effects of pretreatment with Gymnema sylvestre (GS) (100, 200 and 400 mg/kg) and cimitidine (CD) (50 mg/kg) on ulcer index in 80% ethanol-induced gastric ulcers in rats. All treated groups were compared to control (vehicle) group. Six rats were used in each group. Data were expressed as mean ± S.E.M and analyzed using one-way ANOVA and post hoc Student–Newman–Keuls multiple comparisons test. Statistical significane at *p < 0.05, **p < 0.01 and ***p < 0.001.
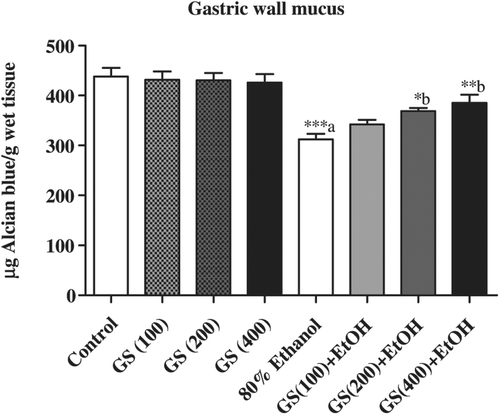
Ethanol treatment significantly (p < 0.001) decreased the Alcian blue binding capacity of gastric wall mucus of rats (312.37 ± 11.01 μg/g Alcian blue/g of tissue) as compared to control rats (437.92 ± 17.53). Pretreatment of rats with G. sylvestre in the doses of 100 mg/kg (342.33 ± 9.30), 200 mg/kg (368.84 ± 6.41) and 400 mg/kg (385.63 ± 16.32) enhanced Alcian blue binding capacity of gastric mucosa. However, the Alcian blue binding capacity remained the same as controls in animals treated only with different doses of G. sylvestre ().
Figure 4. Effects of pretreatment with Gymnema sylvestre (GS) (100, 200 and 400 mg/kg) on the induction of changes in gastric wall mucus by 80% ethanol in rats. (a) Ethanol group to compared to control group. (b) Gymnema sylvestre treated groups were compared to ethanol-treated group. *p < 0.05, **p < 0.01 and ***p < 0.001. Data were expressed as mean±SEM and analyzed one-way ANOVA and post hoc Student–Newman–Keuls multiple comparisons test. Six rats were used in each group.
The NP-SH concentrations in gastric mucosa of control animals was 79.09 ± 4.01 mmol/100 mg wet tissue. Single treatment of G. sylvestre with different doses to fasted rats did not show any significant changes in NP-SH levels compared to controls. The administration of ethanol significantly (p < 0.001) decreased the NP-SH (31.57 ± 1.67 mmol/100 mg) levels in the gastric mucosa. Pretreatment of rats with G. sylvestre in the doses of 100 mg/kg (41.45 ± 2.06), 200 mg/kg (46.67 ± 1.77) and 400 mg/kg (54.04 ± 3.05) attenuated the ethanol-induced depletion of NP-SH ().
Table 2. Effect of Gymnema sylvestre pretreatment on NP-SH and MDA content in glandular stomachs of male Wistar albino rats treated with 80% ethanol.
Ethanol treatment significantly (p < 0.001) increased the concentrations of MDA (29.22 ± 1.16 mmol/100 mg wet tissue) in stomach-wall tissue as compared to the control (19.02 ± 1.94) group of rats. Pretreatment of rats with G. sylvestre at doses of 100 (25.99 ± 0.57), 200 (23.65 ± 0.46), and 400 mg/kg (19.93 ± 0.77) attenuated the ethanol-induced increase of MDA levels. However, a single treatment of G. sylvestre to fasted rats in different doses did not change the levels of MDA compared to controls ().
The treatment with ethanol significantly reduced the total protein (12.24 ± 0.32 mg/100 mg), DNA (296.41 ± 19.33 μg/100 mg) and RNA (342.07 ± 9.59 μg/100 mg) as compared to the control values 15.70 ± 0.74, 421.55 ± 9.73, and 531.10 ± 13.77, respectively. G. sylvestre pretreatment provided dose-dependent protection against the action of ethanol on protein and nucleic acid concentrations ().
Table 3. Effect of Gymnema sylvestre pretreatment on nucleic acids and total protein content in the stomach wall of male Wistar albino rats treated with 80% ethanol.
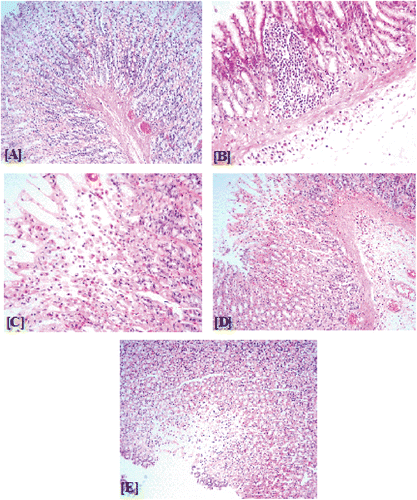
, shows the appearance through gastric mucosa of one of the control rats ( & ; total damage score: 2). Treatment with ethanol to fasted rats caused considerable damage in glandular segments of stomach tissues in the form of necrosis, erosions, congestion, interluminal bleeding, inflammation, muscularis layer degeneration and hemorrhagic mucosal lesions in the stomach walls ( & ; total damage score: 20). Pretreatment with G. sylvestre (100, 200, and 400 mg/kg) showed protection to the damaging action of ethanol evaluated for the same parameters in dose-dependent manner showing total scores 16, 10, and 4, respectively (, and & ; total damage score 16, 10, and 4, respectively).
Table 4. Effect of Gymnema sylvestre pretreatment (100, 200 and 400 mg/kg body weight) on the histopathological examination in gastric mucosal sections in a sample of control and experimental groups.
Figure 5. Histological examination of gastric mucosal sections in control and experimental rats. (A) Section of gastric mucosa obtained from normal rats, (B) section of gastric mucosa obtained from 80% ethanol-treated rat,(C), (D) and (E) sections of gastric mucosa obtained from 80% ethanol-treated rat after pretreatment of Gymnema sylvestre at 100, 200 and 400 mg/kg body weight, respectively.
Discussion
In the present study, the dried ethanolic extract of G. sylvestre leaves, standardized as 25% gymnemic acid, showed potential effects against gastric secretions and gastric ulceration induced by ethanol in male Wistar albino rats. The gastric protection of the higher dose of G. sylvestre was almost comparable to the positive control drug, cimitidine. The results of histopathological assessment also revealed that pretreatment with G. sylvestre at different doses were found to preserve the functional cytoarchitecture of the entire gastric mucosa in dose-dependent manner. Pretreatment with G. sylvestre prevented congestion, hemorrhage, edema, necrosis, inflammatory and dysplastic changes and erosions caused by ethanol in the gastric tissue. Moreover, G. sylvestre treatment significantly inhibited the total gastric secretion and acidity output during 6 h following hypersecretion model by pylorus ligature in dose-dependent manner. Indeed, these results are in agreement with earlier study done with similar constituents, which showed a decrease in gastric secretion protection against experimentally induced gastric damage in animal models (CitationJainu & Devi, 2006; CitationNavarrete et al., 2002; CitationSairam et al., 2002).
Increase of mucus secretions produced by the gastric mucosal cells can prevent gastric ulceration that is closely linked to the pathogenesis and healing of gastrointestinal lesions. It can decrease stomach-wall friction during peristalsis and provide an effective barrier to back diffusion of hydrogen ions (CitationSevak et al., 2002). However, gastric wall mucus plays a more important role in the defense of the gastric mucosa against chemical aggression than the soluble mucus in the lumen of the stomach (CitationAllen et al., 1986). This gastric mucus coat may facilitate the repair of the damaged gastric epithelium (CitationTariq et al., 2006). In the present study, G. sylvestre was found to inhibit the depletion of stomach-wall mucus caused by ethanol treatment. This antiulcer activity could be attributed to G. sylvestre constituents, mainly saponins and flavonoids. Indeed, earlier studies demonstrated that saponins, such as glycyrrhizic acid of licorice, and their triterpene derivatives, arbenoxolone (CitationDoll et al., 1968) have been found to promote ulcer healing by forming protective mucus barrier on the gastric mucosa (CitationTrease & Evans, 1978).
Sulfhydryl compounds of the gastric mucosa constitutes are one of the most important cytoprotective mechanisms participating directly as a potent antioxidant system (CitationCnubben et al., 2001). In addition, these compounds can bind to tissue-generated free radicals following exposure to cytotoxic compounds like ethanol (Citational-Harbi et al., 1997). Reduced levels of endogenous sulfhydryls have been associated with tissue damage in the previous studies and our findings are in agreement with these reports showing depletion in NP-SH concentration in stomach tissue (Citational-Bekairi et al., 1992; Citational-Harbi et al., 1994). Treatment with glutathione inhibitors has been shown to enhanced ulcerogen-induced gastric mucosal injury (CitationAlqasoumi et al., 2009), whereas an increase in mucosal NP-SH exerts a gastroprotective effect (CitationSener-Muratoglu et al., 2001). In the present study, our results pointed toward the mediation of sulfhydryls in G. sylvestre mucosal protection.
Previous studies showed that ethanol-induced tissue damage to the gastrointestinal mucosa might be linked to the generation of toxic reactive species (CitationBagchi et al., 1998) as it following the reactive oxygen species (ROS) can participate in the etiology and pathophysiology of the gastric ulcer disease (CitationRepetto & Llesuy, 2002; CitationKhosla et al., 2004). Similarly, our results showed that ethanol treatment significantly reduced the stomach proteins and nucleic acids contents of the animals, which may be due to the accumulation of toxic free radicals in the mucosal cells. It has been demonstrated that antioxidants protects cellular damage by scavenging free radical formation (CitationSurveswaran et al., 2010; CitationAnanthan et al., 2003). In the present study, ethanol treatment significantly reduced stomach protein and nucleic acid content of the animals, which may be due to the accumulation of toxic free radicals in the mucosal cells (CitationBoyd et al., 1979). In agreement with others (CitationBharavi et al., 2011; CitationPreuss et al., 1998), G. sylvestre pretreatment in our study showed antioxidant and lipid peroxidation lowering properties, which may have offered protection against the action of ethanol through alteration in proteins and nucleic acids, and significant inhibition of lipid peroxidation. The preliminary phytochemical screening of G. sylvestre revealed the presence of triterpenes, flavonoids, glycosides and anthroquinones, and their derivatives. Previous studies have shown these compounds may be related to the antiulcer activity and play a major role in the mechanism of gastroprotection (CitationOyagi et al., 2010; CitationNwafor & Bassey, 2007; CitationAlqasoumi et al., 2009).
In summary, biochemical estimations showed a significant antiperoxidative effect. Thus, from the present investigation, it can be concluded G. sylvestre leaves afforded significant antiulcer activity by enhancing antioxidant potential of the gastric mucosa, thereby reducing mucosal damage.
Acknowledgement
The authors would like to thanks Mr. Mohamed W. Attwa for his assistance performing phytochemical analysis.
Declaration of interest
This study was funded by the Deanship of Scientific Research at King Saud University through the research group project no. RGP-VPP-142.
References
- al-Bekairi AM, Qureshi S, Ahmed MM, Afal M, Shah AH. (1992). A study of uric acid pretreatment for the protection of rat gastric mucosa against toxic damage. Food Chem Toxicol, 30, 525–531.
- al-Harbi MM, Qureshi S, Raza M, Ahmed MM, Afzal M, Shah AH. (1994). Evaluation of Caralluma tuberculata pretreatment for the protection of rat gastric mucosa against toxic damage. Toxicol Appl Pharmacol, 128, 1–8.
- al-Harbi MM, Qureshi S, Raza M, Ahmed MM, Afzal M, Shah AH. (1997). Gastric antiulcer and cytoprotective effect of Commiphora molmol in rats. J Ethnopharmacol, 55, 141–150.
- Allen A, Hutton DA, Leonard AJ, Pearson JP, Sellers LA. (1986). The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl, 125, 71–78.
- Alqasoumi S, Al-Sohaibani M, Al-Howiriny T, Al-Yahya M, Rafatullah S. (2009). Rocket “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J Gastroenterol, 15, 1958–1965.
- Al-Shabanah OA, Qureshi S, Al-Harbi MM, Al-Bekairi AM, Al-Gharably NM, Raza M. (2000). Inhibition of gastric mucosal damage by methylglyoxal pretreatment in rats. Food Chem Toxicol, 38, 577–584.
- Ananthan R, Baskar C, NarmathaBai V, Pari L, Latha M, Ramkumar KM. (2003). Antidiabetic effect of Gymnema montanum leaves: Effect on lipid peroxidation induced oxidative stress in experimental diabetes. Pharmacol Res, 48, 551–556.
- Bagchi D, Carryl OR, Tran MX, Krohn RL, Bagchi DJ, Garg A, Bagchi M, Mitra S, Stohs SJ. (1998). Stress, diet and alcohol-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. J Appl Toxicol, 18, 3–13.
- Bharavi K, Reddy AG, Rao GS, Kumar PR, Kumar DS, Prasadini PP. (2011). Prevention of cadmium bioaccumulation by herbal adaptogens. Indian J Pharmacol, 43, 45–49.
- Boyd J, Easterbrook-Smith SB, Závodszky P, Mountford-Wright C, Dwek RA. (1979). Mobility and symmetry in the Fc and pFc’ fragments as probed by 1H NMR. Mol Immunol, 16, 851–858.
- Bregman AA. (1983). Laboratory Investigation and Cell Biology. New York: John Wiley & Sons. pp. 51–60.
- Cnubben NH, Rietjens IM, Wortelboer H, van Zanden J, van Bladeren PJ. (2001). The interplay of glutathione-related processes in antioxidant defense. Environ Toxicol Pharmacol, 10, 141–152.
- Corne SJ, Morrissey SM, Woods RJ. (1974). Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol (Lond), 242, 116P–117P.
- Culling CFA. (1974). Handbook of Histopathological and Histochemical Techniques. Third edition. London: Butterworth.
- Daisy P, Eliza J, Mohamed Farook KA. (2009). A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J Ethnopharmacol, 126, 339–344.
- Doll R, Langman MJ, Shawdon HH. (1968). Treatment of gastric ulcer with oestrogens. Gut, 9, 46–47.
- Festing MF, Altman DG. (2002). Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J, 43, 244–258.
- Grover JK, Yadav S, Vats V. (2002). Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol, 81, 81–100.
- Jainu M, Devi CS. (2006). Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: Role of proinflammatory cytokines and oxidative damage. Chem Biol Interact, 161, 262–270.
- Kanetkar P, Singhal R, Kamat M. (2007). Gymnema sylvestre: A Memoir. J Clin Biochem Nutr, 41, 77–81.
- Khosla P, Gogia A, Agarwal PK, Jain KP. (2004). Unilateral central retinal artery occlusion followed by contralateral anterior ischemic optic neuropathy. J Postgrad Med, 50, 219–221.
- Konturek PC, Duda A, Brzozowski T, Konturek SJ, Kwiecien S, Drozdowicz D, Pajdo R, Meixner H, Hahn EG. (2000). Activation of genes for superoxide dismutase, interleukin-1β, tumor necrosis factor-α, and intercellular adhesion molecule-1 during healing of ischemia-reperfusion-induced gastric injury. Scand J Gastroenterol, 35, 452–463.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Navarrete A, Trejo-Miranda JL, Reyes-Trejo L. (2002). Principles of root bark of Hippocratea excelsa (Hippocrataceae) with gastroprotective activity. J Ethnopharmacol, 79, 383–388.
- Nwafor PA, Bassey AI. (2007). Evaluation of anti-diarrhoeal and anti-ulcerogenic potential of ethanol extract of Carpolobia lutea leaves in rodents. J Ethnopharmacol, 111, 619–624.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Oyagi A, Ogawa K, Kakino M, Hara H. (2010). Protective effects of a gastrointestinal agent containing Korean red ginseng on gastric ulcer models in mice. BMC Complement Altern Med, 10, 45.
- Porchezhian E, Dobriyal RM. (2003). An overview on the advances of Gymnema sylvestre: Chemistry, pharmacology and patents. Pharmazie, 58, 5–12.
- Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA. (1998). Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr, 17, 116–123.
- Ramkumar KM, Ponmanickam P, Velayuthaprabhu S, Archunan G, Rajaguru P. (2009). Protective effect of Gymnema montanum against renal damage in experimental diabetic rats. Food Chem Toxicol, 47, 2516–2521.
- Rao CV, Sairam K, Goel RK. (2000). Experimental evaluation of Bocopa monniera on rat gastric ulceration and secretion. Indian J Physiol Pharmacol, 44, 435–441.
- Rejaie SS. (2009). Inhibition of ethanol-induced gastric mucosal damage by carvedilol in male Wistar albino rats: Possible biochemical changes. Int J Pharmacol, 5, 146–154.
- Repetto MG, Llesuy SF. (2002). Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz J Med Biol Res, 35, 523–534.
- Sairam K, Rao ChV, Babu MD, Kumar KV, Agrawal VK, K Goel RK. (2002). Antiulcerogenic effect of methanolic extract of Emblica officinalis: An experimental study. J Ethnopharmacol, 82, 1–9.
- Sedlak J, Lindsay RH. (1968). Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem, 25, 192–205.
- Sener-Muratoglu G, Paskaloglu K, Arbak S, Hürdag C, Ayanoglu-Dülger G. (2001). Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in rats. Dig Dis Sci, 46, 318–330.
- Sevak R, Paul A, Goswami S, Santani D. (2002). Gastroprotective effect of β3 adrenoreceptor agonists ZD 7114 and CGP 12177A in rats. Pharmacol Res, 46, 351–356.
- Shay H, Kumarov SA, Fels SA. (1945). A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology, 5, 43–61.
- Sheeba MS, Asha VV. (2006). Effect of Cardiospermum halicacabum on ethanol-induced gastric ulcers in rats. J Ethnopharmacol, 106, 105–110.
- Shigematsu N, Asano R, Shimosaka M, Okazaki M. (2001a). Effect of administration with the extract of Gymnema sylvestre R. Br leaves on lipid metabolism in rats. Biol Pharm Bull, 24, 713–717.
- Shigematsu N, Asano R, Shimosaka M, Okazaki M. (2001b). Effect of long term-administration with Gymnema sylvestre R. BR on plasma and liver lipid in rats. Biol Pharm Bull, 24, 643–649.
- Stermer E. (2002). Alcohol consumption and the gastrointestinal tract. Isr Med Assoc J, 4, 200–202.
- Surveswaran S, Cai YZ, Xing J, Corke H, Sun M. (2010). Antioxidant properties and principal phenolic phytochemicals of Indian medicinal plants from AsclepiadoideaePeriplocoideae. Nat Prod Res, 24, 206–221.
- Takeuchi K, Ueshima K, Ohuchi T, Okabe S. (1994). Induction of gastric lesions and hypoglycemic response by food deprivation in streptozotocin-diabetic rats. Dig Dis Sci, 39, 626–634.
- Tariq M, Elfaki I, Khan HA, Arshaduddin M, Sobki S, Al Moutaery M. (2006). Bromophenacyl bromide, a phospholipase A2 inhibitor attenuates chemically induced gastroduodenal ulcers in rats. World J Gastroenterol, 12, 5798–5804.
- Tashima K, Korolkiewicz R, Kubomi M, Takeuchi K. (1998). Increased susceptibility of gastric mucosa to ulcerogenic stimulation in diabetic rats–role of capsaicin-sensitive sensory neurons. Br J Pharmacol, 124, 1395–1402.
- Trease EG, Evans WE. (1978). Saponins and cardioactive drugs. In: Textbook of Pharmacognosy. London: Bailiere Tindal, 475–506.
- Valcavi U, Caponi R, Brambilla A, Palmira M, Minoja F, Bernini F, Musanti R, Fumagalli R. (1982). Gastric antisecretory, antiulcer and cytoprotective properties of 9-hydroxy-19,20-bis-nor-prostanoic acid in experimental animals. Arzneimittelforschung, 32, 657–663.
- Ye WC, Zhang QW, Liu X, Che CT, Zhao SX. (2000). Oleanane saponins from Gymnema sylvestre. Phytochemistry, 53, 893–899.
- Zakaria ZA, Abdul Hisam EE, Rofiee MS, Norhafizah M, Somchit MN, Teh LK, Salleh MZ. (2011). In vivo antiulcer activity of the aqueous extract of Bauhinia purpurea leaf. J Ethnopharmacol, 137, 1047–1054.