Abstract
Context: One approach to protect human skin against the dangerous effects of solar ultraviolet (UV) irradiation is the use of natural products, such as photoprotectors. Phyllanthus orbicularis Kunth (Euphorbiaceae) is a Cuban endemic plant used in popular medicine. Its antigenotoxicity effect against some harmful agents has been investigated. However, the effect in ultraviolet B (UVB)-irradiated human cells has not been previously assessed.
Objective: The protective effect of a P. orbicularis extract against UVB light-induced damage in human cells was evaluated.
Materials and methods: DNA repair proficient (MRC5-SV) and deficient (XP4PA, complementation group XPC) cell-lines were used. Damaging effects of UVB light were evaluated by clonogenic assay and apoptosis induction by flow cytometry techniques. The extent of DNA repair itself was determined by the removal of cyclobutane pyrimidine dimers (CPDs). The CPDs were detected and quantified by slot-blot assay.
Results: Treatment of UVB-irradiated MRC5-SV cells with P. orbicularis extract increased the percentage of colony-forming cells from 36.03 ± 3.59 and 4.42 ± 1.45 to 53.14 ± 8.8 and 14.52 ± 1.97, for 400 and 600 J/m2, respectively. A decrease in apoptotic cell population was observed in cells maintained within the extract. The P. orbicularis extract enhanced the removal of CPD from genomic DNA. The CPDs remaining were found to be about 27.7 and 1.1%, while with plant extract, treatment these values decreased to 16.1 and 0.2%, for 3 and 24 h, respectively.
Discussion and conclusion: P. orbicularis aqueous extract protects human cells against UVB damage. This protective effect is through the modulation of DNA repair effectiveness.
Keywords::
Introduction
The ultraviolet (UV) irradiation of sunlight constitutes a human environmental carcinogen, the genotoxic effects there from, as well as those from artificial therapeutic lamps, are of major concern in regard human health (Matsumara & Anasthaswamy, Citation2004). This specific irradiation comprises of wavelength in the range of 100–400 nm and, didactically, can be still further divided into three parts, UVA, UVB and UVC, each eliciting distinct biological effects. UVB, with a wavelength varying within the range of 280–315 nm, represents one of the most important environmental factors affecting humans, due to the various biological consequences involved. Lipids, proteins and DNA molecules are all potential targets, either by the way of direct photochemical reactions or indirect oxidative damage from reactive oxygen species (ROS) (He & Häder, Citation2002; Svobodová & Vostálová, Citation2010). The main induced DNA lesions are cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts (6-4PPs) (CitationLima-Bessa & Menck, 2004). These are considered to be the major causes of apoptosis and mutagenesis induction, although the increase in ROS cellular levels resulting from irradiation may also be responsible for some of the deleterious UVB effects (Kulms & Schwarz, Citation2002).
Mammalian cells are equipped with a wide range of mechanisms for modulating the damaging effects of UV irradiation, such as DNA repair, and enzymatic and non-enzymatic antioxidants (Podda et al., Citation1998). Nucleotide excision repair (NER), the main mechanism involved in the repair of UVB-induced DNA damage in these cells, is effective in CPDs and 6-4PPs removal. Patients with the genetic disease xeroderma pigmentosum (XP) manifest genetic defects in various components of the NER complex. They are highly hypersensitive to UV irradiation, apparent through a dramatic increase in the incidence of skin cancer (Costa et al., Citation2003; Lehmann, Citation2003).
Recently, much effort has been dedicated to identify chemopreventive phytochemicals of a dietary and medicinal origin for skin-photoprotection. Natural compounds are among the most promising group of products that can be exploited as ideal preventives against skin cancer. Thus, the systematic evaluation of the photo-protective effect of plant extracts could be a source of important information on their potential use and efficiency (De Flora et al., Citation2001; Svobodová et al., Citation2003; Verschooten et al., Citation2006; Svobodová & Vostálová, Citation2010). The Cuban endemic species Phyllanthus orbicularis Kunth (Euphorbiaceae) is a medicinal plant whose antihepatitis B virus activity has been scientifically confirmed (Del Barrio et al., Citation1994). Furthermore, the antiviral activity of this plant against HSV-1, HSV-2 and bovine herpesvirus-1 (BHV-1) (subfamily Alphaherpesvirinae) (Del Barrio & Parra, Citation2000; Fernández Romero et al., Citation2003; Alvarez et al., Citation2009), as well as its antigenotoxic potential against aromatic amines, hydrogen peroxide, mitomycin C, and physical mutagens, such as X- and γ-rays (Sánchez-Lamar et al., Citation1999; Ferrer et al., Citation2001, Citation2002; Alonso et al., Citation2005), have been demonstrated. Considering these previous works, we report here the protective effects of the Phyllanthus orbicularis extract in UVB-irradiated human cell-lines, which occur most likely due to the modulation of DNA repair of these cells.
Materials and methods
P. orbicularis aqueous extract (POE)
In February, 2007, specimens of P. orbicularis plants were collected at Cajálbana, Pinar del Río, Cuba. These were authenticated by Dr. Rosalina Berazain of Cuban Botanical Gardens (No.7/220 HAJB). The samples were stored in this institution. The lyophilized extract was obtained according to the method described by Del Barrio and Parra (Citation2000). Phytochemichal studies of the P. orbicularis extract have demonstrated the presence of the compounds listed in .
Table 1. Phytochemichal composition of P. orbicularis extract.
Cells lines and culture conditions
Two SV40 transformed cell-lines were used, one NER proficient (MRC5-SV) from lung biopsies of a healthy individual, the other NER-deficient (XP4PA, complementation group XPC) from skin biopsies of a XP patient. Cells were routinely cultured at 37°C, and a 5% CO2 humidified atmosphere, in a Dulbecco modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA), supplemented with a 10% fetal bovine serum (FBS; Cultilab, Campinas, Sao Paulo, Brazil).
UVB irradiation
Vilber Loumart T15M 15 W lamps (emitting mainly at 312 nm, dose rate of 4.8 J/m2·s) were used for UVB irradiation of cell-culture dishes, covered to filter UVC wavelengths. A VLX3W radiometer, equipped with a monochromatic sensor CX-312 (Vilber Loumart, Torcy, France) was used for UVB-dose monitoring. Irradiation doses for each cell line were selected based on similar cell killing. They were 200–400 and 600 J/m2 for MRC5-SV cells and 40, 80 and 120 J/m2 for XP4PA cells. Before UV irradiation, cells were washed with phosphate buffered saline solution (PBS). Cells were irradiated in 1 mL of PBS with or without POE.
Quantification of extract transmittance
In order to evaluate the physical capacity of the extract during UVB irradiation, transmittance in 0.001, 0.01, 0.1, 0.5, 1.0 and 2.0 mg/mL of extract concentration was measured at the 280–326 nm wavelength level, by a Genesis TM Series 10 spectrophotometer (Thermo Electrón Corporación, Waltham, USA). The negative control was sterile miliQ water, the same used for diluting the lyophilized extract.
Clonogenic assay
Cell viability was evaluated by colony formation assay (Franken et al., Citation2006). Before evaluating the possible protective effects of the extracts, the cytotoxicity of their different concentrations were evaluated for both cell-lines. Approximately 1 × 103 exponentially growing cells were platted. After 16 h, the medium was supplemented with the plant extract for an additional 48 h period, and then removed to a medium without plant extract. Survival was expressed in relation to the untreated control. For the protective assessment of POE, cells were plated 17 h before irradiation. The extract (1 µg/mL nontoxic) was added 1 h prior to light-exposure and it was kept 48 h after irradiation. Following growth for 7–10 days, the colonies were colored with 1% violet crystal and counted. The ratio between irradiated colonies treated with POE and untreated was taken as cell-survival ability.
Apoptosis quantification by flow cytometry
The apoptotic cells were analyzed by flow cytometry (Adil et al., Citation2010). Approximately 1 × 105 cells were plated and irradiated as indicated above. After 48 h of cell culture irradiation, the cells were harvested by tripsinization. Dead cells in suspension were also collected. Cell suspensions were centrifuged at 1200 rpm (450g), for 10 min, whereas the pellet was resuspended in PBS and recentrifuged. Cells were then resuspended in ethanol 70%, to thus facilitate storage at −20°C for almost 2 weeks. The following stages involved centrifuging samples at 4000 rpm for 20 min, washing with PBS and incubation in a propidium iodide solution (RNAase 200 µg/mL, PI 20 µg/mL, 0.1% v/v Triton X-100 in PBS) for 30–60 min in the dark. The cells were centrifuged and resuspended in 100 µL of PBS, for subsequent analysis by flow cytometry (Guava Easycyte Plus Technologies, GE Healthcare, Hayward, CA, USA). The frequency of sub-G1 cells or subdiploid nuclei corresponds to apoptotic cells.
The analysis of DNA damage removal by slot blot
Immuno-slot blot assay was performed for the detection and quantification of CPD lesions (Lima-Bessa et al., Citation2008; Schuch et al., Citation2009). Approximately 5 × 105 cells were plated and irradiated as described above. At different intervals, after UVB irradiation, the cells were washed twice with PBS, and once with a solution of Triton X-100 0.5%, NaCl 0.1 M and EDTA 0.01 M, for 1 min at room temperature. They were then incubated with SDS 0.5%, proteinase K 50 μg/μL, NaCl 0.1 M and EDTA 0.01 M for 30 min at 37°C. NaCl was homogenously added to the plate, so as to reach a final concentration of 1 M, whereupon this was incubated for a further 15 min at 37°C. The cells were harvested with a rubber policeman, genomic DNA being extracted twice with chloroform:isoamyl alcohol (24:1), and the aqueous phase precipitated with absolute ethanol. Based on its absorbance at 260 nm, the amount of DNA was determined spectrophotometrically. Approximately 200 ng of total genomic DNA was boiled for 10 min at 100°C, and then spotted onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) using a slot-blot apparatus. Anti-CPD primary antibodies (MBL International Corporation, Nakaku Nagoya, Japan) were used for immunodetection of the number of CPD lesions. An anti-mouse IgG HRP conjugate (R & D Systems, Minneapolis, MN, USA) was employed as a secondary antibody. Blots were developed with a chemiluminescence detection system (Image Quant 300, GE Healthcare).The initial CPD formation and the remaining in DNA cellular repair for 3 and 24 h after UVB radiation were quantified. The damage levels were calculated by comparing the band intensities of the samples.
Statistical analysis
Median values and the corresponding standard deviation were calculated for each treatment. Variance homogeneity and ANOVA tests were also applied. Cytotoxicity median values were compared with the control by way of Dunnett testing (p < 0.05). In order to evaluate the protective effect, data from cells with and without extract were compared through the Student’s t-test (p < 0.05).
Results and discussion
P. orbicularis extract transmitance quantification
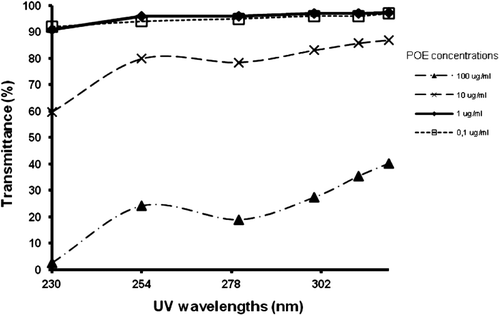
The results of P.orbicularis extract transmittance of various different wavelengths (), indicated that, at a concentration of 0.1–1 µg/mL, the extract permitted the passage of more than 90% UVB radiation, indicating low, if any, light absorption.
P. orbicularis extract cytotoxicity evaluation
The potential cytotoxic was evaluated in order to establish a range of concentration that does not affect cellular viability. The results are shown in . As can be seen, viability is very similar in both cell-lines (MRC5-SV and XP4PA), cytotoxic effects under exposure conditions only being detected at doses higher than 1 µg/mL. Curiously, DNA repair-deficient cells (XP4PA) were more resistant to the extract. The value of LC50 (media lethal concentration) for MRC5-SV and XP4PA cells was 20.4 and 70.5 µg/mL, respectively. The comparative difference in sensitivity (MRC5-SV cells are more sensitive than XP4PA), could be reflect of their different genetic background.
Figure 2. Toxicity of a P. orbicularis extract to human cell-lines. Clonogenic survival of MRC5-SV (squares) and XP4PA (triangles) cell-lines incubated for 48 h with various concentrations of a P. orbicularis extract. Each point represents the means ± SD of three independent experiments. * indicates differences against controls are statistically significant (Dunnet test, p < 0.05).
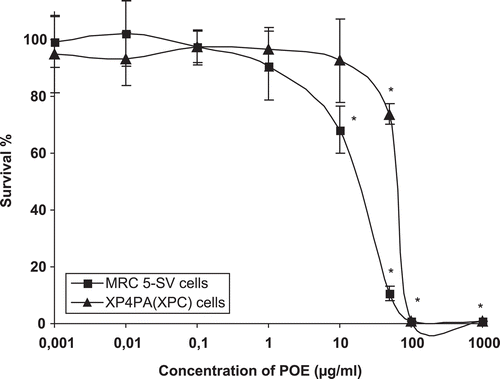
These results confirm previous observations in CHO cells (Sánchez-Lamar et al., Citation1999), where cytotoxicity was observed in doses higher than 100 µg/mL of this extract. Toxic effects have also been observed in rats and mice when high concentrations were intraperitoneally introduced (Rivera et al., Citation1995).
Protective effects of the P. orbicularis extract against UVB radiation in human cells
Based on the results of transmittance and cytotoxicity, a concentration of 1.0 µg/mL was selected for evaluating any protective effects from the extract in UVB irradiation-damaged cells.
The analysis of UVB lethal effects involved clonogenic survival and apoptosis. In both cases, XP4PA cells were irradiated with lower doses of UVB, since these cells, besides being deficient in repair, are more sensitive to UV-induced DNA damage.
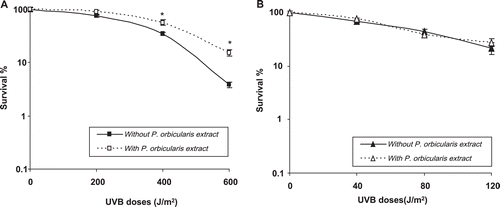
Clonogenic survival was measured in both the absence and presence of the extract ). Obviously, MRC5-SV cells, when irradiated in the presence of the plant extract, presented greater resistance to exposure (significant for 400 and 600 J/m2). Nevertheless, no difference was observed in the DNA repair-deficient cell line XP4PA.
Figure 3. DNA repair proficient cells have enhanced resistance to UVB irradiation in the presence of a P. orbicularis extract. Effects of a P. orbicularis extract (1 µg/mL) on clonogenic survival of MRC5-SV (A) and XP4PA (B) cells irradiated with UVB. Each point represents the means ± SD of three independent experiments. * indicates differences in each treatment that are statistically significant (Student’s t- test, p < 0.05).
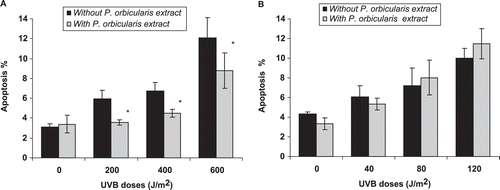
The results related with apoptotic cells indicated that the treatment of cells with the extract in absence of UVB exposure did not elicit any effect on DNA fragmentation.
In both cells lines, the UVB induction of apoptosis increased with UVB doses. However, the MRC5-SV cells treated with P. orbicularis extract were more resistant to the lethal effects of this radiation. The apoptotic population decreased significantly with POE treatment in all UVB doses. This effect was not observed for XP4PA cells ( and ).
Figure 4. Decreased UVB-induced apoptosis in DNA repair proficient cell line when incubated with a P. orbicularis extract. UVB induction of apoptosis in irradiated MRC5-SV (A) and XP4PA (B) cells in the absence (black bars) or presence (grey bars) of 1 µg/mL of plant extract. Each point represents the means ± SD of two independent experiments. * indicates that the differences in each treatment are statistically significant (Student’s t-test, p < 0.05).
The positive effect of some naturally occurring botanicals in the clonogenic survival and antiapoptotic process against different damage inducers has previously been demonstrated. El-Mahdy et al. (2004) has reported that flavonoid as narigenin increases the colony-forming capacity in HaCaT cells and also protects the cells from UVB-induced apoptosis. In this cell line, normal human keratinocyte, flavonoids as 3,4′-dihydroxy flavone and eriodictyol, have exerted antiapoptotic effect against different damaging agents (Lee et al., Citation2005, Citation2007). In addition, anthocyanidin as delphinidin, has been reported to protect HaCaT cells and mouse skin against UVB-induced damage and apoptosis (Afaq et al., Citation2007).
DNA damage removal
CPDs lesions have been described as the main lesions responsible for cell-death induction following UV irradiation in human cells (Chiganças et al., Citation2004; Lima-Bessa et al., Citation2008). In this case, the increased survival rate and diminished of programmed cells death in MRC5-SV could be associated with an enhancement of DNA photolesions repair. Then, we decided to verify the removal of DNA-lesions in MRC5-SV cells irradiated and maintained and not with P. orbicularis extract. The results shown in and indicate that, when the cells were analyzed for CPDs immediately after UVB exposure, no differences were observed in the cells treated with or without P. orbicularis extract, in terms of the number of CPD lesions. This implies the incapacity of the extract to prevent the immediate lesion formation upon exposure, thus eliminating a possible radiation filtering effect. However, after 3 or 24 h of irradiation, the numbers of CPD lesions were significantly reduced in cells maintained within the plant extract. For these times, the CPDs remaining were found to be about 27.7 and 1.1%, respectively. These values were decreased to 16.1 and 0.2% with plant extract treatment. The P. orbicularis extract enhanced the removal of CPDs in a time-dependent manner.
Figure 5. Improved DNA damage (CPD) removal in human cells incubated with a P. orbicularis extract. CPD removal was determined with immunoblots, in MRC5-SV cells irradiated with UVB (600 J/m2) and incubated in the presence or absence of P. orbicularis extract (1 µg/mL). The results are expressed in the relative amount of CPDs induction relative to samples of control DNA and the percentage of the remaining damage. * indicates that the differences in each treatment are statistically significant (Student’s t-test, p < 0.05).
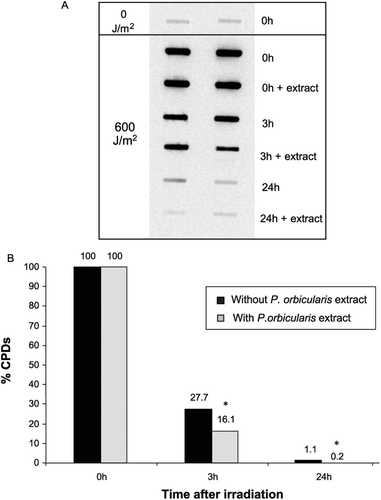
In mammalian cells, the CPD lesions are removed by the NER-system. According to this, our results suggest that the repair of UV-DNA damage by P. orbicularis extract is mediated through the NER mechanism. Also indicate that P. orbicularis might accelerate the repair of UVB-induced CPDs through this repair mechanism, the efficiency of this repair system augments in the presence of extract.
The enhanced DNA repair is one of the described mechanisms for the naturally occurring botanicals to reduce DNA damage and prevent carcinogenesis. In particular, the relation of some phytocompounds and NER-system has been discussed. Studies with catechins found in green tea polyphenols (GTPs) such as (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin-3-gallate and (−)-epigallocatechin-3-gallate have indicated that GTPs reduced significantly the number of CPD lesions in NER proficient cells, suggesting the relation of this compounds with NER mechanism (Meeran et al., Citation2006; Katiyar et al., Citation2007, Citation2010; Katiyar, Citation2011). Similar activity described above has been demonstrated for flavonoids, as narigenin and silimaryn (El-Mahdy et al., 2009; Vaid & Katiyar, Citation2010; Katiyar et al., Citation2011) and proanthocianidins (Vaid et al., Citation2010).
Exposure to the UV component of sunlight has been established as the major cause of human skin cancer. Thus, adequate photoprotection is essential in the control of UV-related deleterious effects. The avoidance of sunlight, skin-protection with clothing and sunscreens are, at present, and assuming their adequate use, the best ways of achieving efficient photoprotection. Moreover, novel approaches include the search for agents that favor endogenous protective responses to UV light. Currently, there is increased interest in using plant-derived agents in the prevention of skin damage caused by solar UV irradiation, the most promising chemical substances apparently being vitamins and elements of the polyphenol family (Svobodová et al., Citation2003; CitationLima-Bessa & Menck, 2004; Verschooten et al., Citation2006; Adil, et al Citation2010; Svobodová & Vostálová, Citation2010; Vaid et al., Citation2010; Afaq, Citation2011; Katiyar et al., Citation2011).
The P. orbicularis extract is a complex mixture of natural substances, rich in polyphenolic compounds. Phytochemistry studies have revealed the presence of flavanols, quercetin glycosides, condensed tannins, gallic acid-derivatives, quercetin-3-O-rutinoside, kaempherol-3-O-rutinoside, procyanidin B1, procyanidin B2, catechin, epicatechin, and protocatechuic acid (Gutiérrez et al., Citation2000, Citation2010; Alvarez et al., Citation2009). Several studies have demonstrated the beneficial properties of this extract. Some of these properties have been attributed to polyphenols present in the extract (Sánchez-Lamar et al., Citation1999; Del Barrio & Parra, Citation2000; Gutiérrez et al., Citation2000, Citation2010; Ferrer et al., Citation2001, Citation2002; Fernández Romero et al., Citation2003; Alonso et al., Citation2005; Alvarez et al., Citation2009).
In our investigation, we cannot exactly describe which compound(s) is/are responsible for the observed effect. However, since such effects has been reported in the case of catechins and flavonoids, the NER-related protective effect of P. orbicularis could be attributed to the presence of these compounds in our extract.
On the other hand, due consideration should also be given to the antioxidant properties of plant extracts (Sánchez-Lamar et al., Citation1999; Ferrer et al., Citation2002). The inactivation or scavenging of oxygen-free radicals generated during irradiation, by polyphenols, has been reported (Vayalil et al., Citation2004; Baliga & Katiyar Citation2006). Nevertheless, the experimental design employed here does not allow for evaluating this form of protection.
Conclusion
In this work, the effects of a P. orbicularis extract on the capacity of human cell-lines to respond to UVB irradiation were assessed. The present study constitutes the first report on the characterization of a P. orbicularis extract, as a protector against DNA-lesions in human cells, induced by UVB. It was also demonstrated that this protective effect is through modulation of NER-system effectiveness, thereby demonstrating the bioantimutagenic capacity of this extract against UV radiation. The results obtained with human cells, in an experimental-model form, constitute an important approach, thereby indicating the usefulness of this extract as a source of possible protective agents for humans.
Declaration of interest
This work was supported by CAPES (Brazil) -MES (Cuba) International Collaboration Project, FAPESP (São Paulo, Brazil) and CNPq (Brasília, Brazil). We thank Ph.D. Rosalina Berazain for authentication of specimen collected.
References
- Adil MD, Kaiser P, Satti NK, Zargar AM, Vishwakarma RA, Tasduq SA. (2010). Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. J Ethnopharmacol, 132, 109–114.
- Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. (2007). Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol, 127, 222–232.
- Afaq F.. (2011). Natural agents: cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys, 508, 144–151.
- Alonso A, Fuentes JL, Prieto E, Sánchez-Lamar A, Ferrer M, Llagostera M. (2005). Antigenotoxic effect of the aqueous extract of Phyllanthus orbicularis HBK in γ irradiated Escherichia coli cells. Recent Prog Med Plant, 10, 15–23.
- Alvarez AL, del Barrio G, Kourí V, Martínez PA, Suárez B, Parra F. (2009). In vitro anti-herpetic activity of an aqueous extract from the plant Phyllanthus orbicularis. Phytomedicine, 16, 960–966.
- Baliga MS, Katiyar SK. (2006). Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci, 5, 243–253.
- Chiganças V, Sarasin A, Menck CF. (2004). CPD-photolyase adenovirus-mediated gene transfer in normal and DNA-repair-deficient human cells. J Cell Sci, 117, 3579–3592.
- Costa RM, Chiganças V, Galhardo Rda S, Carvalho H, Menck CF. (2003). The eukaryotic nucleotide excision repair pathway. Biochimie, 85, 1083–1099.
- De Flora S, Izzotti A, D´Agostini F, Balansky RM, Noonan D, Albini A. (2001). Multiple points of intervention in the prevention of cancer and other mutation-related diseases. Mutat Res, 480–481, 19–22.
- Del Barrio G, Caballero O, Rivas LD, García D. (1994). Efectos de Phyllanthus orbicularis sobre el HBsAg del virus de la hepatitis B. Avances Biotecnol Modern, 2, 236.
- Del Barrio G, Parra F. (2000). Evaluation of the antiviral activity of an aqueous extract from Phyllanthus orbicularis. J Ethnopharmacol, 72, 317–322.
- El-Mahdy MA, Zhu Q, Wang QE, Wani G, Patnaik S, Zhao Q, Arafa el-S, Barakat B, Mir SN, Wani AA. (2008). Naringenin protects HaCaT human keratinocytes against UVB-induced apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome. Photochem Photobiol, 84, 307–316.
- Fernández Romero JA, Del Barrio Alonso G, Romeu Alvarez B, Gutiérrez Y, Valdés VS, Parra F. (2003). In vitro antiviral activity of Phyllanthus orbicularis extracts against herpes simplex virus type 1. Phytother Res, 17, 980–982.
- Ferrer M, Sánchez-Lamar A, Fuentes JL, Barbé J, Llagostera M. (2002). Antimutagenic mechanisms of Phyllanthus orbicularis when hydrogen peroxide is tested using Salmonella assay. Mutat Res, 400452, 1–4.
- Ferrer M, Sánchez-Lamar A, Fuentes JL, Barbé J, Llagostera M. (2001). Studies on the antimutagenesis of Phyllanthus orbicularis: Mechanisms involved against aromatic amines. Mutat Res, 498, 99–105.
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. (2006). Clonogenic assay of cells in vitro. Nat Protoc, 1, 2315–2319.
- Gutiérrez Y, Miranda M, del Barrio G, Varona N, Mayoral JL. (2000). Evaluación farmacognóstica y fitoquímica preliminar de Phyllanthus orbicularis. Rev Cubana Farm, 34, 56–62.
- Gutiérrez Y, Miranda M, del Barrio G. (2010). Análisis de flavonoides en una fracción butanólica obtenida de Phyllanthus orbicularis HBK. Rev Cubana Farm, 44, 367–373.
- He YY, Häder D. (2002). Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochem Photobiol Sci, 1, 729–736.
- Katiyar S, Elmets CA, Katiyar SK. (2007). Green tea and skin cancer: Photoimmunology, angiogenesis and DNA repair. J Nutr Biochem, 18, 287–296.
- Katiyar SK, Vaid M, van Steeg H, Meeran SM. (2010). Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res (Phila), 3, 179–189.
- Katiyar SK, Mantena SK, Meeran SM. (2011). Silymarin protects epidermal keratinocytes from ultraviolet radiation-induced apoptosis and DNA damage by nucleotide excision repair mechanism. PLoS ONE, 6, e21410.
- Katiyar SK. (2011). Green tea prevents non-melanoma skin cancer by enhancing DNA repair. Arch Biochem Biophys, 508, 152–158.
- Kulms D, Schwarz T. (2002). Independent contribution of three different pathways to ultraviolet-B-induced apoptosis. Biochem Pharmacol, 64, 837–841.
- Lee ER, Kang YJ, Kim JH, Lee HT, Cho SG. (2005). Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. J Biol Chem, 280, 31498–31507.
- Lee ER, Kim JH, Kang YJ, Cho SG. (2007). The anti-apoptotic and anti-oxidant effect of eriodictyol on UV-induced apoptosis in keratinocytes. Biol Pharm Bull, 30, 32–37.
- Lehmann AR. (2003). DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie, 85, 1101–1111.
- de Lima-Bessa KM, Armelini MG, Chiganças V, Jacysyn JF, Amarante-Mendes GP, Sarasin A, Menck CF. (2008). CPDs and 6-4PPs play different roles in UV-induced cell death in normal and NER-deficient human cells. DNA Repair (Amst), 7, 303–312.
- Lima-Bessa K, Menck CFM. (2004), Skin cancer: Lights on genome lesions. Curr Biol, 15, 58–61.
- Matsumara Y, Anasthaswamy HN. (2004). Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol, 195, 298–308.
- Meeran SM, Mantena SK, Elmets CA, Katiyar SK. (2006). (-)-Epigallocatechin-3-gallate prevents photocarcinogenesis in mice through interleukin-12-dependent DNA repair. Cancer Res, 66, 5512–5520.
- Podda M, Traber MG, Weber C, Yan LJ, Packer L. (1998). UV-irradiation depletes antioxidants and causes oxidative damage in a model of human skin. Free Radic Biol Med, 24, 55–65.
- Rivera F, Vidal AT, Lòpez EM, Ferrer M, Sotolongo ME. (1995). Toxicidad aguda y subcrònica de un extracto de Phyllanthus orbicularis. Rev Cubana Farm Suppl Especial, 30, 35–36.
- Sánchez-LamarA, Fiore M, Cundari E, Ricordy R, Cozzi R, De Salvia R. (1999). Phyllanthus orbicularis aqueous extract: cytotoxic, genotoxic, and antimutagenic effects in the CHO cell line. Toxicol Appl Pharmacol, 161, 231–239.
- Schuch AP, da Silva Galhardo R, de Lima-Bessa KM, Schuch NJ, Menck CF. (2009). Development of a DNA-dosimeter system for monitoring the effects of solar-ultraviolet radiation. Photochem Photobiol Sci, 8, 111–120.
- Svobodová A, Psotová J, Walterová D. (2003). Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 147, 137–145.
- Svobodová A, Vostálová J. (2010). Solar radiation induced skin damage: Review of protective and preventive options. Int J Radiat Biol, 86, 999–1030.
- Vaid M, Katiyar SK. (2010). Molecular mechanisms of inhibition of photocarcinogenesis by silymarin, a phytochemical from milk thistle (Silybum marianum L. Gaertn.) (Review). Int J Oncol, 36, 1053–1060.
- Vaid M, Sharma SD, Katiyar SK. (2010). Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum group A-dependent mechanism. Cancer Prev Res (Phila), 3, 1621–1629.
- Vayalil PK, Mittal A, Hara Y, Elmets CA, Katiyar SK. (2004). Green tea polyphenols prevent ultraviolet light-induced oxidative damage and matrix metalloproteinases expression in mouse skin. J Invest Dermatol, 122, 1480–1487.
- Verschooten L, Claerhout S, Van Laethem A, Agostinis P, Garmyn M. (2006). New strategies of photoprotection. Photochem Photobiol, 82, 1016–1023.