Abstract
Context: Daidzein is a naturally occurring compound and has various health benefits. However, its effects on intestinal smooth muscle contractility remain unknown.
Aims: The present study was to characterize the effects of daidzein on the contractility of isolated jejunal smooth muscle and its underlying mechanisms.
Methods: Ex vivo assay was selected as the major method to determine the effects of daidzein on the contractility of isolated jejunal smooth muscle fragment (JSMF).
Results: Daidzein (5–160 µmol/L) inhibited the contractility of JSMF in normal contractile state and in a dose-dependent manner. Daidzein also inhibited the contractility of JSMF induced by ACh, histamine, erythromycin and high Ca2+, respectively, and decreased charcoal propulsion in the small intestine in vivo. The inhibitory effects of daidzein were partially blocked by phentolamine or propranolol and were abolished in the presence of varapamil or at Ca2+-free assay condition. However, the inhibitory effects of daidzein on jejunal contraction were not significantly influenced by nitric oxide (NO) synthase inhibitor L-NG-nitro-arginine (l-NNA). Daidzein was also found to directly inhibit the phosphorylation and Mg2+-ATPase activity of smooth muscle myosin.
Discussion and Conclusion: The results implicated that α- and β-adrenergic receptors were involved in the inhibitory effects produced by daidzein rather than via NO pathway. As a phytoestrogen, daidzein has shown its potential value in relieving the hypercontractility of small intestine.
Introduction
Daidzein, which belongs to the group of isoflavones, is a naturally occurring compound in a number of plants including Thai Kwao Krua and Pueraria mirifica, and in food sources such as soybeans. Isoflavones have a wide spectrum of physiological and pharmacological functions (CitationDwiecki et al., 2009; CitationGottstein et al., 2003). They are known to have various health benefits, such as preventing osteoporosis, alleviating menopausal symptoms, producing immunomodulatory and differentiation-inducing effects, (CitationValachovicova et al., 2004; CitationMcCue & Shetty, 2004), and anti-cancer properties (Park & Surh, 2004; CitationSarkar & Li, 2003; CitationToyomura & Kono, 2002). Daidzein is also found to dilate arteries via the inhibition of Ca2+ inflow (CitationTorregrosa et al., 2003). As a phytoestrogen, daidzein acts either as an estrogen agonist or as an antagonist, and thus used for natural estrogen replacement therapy (CitationLethaby et al., 2007; CitationSetchell & Cassidy, 1999). Estrogen is found to relax duodenum in an estrogen receptor-independent way and to dramatically improve the stool scores of HLA-B27 transgenic rats with chronic diarrhea (CitationSetchell & Cassidy, 1999; CitationHarnish et al., 2004). However, the effects of daidzein on intestinal smooth muscle contractility and the underlying mechanism(s) remain to be clarified.
Based upon the aforementioned information, the aim of the present study was to characterize the effects of daidzein on the contractility of isolated jejunal smooth muscle fragment (JSMF) and to reveal the underlying mechanisms. As motility-related diarrhea is caused by the hypercontractility of bowels and jejunum could be regarded as a typical region of the small intestine, the effects of daidzein were tested on JSMF in normal contractile state and high contractile states induced in different assay conditions respectively. The effects of daidzein on intestinal propulsion in vivo and on myosin light chain phosphorylation in vitro were also investigated.
Materials and methods
Animals
The experiments were performed according to the rules of animal care and were approved by the animal protection committee of Dalian Medical University. Four-week-old Kunming mice (20–30 g) and Sprague-Dawley rats (180–220 g) were used in the experiments. Animals were housed in a temperature-controlled room with a 12-h light–dark cycle.
Drug and agents
Daidzein was bought from Chengdu Biopurify Phytochemicals Ltd., Chengdu, China. Krebs buffer ingredient (mmol/L): sodium chloride 114.0, potassium chloride 4.7, magnesium chloride 1.2, calcium chloride 2.5, sodium dehydrogenate phosphate 1.8, glucose 11.5, and sodium bicarbonate 4.2, pH 7.4. Unless otherwise indicated, chemicals were obtained from Sigma, Chicago, IL, USA.
Tissue preparation and contraction determination
Jejunum was isolated and cut into approximately 2 cm in length (tubes) according to the methods previously described (CitationMathison & Shaffer, 2006; CitationLin et al., 2011). One end of isolated jejunal segment was fixed to the wall of a tissue bath chamber (20 mL volume), and the other end was connected to a force-displacement transducer to determine the contraction. The organ bath was maintained at 37°C, and the resting tension was set optimally at 1.0 g. Preliminary experiments showed that this load stretched the tissues to their optimal length for force development during contraction. The preparation was allowed to equilibrate in aerated Krebs buffer for 50 min and the bath solution was replaced every 10 min. Contractile amplitude of JSMF was measured from the baseline to the peak and was expressed as a percentage of the normal contractile amplitude. Contractile amplitude of JSMF was recorded and the identical time-interval of each assay with the same start and stop time was chosen to compare the amplitude of contractions before and after drug treatment under different assay conditions. The mean amplitude was calculated from the results of six separated assays.
Assay for small intestinal propulsion
Effects of daidzein in different doses on small intestinal propulsion of overnight fasted mice were tested in the absence and presence of carbachol, respectively. For group A, mice were randomly divided into four subgroups and were administered by gavage of daidzein in a dose of 0, 20, 40, and 80 mg/kg, respectively, and after 30 min, mice were orally administered 10 mL/kg of 10% activated charcoal powder suspended in 5% arabic gum. The same procedures were performed for group B, except that carbachol (1 mg/kg, i.p.) was injected 15 min before daidzein administration. Mice were sacrificed 30 min after given activated charcoal, and the entire small intestine was removed. The rate of small intestinal propulsion was calculated from the distance of the activated charcoal migration divided by the total length of the small intestine as previously described (CitationKase et al., 1996).
Measurement of phosphorylation and Mg2+-ATPase activity of myosin
Myosin and myosin light chain kinase (MLCK) were purified from the chicken gizzard smooth muscle to homogeneity as described previously (CitationTang et al., 2005) and used in the study. The purity analysis of protein samples was assessed by SDS-PAGE. Protein concentrations were determined by using the method of Bradford (CitationBradford, 1976; CitationLin et al., 2000).
Determination of myosin light chain phosphorylation (P-LC20) and of Mg2+-ATPase activity was carried out in a 20 mmol/L Tris–HCl (pH 7.4) buffer containing 1 mmol/L dithiothreitol, 5 mmol/L MgCl2, 60 mmol/L KCl, 2 mmol/L ethylene glycol bis (β-aminoethyl ether) N,N′-tetraacetic acid, 4 μmol/L myosin, 2 μmol/L MLCK and 2 mmol/L ATP (CitationTang et al., 2005).
Glycerol polyacrylamide gel electrophoresis was used to measure the extent of P-LC20. The densitometry extent of P-LC20 was measured with the Scion Image Software from Scion Co. Ltd, Shanghai, China (CitationYang et al., 2003).
Statistical analysis
Student’s t-test was used to compare statistical differences between two samples, p < 0.05 was considered to indicate statistical significance.
Results
Effects of daidzein on jejunal contraction
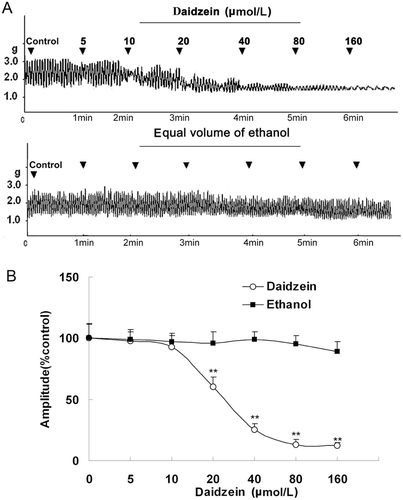
At bath concentrations of 5–160 μmol/L, daidzein decreased contractile amplitude of JSMF (from 100 ± 10.4 to 19 ± 3.41%) in a dose dependent manner ().
Figure 1. Cumulative dose-response curve for the inhibitory effects of daidzein on the contractility of jejunal smooth muscle fragment (JSMF). Representative traces (A) and statistical analysis (B) of 5, 10, 20, 40, 80, and 160 μmol/L of daidzein on the contractility of JSMF are illustrated; the contractile amplitude of JSMF in normal contractile state is chosen as 100% (control); other data are the relative values compared with the control (n = 6). **p < 0.01 compared with the corresponding control.
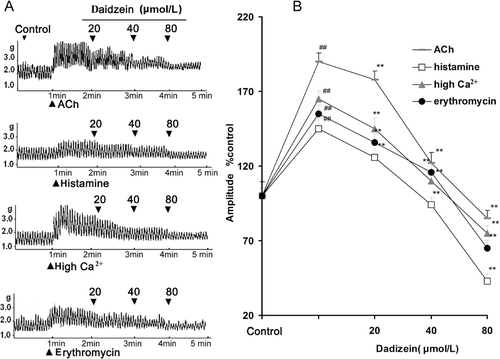
Daidzein, at bath concentrations of 20–80 μmol/L, was chosen to investigate its effects on JSMF contracted by 10 μmol/L ACh, 20 μmol/L histamine, 5 μmol/L erythromycin (motilin receptor agonist), and high Ca2+ (Krebs buffer contained 5 mmol/L Ca2+), respectively (). The results showed that daidzein (20–80 μmol/L) decreased the contractility of JSMF induced by ACh, histamine, erythromycin and high Ca2+ in a dose-dependent manner, respectively.
Figure 2. Effects of daizein on the contractility of jejunal smooth muscle fragment (JSMF) in different high contractile states. Representative traces (A) and statistical analysis (B) of daidzein on the contractility of JSMF pretreated with ACh, histamine, high Ca2+ and erythromycin, respectively are illustrated. The mean contractile amplitude of JSMF in normal contractile state is chosen as 100% (control), other data are the relative value compared with the control. Data are expressed as mean ± SEM. (n = 6). ##p < 0.01 compared with control, **p < 0.01 compared with ACh, histamine, high Ca2+ and erythromycin groups before given daizein, respectively.
Underlying mechanism involved in daidzein-induced inhibition
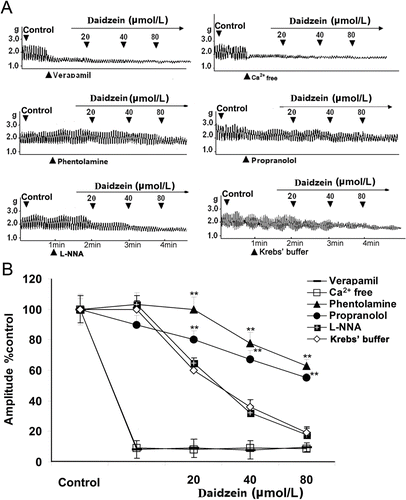
As shown in , daidzein at bath concentrations of 20, 40, and 80 µmol/L did not affect jejunal contraction significantly under Ca2+-free assay conditions, and it did not significantly affect jejunal contraction preincubated with Ca2+ channel blocker verapamil (0.1 μmol/L). The results also showed that compared with daidzein control, phentolamine (10 μmol/L) and propranolol (10 μmol/L) partially blocked the inhibitory effects produced by 20, 40, and 80 μmol/L daidzein, respectively. However, nitric oxide (NO) synthase inhibitor L-NNA (10 μmol/L) did not significantly affect the inhibitory effects of daidzein on jejunal contractility ().
Figure 3. Underlying mechanisms involved in daidzein-induced inhibition on the contractility of jejunal smooth muscle fragment (JSMF). Representative traces (A) and statistical analysis (B) of daidzein on the contractility of JSMF in the conditions of using Ca2+-free Krebs buffer, pretreatment with verapamil, phentolamine, propranolol, and L- NNA, respectively. The contractile amplitude of JSMF in normal contractile state is chosen as 100% (control), other data are the relative value compared to the control. **p < 0.01 indicates the data of the treatment groups compared with the corresponding data of daidzein control.
Effects of daidzein on small intestinal propulsion
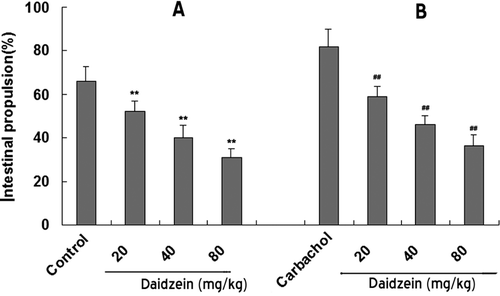
Daidzein administered by gavage significantly inhibited normal intestinal propulsion and the increased propulsion induced by carbachol stimulation ().
Figure 4. Effects of daidzein on intestinal propulsion in (A) normal contractile state and in (B) high contractile state (pretreated with carbachol). Each column represents the mean ± SEM (n = 6). **p < 0.01 compared with the propulsion before the treatment of daidzein (normal control). ##p < 0.01 compared with the carbachol-induced propulsion before the treatment of daidzein (carbachol control).
Effects of daidzein on myosin phosphorylation and Mg2+-ATPase activity of myosin
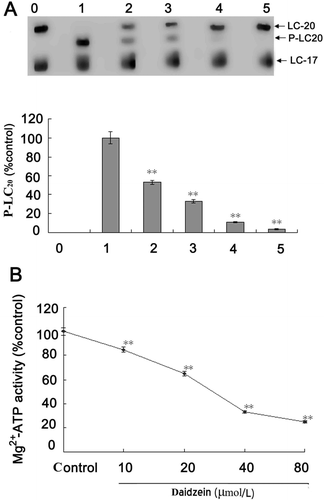
The results showed that daidzein directly decreased both the myosin light chain phosphorylation (, lane 2, 3, 4, 5) and the Mg2+-ATPase activities of myosin () in a dose-dependent manner (**p < 0.01).
Figure 5. Effects of daidzein on myosin phosphorylation and myosin Mg2+-ATPase activity. LC20, 20 kDa unphosphorylated myosin light chain; P-LC20, phosphorylated 20 kDa myosin light chain; LC17, 17 kDa myosin essential light chains. (A) Effects of daidzein on myosin light chain phosphorylation; lane 0–5 and column 0–5 represent unphosphorylated myosin (without MLCK and daidzein), control (without daidzein), 10, 20, 40, and 80 μmol/L daidzein, respectively. (B) Effects of daidzein on myosin Mg2+-ATPase activities. **p < 0.01 compared with the control of phosphorylated myosin.
Discussion
The selection of isolated JSMF to observe the effects of daidzein in the study was based on the fact that the motility of intestinal smooth muscle is modulated by the enteric nervous system (ENS), which is able to fulfill pivotal functions even when isolated from the body (CitationSchemann, 2005). The results showed that daidzein, at bath concentrations of 20–80 μmol/L, produced inhibitory effects on JSMF in normal contractile state and in a dose-dependent manner and inhibited the contractile responses induced by high Ca2+, ACh, histamine, and motilin receptor agonist erythromycin, respectively, indicating that daidzein can inhibit intestinal motility in both normal and high states. The inhibitory effects of daidzein on intestinal smooth muscle were also observed in vivo, both in the absence and presence of carbachol.
It is known that activation of α- or β-adrenoceptor inhibits intestinal contractility. The α-adrenergic antagonist phentolamine and the β-adrenergic antagonist propranolol partially blocked the inhibitory effects induced by daidzein, respectively, and this evidence implicates that the inhibitory effect of daidzein on intestinal motility is correlated with the stimulation of adrenergic α, and β-adrenergic receptors. Inhibition of intestinal motility is also mediated by NO which is a nonadrenergic, noncholinergic neurotransmitter, producing its effect by directly acting on smooth muscle and by indirectly inhibiting acetylcholine and substance P releasing (CitationMang et al., 2002; CitationLi et al., 2002). Our study showed that NO synthase inhibitor L-NNA did not affect the inhibitory effects produced by daidzein significantly; implicating that NO nonadrenergic, noncholinergic inhibitory pathways were not involved in daidzein-induced inhibitory effects. The inhibitory effects of daidzein on jejunal contracton were not found after the pretreatment of Ca2+ channel blocker verapamil or under Ca2+-free assay condition, implicating that the modulation of daidzein on jejunal contraction was dependent on the influx of extracellular Ca2+. The results indicated that the mechanisms underlying the inhibitory effects of daidzein were also correlated to the direct inhibition of smooth muscle myosin light chain phosphorylation and of myosin Mg2+-ATPase activity.
Conclusion
Our results indicated that daidzein relaxed intestinal smooth muscle both in normal and high contractile states and under ex vivo and in vivo assay conditions. α- and β-Adrenergic receptors were found involved in the inhibitory effects produced by daidzein rather than via NO pathway. The inhibitory effects of daidzein were found to correlate to the inhibition of both myosin light chain phosphorylation and Mg2+-ATPase activities. However, since the highly interconnected ENS is responsible for secreting at least 50 different substances (CitationGoldstein & Nagy, 2008), and the relationship between these substances in regulating intestinal function remains unclear, these results should be considered preliminary. The detailed mechanisms and the potential value of daidzein in relieving the hypercontractility of intestinal smooth muscle in clinics need further investigation.
Acknowledgement
We thank Lin Zhi, Yuan Fun, and Jiang Xin for their valuable comments.
Declaration of interest
This study was supported by the National Natural Science Foundation of China (No. 30772601).
References
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72, 248–254.
- Dwiecki K, Neunert G, Polewski P, Polewski K. (2009). Antioxidant activity of daidzein, a natural antioxidant, and its spectroscopic properties in organic solvents and phosphatidylcholine liposomes. J Photochem Photobiol B, Biol, 96, 242–248.
- Goldstein AM, Nagy N. (2008). A bird’s eye view of enteric nervous system development: Lessons from the avian embryo. Pediatr Res, 64, 326–333.
- Gottstein N, Ewins BA, Eccleston C, Hubbard GP, Kavanagh IC, Minihane AM, Weinberg PD, Rimbach G. (2003). Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Br J Nutr, 89, 607–616.
- Harnish DC, Albert LM, Leathurby Y, Eckert AM, Ciarletta A, Kasaian M, Keith JC Jr. (2004). Beneficial effects of estrogen treatment in the HLA-B27 transgenic rat model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol, 286, G118–G125.
- Kase Y, Hayakawa T, Takeda S, Ishige A, Aburada M, Okada M. (1996). Pharmacological studies on antidiarrheal effects of Hange-shashin-to. Biol Pharm Bull, 19, 1367–1370.
- Lethaby AE, Brown J, Marjoribanks J, Kronenberg F, Roberts H, Eden J. (2007). Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev, CD001395.
- Li M, Johnson CP, Adams MB, Sarna SK. (2002). Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol, 283, G544–G552.
- Lin GM, Xu ZL, Zhang X, Gao H, Hou Y, Yin LB, Zhang XD, Yang JX, Jia TZ. (2011). Epoxyaurapten inhibition of smooth muscle contraction and phosphorylation of myosin light chain by myosin light chain kinase. J Med Plants Res, 5, 3412–3420.
- Lin Y, Sun H, Dai S, Tang Z, He X, Chen H. (2000). The bi-directional regulation of filamin on the ATPase activity of smooth muscle myosin. Chin Med Sci J, 15, 162–164.
- Mang CF, Truempler S, Erbelding D, Kilbinger H. (2002). Modulation by NO of acetylcholine release in the ileum of wild-type and NOS gene knockout mice. Am J Physiol Gastrointest Liver Physiol, 283, G1132–G1138.
- Mathison R, Shaffer E. (2006). Increased cholinergic contractions of jejunal smooth muscle caused by a high cholesterol diet are prevented by the 5-HT4 agonist–tegaserod. BMC Gastroenterol, 6, 8.
- McCue P, Shetty K. (2004). Health benefits of soy isoflavonoids and strategies for enhancement: A review. Crit Rev Food Sci Nutr, 44, 361–367.
- Park OJ, Surh YJ. (2004). Chemopreventive potential of epigallocatechin gallate and genistein: Evidence from epidemiological and laboratory studies. Toxicol Lett, 150, 43–56.
- Sarkar FH, Li Y. (2003). Soy isoflavones and cancer prevention. Cancer Invest, 21, 744–757.
- Schemann M. (2005). Control of gastrointestinal motility by the “gut brain”–the enteric nervous system. J Pediatr Gastroenterol Nutr, 41 Suppl 1, S4–S6.
- Setchell KD, Cassidy A. (1999). Dietary isoflavones: Biological effects and relevance to human health. J Nutr, 129, 758S–767S.
- Tang Z, Chen H, Yang J, Dai S, Lin Y. (2005). The comparison of Ca2+/CaM-independent and Ca2+/CaM-dependent phosphorylation of myosin light chains by MLCK. Physiol Res, 54, 671–678.
- Torregrosa G, Burguete MC, Pérez-Asensio FJ, Salom JB, Gil JV, Alborch E. (2003). Pharmacological profile of phytoestrogens in cerebral vessels: in vitro study with rabbit basilar artery. Eur J Pharmacol, 482, 227–234.
- Toyomura K, Kono S. (2002). Soybeans, soy foods, isoflavones and risk of colorectal cancer: A review of experimental and epidemiological data. Asian Pac J Cancer Prev, 3, 125–132.
- Valachovicova T, Slivova V, Sliva D. (2004). Cellular and physiological effects of soy flavonoids. Mini Rev Med Chem, 4, 881–887.
- Yang JX, Wang XM, Tang ZY, Chen H, Xu H, Lin Y. (2003). The characterization of Ca(2+)-calmodulin independent phosphory-lation of myosin light chains by a fragment from myosin light chain kinase. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao, 35, 793–800.