Abstract
Context: As microtubules are highly involved in cellular growth, it appears to be a preferential target for cancer treatment. Therefore, many efforts have been performed to discover drugs that affect on microtubule function. Several microtubule inhibitors are in various stages of laboratory evaluations and clinical trials.
Objective: A series of chromene-based chalcones with chlorine, methoxy, fluorine, tetrahydropyranyloxy and cyanide substituents were prepared and evaluated for cytotoxic effects against K562 and SK-N-MC cell lines, and the inhibitory effect on tubulin polymerization was studied as well.
Materials and methods: MTT, tubulin polymerization assays and binding measurements were evaluated by using related spectroscopy. Immunocytochemical study, morphological observations and apoptosis assay were examined using a fluorescence microscope and a flow cytometer.
Results: (E)-3-(6-Chloro-2H-chromen-3-yl)-1-(3,4,5-trimethoxyphenyl) prop-2-en-1-one (compound 14) proved to be the most active in this series as an inhibitor of tubulin assembly [IC50, 19.6 µM] and cytotoxic agent on K562 cells [IC50, 38.7 µM]. Furthermore, these compounds exhibited a strong inhibitory effect on tubulin polymerization and reduced the in vitro assembly and bundling of proto-filaments. Also, compound 14 bound to the tubulin with a dissociation constant of 9.4 ± 0.7 µM and induced conformational changes in this protein.
Discussion and conclusion: This study suggests that the compound 14 could be a good antitumor agent because of its biological functions. Compound 14 appears to bind directly to tubulin and thereby perturbs microtubule stability and the function of the spindle apparatus, which causes cancer cells to arrest and undergo apoptosis.
Keywords::
Introduction
Chalcones (1,3-diphenyl-2-propen-1-ones) are open-chain flavonoids in which two aromatic rings are joined by a three-carbon α,β-unsaturated carbonyl system (CitationAvila et al., 2008). They serve as precursors for the synthesis of different classes of flavonoids, which are common substances in plants. They have been reported to be the major bioactive constituents of medicinal herbs because of their anti-inflammatory, antioxidant (CitationCheng et al., 2008), antibacterial (CitationAvila et al., 2008), antifungal (CitationSortino et al., 2007), and anticarcinogenic activities (CitationModzelewska et al., 2006; CitationKumar et al., 2003). These activities are largely attributed to the α,β-unsaturated ketone moiety (CitationRao et al., 2009). Their beneficial effects on health, and particularly the anticarcinogenic potential, have been credited to their inhibitory efficiency demonstrated in bacterial and mutagenicity tests (CitationAvila et al., 2008; CitationModzelewska et al., 2006), chemopreventive activity against chemically-induced tumorigenesis (CitationGuo et al., 2008; CitationAkihisa et al., 2003), decreased reactive oxygen species (CitationPanchatcharam et al., 2006), and with their antioxidant activity (CitationYang et al., 2007). There have been a number of bioassay-guided searches for cytotoxic antitumor chalcones. In a search for the cytotoxic constituents of the aerial parts of Ononis natrix, 4,2′,6′-trihydroxy-4′-methoxydihy-drochalcone, 2′,6′-dihydroxy-4′-methoxydihydrochalcone and 2′,4′-diacetoxychalcone were identified as having moderate activity against P-388, A-549 and HT-29 cancer cell lines (CitationHarborne & Williams, 2000). The chalcone, pedicin, from the leaves of Fissistigma languinosum, was found to inhibit the assembly of tubulins into microtubules (CitationAlias et al., 1995).
Here, we demonstrate cytotoxic effects of several new analogues of chalcones against K562 and SK-N-MC human cancer cell lines and show that these compounds function through the inhibition of tubulin polymerization. Another purpose of this study was to carry out a detailed investigation on the type of cell death (apoptotic/necrotic) after exposure of experimental cell lines to the test compounds by performing flow cytometry and visualization with acridine orange/ethidium bromide (AO/EB) double staining with the help of fluorescence microscopy.
Materials and methods
Reagents
Mouse monoclonal anti-α-tubulin antibody, FITC-conjugated goat anti-mouse IgG, Hoechst 33258, guanosine-5′-triphosphate (GTP) and [piperazine-1,4-bis (2-ethanesulfonic acid)] PIPES were purchased from Sigma-Aldrich [http://www.sigmaaldrich.com, USA]. All other chemicals were of analytical grade. All the test compounds used in this study were dissolved in dimethyl sulfoxide (DMSO) as 1 mM stock. For in vitro and cell culture tests, DMSO was used at the final concentrations of <4 and <0.1% (v/v), respectively. The solutions were kept in a refrigerator at 4°C and protected from light. RPMI-1640 growth medium was provided by Invitrogen. The Annexin-V-FLUOS staining kit and [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyltetrazolium bromide] MTT kit were purchased from Roche Corporation [www.roche-applied-science.com, Germany]. The K562 and SK-N-MC cancer cell lines were obtained from Pasture Research Institute, Tehran, Iran.
General procedure for the synthesis of chalcones 1–16
The two types of chromene-based chalcones 1–4 and 5–16 were prepared according to the previously described method. Briefly, Claisen-Schmidt condensation of 1-(6-methoxy-2H-chromen-3-yl) ethanone with different aldehydes in ethanol solution of NaOH yielded corresponding chalcones 1–4 (CitationNazarian et al., 2010; CitationForoumadi et al., 2010). Condensation of 6-methoxy-2H-chromene-3-carbaldehyde or 6-chloro-2H-chromene-3-carbaldehyde with appropriate acetophenone in ethanol solution of NaOH yielded the chalcones 5–16. The structures of the target compounds were established with IR, NMR, mass spectrometry and elemental analyses.
Tubulin purification
Tubulin was purified from sheep brain through two cycles of polymerization–depolymerization in the PEM buffer [100 mM PIPES, pH 6.9, 1mM MgSO4 and 1 mM ethylene glycol tetraacetic acid (EGTA)], according to the method described by CitationSengupta et al. (2005). Then microtubule-associated proteins (MAPs)-free tubulin were purified from the microtubule protein by DEAE chromatography aliquots and stored in liquid nitrogen when not in use (CitationMurphy et al., 1977). The protein content was estimated by the Bradford method using bovine serum albumin (BSA) as a standard (CitationBradford, 1976). The purity of tubulin from sheep brain was determined using polyacrylamide gel electrophoresis, which was performed by the method of CitationLaemmli (1970).
Tubulin polymerization assay
Tubulin polymerization was followed with a Varian Cary 100 recording spectrophotometer equipped with electronic temperature controller [www.varianinc.com, USA]. The assay was carried out based on previous procedures with some modifications (CitationMa et al., 2008). Briefly, a drug/DMSO-tubulin preincubation, without GTP, was performed for 15 min at 0°C in ice. Three microliter of GTP was added (final concentration, 1 mM) and polymerization was initiated by setting the temperature controller at 37°C. Turbidity development was followed at 350 nm. Turbidity (absorbance) readings were recorded after every 10 sec throughout the incubation time and the changes in absorbance were used to calculate the extent of polymerization [%inhibition = (1−A350 sample/A350 control) × 100]. The IC50 value was defined as the drug concentration required to inhibit the extent of assembly by 50% after 30 min of incubation.
Transmission electron microscopic analysis
The electron microscopic pictures were taken according to the method of CitationRai et al. (2008). Tubulin (1 mg/mL) was polymerized in the absence and presence of 30 µM of compound 14 in the PEM buffer at 37°C. After 15 min of polymerization, the microtubule polymers (20 µL) were transferred to the formvar-carbon-coated copper grids (400 meshes) for 45 s and blotted dry. The grids were subsequently stained negatively with a 1% uranyl acetate solution for 35 s, air-dried and observed under transmission electron microscope (Hitachi HU-12A) at 15,000× magnification.
Binding measurements
All the fluorescence measurements were performed in a Varian’s Cary Eclipse fluorescence spectrophotometer. The increased fluorescence of compound 14 at 465 nm, upon binding to tubulin, was used to determine the ligand affinity with tubulin (CitationRai et al., 2008; CitationGupta et al., 2006). Tubulin (1 µM) was incubated with varying concentrations of ligand (1–50 µM) in 25 mM PIPES at 25°C for 30 min. The excitation and emission wavelengths were 420 and 465 nm, respectively. The binding parameters of compound 14 to tubulin were determined via the equation of CitationScatchard (1949). Scatchard analysis is a method of linearizing the data from a saturation binding experiment (plots of fluorescence intensity against the concentration of chalcone analogues), in order to determine the binding constants.
Cell culture
The K562 and SK-N-MC cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 50 IU/mL penicillin and 50 IU/mL streptomycin. For morphological studies, the cells were seeded in 6-well plates at a concentration of 1 × 105 cells/well in 2 mL of the growth medium. When cells reached 60% confluency, the test compounds were added to the cells at different concentrations. After 72 h of incubation, the cell morphology was assessed with a Zeiss Axiovert 25 phase-contrast microscope. For the MTT assay, the cells were seeded onto 96-well plates at a density of 1 × 104 cells/well. The cells were incubated in a humidified incubator at 37°C and 5% CO2 atmosphere for 24 h before the assays. For the apoptosis/necrosis assay and acridine orange/ethidium bromide (AO/EB) double staining, the cells were seeded into 6-well plates at a density of 1 × 105 cells/well and cultured for 24 h before the assays.
Immunocytochemical study
Microtubular cytoskeleton was observed as described by CitationMa et al. (2008) with some modifications. Cells were seeded on sterile glass cover slips in 6-well tissue culture plate at a density of 1 × 105 cells/well and allowed to adhere and grow for 24 h at 37°C and 5% CO2 before the assays. Attached cells were treated with or without test compounds for 48 h. Medium was aspirated and cells washed with PBS prior to fixation in cold methanol for 15 min at −20°C. Cover slips were placed on parafilm in a covered container and the fixed cells were washed thrice with PBS followed by washing with antibody buffer (PBS, 3% BSA, 0.05% Tween 20, 0.04% NaN3) for 5 min each. Cells were incubated with the primary antibody (mouse monoclonal anti-α-tubulin antibody, diluted at 1:100 in antibody buffer) for 1.5 h. Following three rinses in antibody buffer, cells were incubated with the secondary antibody in the dark for 45 min (FITC-conjugated goat anti-mouse IgG, diluted at 1:100 in antibody buffer). All incubations were carried out at room temperature. Cover slips were gently washed for 5 min with antibody buffer in the dark. DNA was also stained with Hoechst 33258 (1 mg/mL) in PBS for 5 min and then washed with PBS twice for 5 min each. Cover slips were mounted on microscope slides by inverting over a drop of vectashield mounting medium. The cover slips were sealed on the slide with a light coat of nail polish at the edge and allowed to dry for 5–10 min. Finally, the cover slips were examined with a Leica fluorescence microscope (Zeiss Axioskop 2 plus, Germany) equipped with appropriate filter sets. Photographs were taken with a digital camera attached to the microscope.
Acridine orange/ethidium bromide double staining
After treatment for 48 h, the cells were washed with PBS and fixed in 4% chilled paraformaldehyde for 20 min and washed twice with PBS. Cells were stained by adding 1 mL AO/EB mixture (100 μg/mL AO and 100 μg/mL EB in PBS). After 2 min of incubation, the cells were washed with PBS and visualized under a fluorescence microscope (Zeiss Axioskop 2 plus, Germany) at 100× magnification (CitationArora et al., 2009).
In vitro cytotoxicity studies
MTT colorimetric assay was employed according to the methods of CitationScudiero et al. (1988). MTT is a yellow dye, taken up by viable cells and converted to formazen 8 (purple crystals). Change in color can be measured spectrophotometrically to give an assessment of metabolic activity as a function of cytotoxicity. The cells were treated with different concentrations of test compounds in a CO2 incubator for 72 h. At the end of this period, 10 µL of MTT (final concentration, 0.5 mg/mL) was added to each well and the plates were incubated for 4 h at 37°C. Afterwards, 100 µL of the solubilization solution was added into each well and kept overnight at 37°C. Absorbance (A570) was determined, at 570 nm, for each well using microplate reader (Model Expert 96, Asys Hitech, Ec. Salzburg, Austria). The IC50 (concentration reduction by 50% at the absorbance of 570 nm) was calculated by linear regression, performed on the linear zone of the dose–response curve. Control wells contained all the components presented in the treated wells except the target compounds.
Apoptosis/necrosis assay
Annexin-V-FLUOS staining kit (Roche Applied Science, www.roche-applied-science.com, Germany) was used to determine the cellular apoptosis or necrosis. The analysis of phosphatidyl-serine on the outer leaflet of apoptotic cell-membranes was performed by using annexin-V-fluorescein and proidium iodide (PI) for the differentiation of necrotic cells. Cells (106) were washed twice with PBS and centrifuged at 200×g for 5 min. Afterwards, the cell pellet was re-suspended in 100 μL annexin-V FLUOS labeling solution and incubated for 10–15 min at 15–25°C and centrifuged. Finally, 0.5 mL incubation buffer per 106 cells was added to the cell pellet and analyzed with the help of flow cytometer (Becton Dickinson) using the excitation wavelength of 488 nm and a 515 nm band-pass filter for fluorescein detection and a filter >600 nm for PI detection.
DNA laddering
DNA samples were extracted by a previously reported method with some modifications (CitationJiang et al., 1998). Briefly, cells were washed with PBS and incubated in 1 mL of lysis buffer [0.01 M Tris–HCl (pH 8.0), 0.1 M NaCl, 0.025 M EDTA (pH 8.0), and 1% SDS] containing 0.2 mg/mL protease K for 2 h at 56°C. After centrifugation at 12,000×g, the supernatant was first extracted with phenol and then with phenol–chloroform–isoamyl alcohol (25:24:1) to remove proteins and lipids. DNA in the supernatants was then precipitated in ethanol overnight at −20°C and pelleted at 15,000×g. The dry sample pellets were resuspended in 10 mM Tris–HCl/1 mM EDTA solution. The DNA (5 µg/lane) was treated with RNase at a final concentration of 0.1 µg/µL at 37°C for 20 min. Equal fractions of extraction volumes were loaded onto 1.5% agarose gel and run in Tris–boric acid–EDTA buffer under 100 V. DNA was stained with ethidium bromide, and photographs were taken under UV light.
Results
Microtubular assembly in the presence of synthetic chalcones
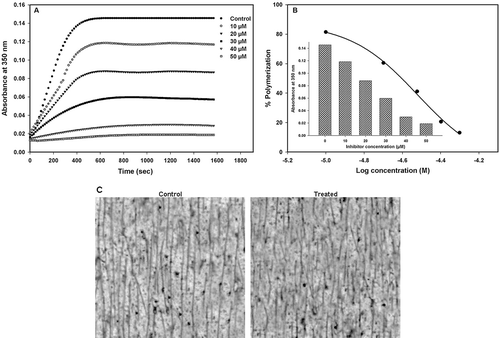
To determine the microtubule assembly, purified tubulin from sheep brain was treated with different concentrations of the synthetic chalcones (10–50 µM). As shown in , kinetics of microtubule proteins (30 µM) were measured at the turbidity of 350 nm in PEM buffer with different concentrations of compound 14 (10–50 µM). Microtubule assembly was induced by adding GTP and increasing the temperature from 0 to 37°C. The inhibition was attested by a decrease in both the rate and the plateau of assembly. The half-maximal concentration (IC50) of polymerization inhibition was calculated from the semi-logarithmic dose–response plots using nonlinear regression program SigmaPlot. The IC50 value of compound 14 was found to be 30 µM (), while an inhibition higher than 80% was observed at the concentration of 50 µM. Observation of microtubules under an electron microscope showed that the microtubules were shortened in the presence of high concentrations of compound 14 ().
Figure 1. Inhibition of tubulin polymerization by compound 14. (A) Kinetics of the inhibition of microtubule assembly by increasing concentrations of compound 14. (B) The IC50 value was obtained from a plot of percent tubulin polymerization against log concentration (M) of compound 14 at 37°C. Inset: column scatter graph of absorbance against the concentration of compound 14 in the reaction mixture. IC50 = 30.14 ± 0.1 µM. (C) TEM micrographs of microtubule polymers in the absence (control) and presence of 30 µM (treated) of compound 14. Scale bars, 2 µm.
Binding of the compound 14 to tubulin
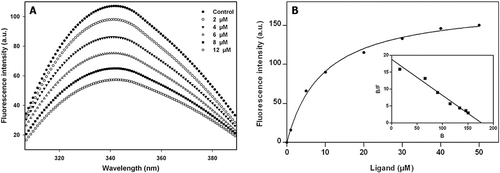
We wanted to know whether the observed effects of compound 14 on microtubular assembly are due to its binding to tubulin. The tryptophan residues in tubulin are intrinsically fluorescent (eight tryptophan residues per tubulin dimer), and change in the tryptophan fluorescence by ligands, as determined by fluorescence spectroscopy, has been used extensively to investigate the binding of ligands to tubulin. Compound 14 reduces the intrinsic fluorescence of tryptophan residue within the tubulin in a concentration-dependent manner, suggesting that it induces conformational change in tubulin (). In addition, the binding constants and stoichiometries were calculated by the titration of tubulin with compound 14 at 25°C. The effects of different concentrations of compound 14 on its fluorescence are shown in . The fluorescence intensity of the tubulin–compound 14 complex increased with increase in concentration of compound 14. The dissociation constant (Kd) value of 9.4 ± 0.7 µM was calculated by the Scatchard plot using “Saturation Binding Curves and Scatchard Plots” mode of GraphPad Prism version 5.03 ().
Figure 2. Characterization of the binding of compound 14 with tubulin. (A) Effects of compound 14 on the intrinsic tryptophan fluorescence of tubulin. Tubulin (2 µM) was incubated without or with compound 14 for 30 min at 25°C. The excitation wavelength was 295 nm. (B) Measurement of compound 14 binding to tubulin by fluorescence spectroscopy. Tubulin (1 μM) was incubated with different concentrations of compound 14 and the fluorescence intensity was measured as described in “Materials and Methods”. Inset shows the Scatchard plot of compound 14 binding to tubulin. The λex and λem values were taken at 420 and 500 nm, respectively.
Cytotoxic activity of chalcones
Two tumorigenic human cell lines, SK-N-MC and K562, were selected for the determination of cytotoxic activity of new chalcones. Inhibition of cell growth was observed following the treatment of different doses of test compounds for 72 h. IC50 values were calculated from the semi-logarithmic dose–response plots using nonlinear regression program SigmaPlot. SK-N-MC cells were more resistant to the cytotoxic effect of the chalcones ().
Table 1. Structure, inhibition of tubulin polymerization and cytotoxic activity of new chalcones.
The microtubule organization of the cells in the presence of synthetic chalcones
Organization of cytoplasmic microtubular network was investigated in SK-N-MC and K562 cancer cells treated with compound 14. The cells were exposed to compound 14 for 48 h at 37°C. After fixing and permeabilization, the microtubules were visualized by immunofluorescence microscope using an antibody against α-tubulin. shows the control cells (DMSO treated) with the microtubule network constituting the cell shape at interphase ( and ). In contrast, the cells exposed to 60 µM of compound 14 showed clear disruption with the decreasing amount of microtubule polymers ( and ). Hoechst 33258 staining of cells exposed to compound 14 demonstrated condensation and fragmentation of nuclear chromatin ( and ).
Figure 3. Immunofluorescence (1,3) and Hoechst (2,4) images of SK-N-MC (A) and K562 (B) cells treated with compound 14. Cells were cultured as described in ‘‘Materials and Methods’’ and then incubated without or with 60 µM compound 14. The fixed cells were stained with the antitubulin primary antibody and the secondary antibody. Effects of compound 14 on microtubules and nuclear organization of SK-N-MC (A3,4) and K562 (B3,4) cells are shown (Magnification ×100).
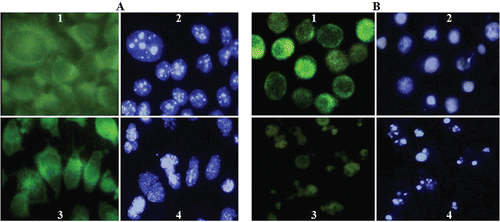
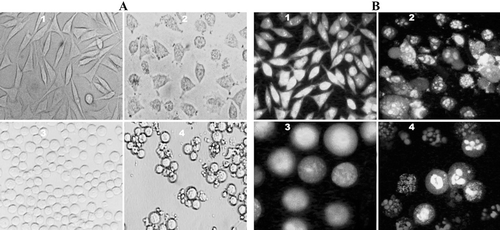
Figure 4. Phase-contrast micrographs and fluorescence of SK-N-MC and K562 cells treated with compound 14. (A) Phase-contrast images of SK-N-MC (1, 2) and K562 (3, 4) cells. Unexposed cells have a polyhedral (1) and round (3) shape indicating live cells. While, exposed cells (60 µM) have a shrunken and condensed form (2, 4). (B) Fluorescence images of AO/EB stained SK-N-MC (1, 2) and K562 (3, 4) cells. Unexposed (1, 3) cells have a normal green nucleus indicating live cells while exposed cells (60 µM) have a bright green nucleus with condensed or fragmented chromatin suggesting apoptosis. (Magnification, ×100).
Morphological study of the cells
Under phase-contrast microscopy, SK-N-MC (control) appeared fusiform and polyhedral () that joined neighboring cells. Compound 14-treated cells lost their polyhedral shape and became shrunken (A2). K562 cells (control) were found in a round form (), while the treated cells decreased adhesiveness to glass and condensed, became smaller than control cells (A4). The cells also stained by AO/EB to detect of the apoptotic cells (). Fluorescence light microscopy with differential uptake of fluorescent DNA binding dyes (AO/EB staining) is the method of choice (for its simplicity, rapidity, and accuracy) that distinguishes the apoptotic and necrotic cell populations. Acridine orange permeates all cells and makes the nuclei appears green. Ethidium bromide is only taken up by cells when cytoplasmic membrane integrity is lost, and stains the nucleus red. EB also dominates over AO. Thus live cells have a normal green nucleus; early apoptotic cells have bright green nucleus with condensed or fragmented chromatin; late apoptotic cells display condensed and fragmented orange chromatin; while the cells that have died due to necrosis, have a structurally normal orange nucleus (CitationRenvoizé et al., 1998). To support these findings, typical apoptotic or necrotic features in cells were also observed under fluorescence spectroscopy. Compound 14 induced apoptosis in both cell lines. Under fluorescence microscope, normal green nucleus was observed in control cells ( and ). Exposure to compound 14 caused apoptosis in the cells (bright green nucleus with condensed or fragmented chromatin). Late apoptotic cells display condensed and fragmented orange chromatin, while necrotic cells have a structurally normal orange nucleus ( and ).
Apoptosis assay
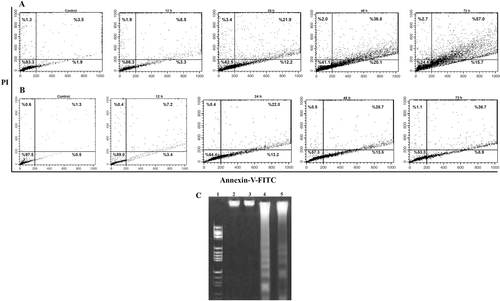
To further confirm apoptosis, flow cytometric was used. The detection of surface exposed phosphatidyl-serine (PS) by annexin-V-FITC has been shown to be a general and early marker of apoptosis as a result of redistribution of the plasma membrane of cells following the occurrence of apoptosis. Consistent with previous data on morphological changing of cells, apoptosis was mostly observed time-dependently after 12–72 h of exposure to compound 14. Shifts from early (lower right quadrant) to late apoptosis or necrosis (upper right quadrant) were clearly evident in dot plots of . Flow cytometric analysis with annexin V/PI staining showed that when cells were exposed to compound 14, the proportion of AV+/PI− (apoptotic cells) in K562 and SK-N-MC cells increased from 1.9 to 20.1% and 0.5 to 13.5%, respectively. During apoptosis, DNA is often degraded by endonucleases, and DNA fragments, in the multiples of approximately 180 bp (oligonucleosomes), are produced (CitationWyllie et al., 1984). Treatment of K562 and SK-N-MC cells with 60 µM compound 14 for 72 h resulted in a DNA laddering of multiples of about 180 bp ().
Figure 5. Treatment with compound 14 in a time-dependent manner (12–72 h) induced apoptosis in K562 and SK-N-MC cells. The cells were cultured for 24 h and incubated with 60 µM of compound 14. The cells were harvested after 12, 24, 48, and 72 h, stained with annexin V–FITC and propidium iodide, and analyzed by flow cytometry. Time-dependent treatment of compound 14 increased the percentage of apoptotic cells in K562 (A) and SK-N-MC (B) cells. LR quadrant indicates the percentage of early apoptotic cells, and UR quadrant indicates the percentage of late apoptotic cells. Representative of two independent experiments (C). DNA fragmentation after 72 h of treatment of cells with 60 μmol compound 14. Lane 1: Marker, (HaeIII); Lane 2 (K562) and 3 (SK-N-MC), Control cells; Lane 4 (K562) and 5 (SK-N-MC) treated with compound 14.
Discussion
Chalcones display a vast array of cellular effects. They can affect the overall process of carcinogenesis by several mechanisms, including cell cycle arrest, the release of cytochrome c and the activation of caspase-9 and caspase-3 (CitationShen et al., 2007; CitationYun et al., 2006; CitationTabata et al., 2005; CitationZi & Simoneau, 2005), increased expression of p21, p27, Bax and Bak proteins (CitationShen et al., 2007; CitationYun et al., 2006), decreased expression of cyclin B1, cyclin A and Cdc2 proteins (CitationHsu et al., 2006). Flavokawain B significantly inhibits the growth of colon cancer cells. This chalcone exerts its apoptotic action through ROS generation and GADD153 up-regulation, which lead to mitochondria-dependent apoptosis, characterized by the release of cytochrome c and the translocation of Bak (CitationKuo et al., 2010). Flavokawain B also induces apoptosis via the up-regulation of death-receptor 5 and Bim expression in prostate cancer cell lines (CitationTang et al., 2010). Therefore, inhibition of cell proliferation and the induction of differentiation and apoptosis are main preventive approaches, and the induction of programmed cell death is currently considered as one relevant target in a preventive approach. It has been demonstrated that chalcone compounds can trigger apoptosis (CitationWatson et al., 2000; CitationRamos et al., 2005); although the regulation and induction of the apoptotic process by these natural products remain to be elucidated.
In our systematic effort to discover novel anticancer agents from synthetic small molecules, a novel class of chromene-based chalcones has been synthesized (CitationNazarian et al., 2010; CitationForoumadi et al., 2010). These compounds show properties in inhibiting the tumor cell growth. The anti-proliferative activity of these compounds correlates well with its ability to depolymerize the cellular microtubular network. In this study, we reported that these compounds inhibit tubulin polymerization and found the inhibition concentration (IC50) values at the micromolar level (). Among these compounds, the trimethoxy analogue (compound 14) with an IC50 value of 30 µM in tubulin inhibition assay was selected for further studies. At its lowest effective inhibitory concentration range, compound 14 strongly depolymerized interphase-state microtubules of both K562 and SK-N-MC cell lines () and bound to tubulin with dissociation constant (Kd) value of 9.4 ± 0.7 µM () and then inhibited the in vitro assembly of tubulin into microtubules (). The fact that compound 14 has antiproliferative activity and induced apoptosis may be related to its effect on mitotic spindles in the cells (CitationEstève et al., 2007). It seems that compound 14 disrupts microtubule assembly. The in vitro polymerization study of the tubulin shows that the compound 14 blocked microtubule assembly. The microtubule network in cytoplasm was strongly disrupted when the tumor cells were treated with compound 14. This compound induced typical chromatin condensation and nuclear fragmentation, as shown by the Hoechst and AO/EB staining, and an increase in the number of apoptotic cells as detected by the annexin V/PI assay. The detectable fragmented-DNA appeared after 72 h. The 180–200 bp DNA ladder represents the length of each oligonucleosome, indicating the breakage of double stranded DNA at the linker region between nucleosomes and the involvement of endonucleases in this process (CitationKhodarev & Ashwell, 1996).
The structure–activity studies of agents which exhibit cytotoxicity through the inhibition of tubulin polymerization have corroborated that most of them have a biaryl pharmacophore (CitationMiller et al., 1998). Structurally, the test compounds 1–16 are chalcone-type compounds containing a chromene scaffold with a biaryl pharmacophore. The spacer of two aryl rings in compounds 1–4 is a retroisomer of the spacer in compounds 5–16. By comparing the IC50s of these series of compounds, it seems that the type of spacer could affect the activity. As shown in the , the 2-chlorophenyl analogue 6 exhibited more potent activity than unsubstituted counterpart 5. This finding revealed that ortho-chloro substituent slightly improves the tubulin inhibition activity. While chlorine substitution at meta- and para-positions of phenyl ring (compounds 7 and 9, respectively) could not improve the activity. In the 6-chlorochromene derivatives, compound 13 (4-methoxyphenyl derivative) was more potent than compound 10 (unsubstituted phenyl derivative). Thus, the introduction of a 4-methoxy group on phenyl ring in 6-chlorochromene series increases the tubulin inhibitory and cytotoxic activities. Moreover, the introduction of a 3,4,5-trimethoxyphenyl group more significantly improves the activities (compound 14 vs. 10 and 13). The previous studies on tubulin polymerization inhibitors revealed that the most active compounds are methoxy analogues, thus methoxy substituents play a critical role in binding of colchicine and its biphenyl analogue to tubulin (CitationBai et al., 1996). Therefore, methoxyphenyl and trimethoxyphenyl moieties of compounds 13 and 14 maybe act as A-ring in colchicine. Based on the results obtained for compound 14, a trimethoxyphenyl adduct that possesses greater activity for the inhibition of tubulin polymerization, we have postulated that this domain possesses structural correspondence with the trimethoxyaryl substituents found in many biaryl tubulin inhibitors.
Conclusion
This study suggests that the compound 14 [(E)-3-(6-chloro-2H-chromen-3-yl)-1-(3,4,5-trimethoxyphenyl) prop-2-en-1-one] could be a good antitumor agent because of its biological functions. Compound 14 or its metabolites may bind directly to the tubulin and thereby perturb microtubule polymerization and the function of the spindle apparatus, which causes cancer cells to arrest and undergo apoptosis. Given the respectable activity of compound 14 for the inhibition of tubulin polymerization, the chromene-based chalcones provide a new avenue for the exploration of the development of new agents, which possess tubulin-mediated antimitotic activity.
Acknowledgements
The authors appreciate Amaneh Javid for the critical reading of manuscript and Yaghoub Pazhang for cooperating in immunocytochemical tests. We are also grateful to Nasibeh Davari for preparing photographs and cooperating in the experiments.
Declaration of interest: This investigation was supported by a grant from the Institute of Biochemistry and Biophysics, University of Tehran, Tehran, Iran.
References
- Akihisa T, Tokuda H, Ukiya M, Iizuka M, Schneider S, Ogasawara K, Mukainaka T, Iwatsuki K, Suzuki T, Nishino H. (2003). Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett, 201, 133–137.
- Alias Y, Awang K, Hadi AH, Thoison O, Sévenet T, Païs M. (1995). An antimitotic and cytotoxic chalcone from Fissistigma lanuginosum. J Nat Prod, 58, 1160–1166.
- Arora S, Jain J, Rajwade JM, Paknikar KM. (2009). Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol, 236, 310–318.
- Avila HP, Smânia Ede F, Monache FD, Smânia A Jr. (2008). Structure-activity relationship of antibacterial chalcones. Bioorg Med Chem, 16, 9790–9794.
- Bai R, Pei XF, Boyé O, Getahun Z, Grover S, Bekisz J, Nguyen NY, Brossi A, Hamel E. (1996). Identification of cysteine 354 of beta-tubulin as part of the binding site for the A ring of colchicine. J Biol Chem, 271, 12639–12645.
- Barrero AF, Herrador MM, Arteaga P, Cabrera E, Rodriguez-Garcia I, Garcia-Moreno M, Gravalos DG. (1997). Cytotoxic activity of favonoids from Carthamus arborescens, Ononis natrix ssp. ramosissima and Centiaurea malcitana. Fitoterapia, 68, 281–283.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248–254.
- Cheng JH, Hung CF, Yang SC, Wang JP, Won SJ, Lin CN. (2008). Synthesis and cytotoxic, anti-inflammatory, and anti-oxidant activities of 2′,5′-dialkoxylchalcones as cancer chemopreventive agents. Bioorg Med Chem, 16, 7270–7276.
- Estève MA, Carré M, Braguer D. (2007). Microtubules in apoptosis induction: Are they necessary? Curr Cancer Drug Targets, 7, 713–729.
- Foroumadi A, Emami S, Sorkhi M, Nakhjiri M, Nazarian Z, Heydari S, Ardestani SK, Poorrajab F, Shafiee A. (2010). Chromene-based synthetic chalcones as potent antileishmanial agents: Synthesis and biological activity. Chem Biol Drug Des, 75, 590–596.
- Guo J, Liu A, Cao H, Luo Y, Pezzuto JM, van Breemen RB. (2008). Biotransformation of the chemopreventive agent 2′,4′,4-trihydroxychalcone (isoliquiritigenin) by UDP-glucuronosyltransferases. Drug Metab Dispos, 36, 2104–2112.
- Gupta KK, Bharne SS, Rathinasamy K, Naik NR, Panda D. (2006). Dietary antioxidant curcumin inhibits microtubule assembly through tubulin binding. FEBS J, 273, 5320–5332.
- Harborne JB, Williams CA. (2000). Advances in flavonoid research since 1992. Phytochemistry, 55, 481–504.
- Hsu YL, Kuo PL, Tzeng WS, Lin CC. (2006). Chalcone inhibits the proliferation of human breast cancer cell by blocking cell cycle progression and inducing apoptosis. Food Chem Toxicol, 44, 704–713.
- Jiang JD, Davis AS, Middleton K, Ling YH, Perez-Soler R, Holland JF, Bekesi JG. (1998). 3-(Iodoacetamido)-benzoylurea: A novel cancericidal tubulin ligand that inhibits microtubule polymerization, phosphorylates bcl-2, and induces apoptosis in tumor cells. Cancer Res, 58, 5389–5395.
- Khodarev NN, Ashwell JD. (1996). An inducible lymphocyte nuclear Ca2+/Mg(2+)-dependent endonuclease associated with apoptosis. J Immunol, 156, 922–931.
- Kumar SK, Hager E, Pettit C, Gurulingappa H, Davidson NE, Khan SR. (2003). Design, synthesis, and evaluation of novel boronic-chalcone derivatives as antitumor agents. J Med Chem, 46, 2813–2815.
- Kuo YF, Su YZ, Tseng YH, Wang SY, Wang HM, Chueh PJ. (2010). Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: Involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radic Biol Med, 49, 214–226.
- Laemmli UK. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
- Ma R, Song G, You W, Yu L, Su W, Liao M, Zhang Y, Huang L, Zhang X, Yu T. (2008). Anti-microtubule activity of tubeimoside I and its colchicine binding site of tubulin. Cancer Chemother Pharmacol, 62, 559–568.
- Miller TA, Vachaspati PR, Labroli MA, Thompson CD, Bulman AL, Macdonald TL. (1998). The synthesis and evaluation of benzannelated-azatoxins: The benzazatoxins. Bioorg Med Chem Lett, 8, 1065–1070.
- Modzelewska A, Pettit C, Achanta G, Davidson NE, Huang P, Khan SR. (2006). Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg Med Chem, 14, 3491–3495.
- Murphy DB, Johnson KA, Borisy GG. (1977). Role of tubulin-associated proteins in microtubule nucleation and elongation. J Mol Biol, 117, 33–52.
- Nazarian Z, Emami S, Heydari S, Ardestani SK, Nakhjiri M, Poorrajab F, Shafiee A, Foroumadi A. (2010). Novel antileishmanial chalconoids: Synthesis and biological activity of 1- or 3-(6-chloro-2H-chromen-3-yl)propen-1-ones. Eur J Med Chem, 45, 1424–1429.
- Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. (2006). Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem, 290, 87–96.
- Rai D, Singh JK, Roy N, Panda D. (2008). Curcumin inhibits FtsZ assembly: An attractive mechanism for its antibacterial activity. Biochem J, 410, 147–155.
- Ramos S, Alía M, Bravo L, Goya L. (2005). Comparative effects of food-derived polyphenols on the viability and apoptosis of a human hepatoma cell line (HepG2). J Agric Food Chem, 53, 1271–1280.
- Rao YK, Fang SH, Tzeng YM. (2009). Synthesis and biological evaluation of 3′,4′,5′-trimethoxychalcone analogues as inhibitors of nitric oxide production and tumor cell proliferation. Bioorg Med Chem, 17, 7909–7914.
- Renvoizé C, Biola A, Pallardy M, Bréard J. (1998). Apoptosis: Identification of dying cells. Cell Biol Toxicol, 14, 111–120.
- Scatchard G. (1949). The attractions of proteins for small molecules ions. Ann N Y Acad Sci, 51, 660–672.
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. (1988). Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res, 48, 4827–4833.
- Sengupta S, Smitha SL, Thomas NE, Santhoshkumar TR, Devi SK, Sreejalekshmi KG, Rajasekharan KN. (2005). 4-Amino-5-benzoyl-2-(4-methoxyphenylamino)thiazole (DAT1): A cytotoxic agent towards cancer cells and a probe for tubulin-microtubule system. Br J Pharmacol, 145, 1076–1083.
- Shen KH, Chang JK, Hsu YL, Kuo PL. (2007). Chalcone arrests cell cycle progression and induces apoptosis through induction of mitochondrial pathway and inhibition of nuclear factor kappa B signalling in human bladder cancer cells. Basic Clin Pharmacol Toxicol, 101, 254–261.
- Sortino M, Delgado P, Juárez S, Quiroga J, Abonía R, Insuasty B, Nogueras M, Rodero L, Garibotto FM, Enriz RD, Zacchino SA. (2007). Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg Med Chem, 15, 484–494.
- Tabata K, Motani K, Takayanagi N, Nishimura R, Asami S, Kimura Y, Ukiya M, Hasegawa D, Akihisa T, Suzuki T. (2005). Xanthoangelol, a major chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma and leukemia cells. Biol Pharm Bull, 28, 1404–1407.
- Tang Y, Li X, Liu Z, Simoneau AR, Xie J, Zi X. (2010). Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int J Cancer, 127, 1758–1768.
- Watson WH, Cai J, Jones DP. (2000). Diet and apoptosis. Annu Rev Nutr, 20, 485–505.
- Wyllie AH, Morris RG, Smith AL, Dunlop D. (1984). Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol, 142, 67–77.
- Yang XW, Wang JS, Wang YH, Xiao HT, Hu XJ, Mu SZ, Ma YL, Lin H, He HP, Li L, Hao XJ. (2007). Tarennane and tarennone, two novel chalcone constituents from Tarenna attenuata. Planta Med, 73, 496–498.
- Yun JM, Kweon MH, Kwon H, Hwang JK, Mukhtar H. (2006). Induction of apoptosis and cell cycle arrest by a chalcone panduratin A isolated from Kaempferia pandurata in androgen-independent human prostate cancer cells PC3 and DU145. Carcinogenesis, 27, 1454–1464.
- Zi X, Simoneau AR. (2005). Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res, 65, 3479–3486.