Abstract
Context: Clematichinenoside (AR-6) is a triterpene saponin from an anti-arthritic herbal formula Wei-Ling-Xian in Chinese, which is an herbal medicine derived from the dried root and rhizome of Clematis chinensis Osbeck, C. hexapetala Pall., or C. manshurica Rupr. (Ranunculaceae).
Objective: To investigate the modulating effect and explored the potential mechanism of AR-6 in rheumatoid arthritis (RA), using collagen-induced arthritis (CIA) in a rat model.
Materials and methods: CIA was evaluated by measuring body weight, paw swelling and organ index. Expression of TNF-α, PI3K and p-Akt in synovium tissue was measured by immunohistochemistry. Furthermore, expression of TNF-α mRNA, PI3K mRNA and p-Akt mRNA was measured with RT-PCR.
Results: The intragastric administration of AR-6 (32, 16 and 8 mg/kg), especially the high dose level of 32 mg/kg, significantly suppressed the swelling of hind paws of CIA rats (p < 0.01) and inhibited their body weight loss (p < 0.01). Based on histopathological observation, all AR-6 groups showed great amelioration compared with model group. Moreover, AR-6 significantly reduced the production of TNF-α, PI3K and p-Akt expression by immunohistochemistry (p < 0.01), and decreased TNF-α mRNA, PI3K mRNA and p-Akt mRNA in CIA rat synovium (p < 0.01).
Discussion: Our study indicates the mechanism of AR-6 is associated with PI3K/Akt signaling pathway and TNF-α.
Conclusions: Such characteristics relating to AR-6 curing chronic inflammation of CIA, may be effectively applied to the therapeutic potential in patients with inactive RA.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune disorder of the joints (CitationArntz et al., 2010), for which current treatment strategies remain suboptimal (CitationO’Dell, 2004). Although the pathogenesis of the disease is not fully understood, it involves cellular infiltration into synovial tissue and increased inflammatory cytokines that lead to cartilage and bone erosion through the induction of matrix metalloproteinases (MMPs) and dysregulated chondrocyte function (CitationGoldring, 2003).
The pathogenic mechanism is incompletely understood, but cytokine networks are known to play a substantial part in synovitis and bone destruction, and are persistent factors in disease progression (CitationMiossec, 2004). Among the inflammatory cytokines, tumor necrosis factor-α (TNF-α) seems to be on top of a cytokine cascade. Many studies have shown that TNF-α is a principal mediator of inflammation in RA (CitationMontalban et al., 2010), contributing to joint effusion, synovial proliferation, and bone and cartilage damage in addition to systemic inflammation (CitationBingham, 2002) by enhancing the generation of MMP and supporting the formation of osteoclasts (CitationZwerina et al., 2006). Many inflammatory proteins are expressed through activation of transcription factors by upstream signaling such as TNF-α signaling pathway. Therefore, the inhibition of TNF-α may help prevent inflammation.
Mediators released during inflammatory disease states initiate intracellular signaling cascades regulated by kinases and phosphatases (CitationHerlaar & Brown, 1999). Among the different signaling pathways implicated in systemic autoimmunity, the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway occupies a central position in the regulation of multiple pathogenic cascades that can lead to the development of autoimmunity (CitationWu & Mohan, 2009). PI3K is a family of evolutionary conserved lipid kinases, which play a crucial role in controlling a wide variety of intracellular signaling events. Akt, as a downstream kinase of PI3K (CitationKim et al., 2010), is involved in regulating the cell cycle and glucose metabolism and in modulation of cell growth and survival. Moreover, Akt controls the translational machinery through the mammalian target of rapamycin (mTOR), the ribosomal protein S6 kinase (S6K) and the 4E-binding protein (4E-BP) (CitationFingar & Blenis, 2004). Downstream events controlled by Akt further include the control of apoptosis through the regulation of proteins such as forkhead (FKHR), BAD and NF-κB (CitationDownward, 2004).
On the contrary, traditional Chinese medicine (TCM) is accepted as the most prevalent and effective treatment in the management of RA in many Asian countries. Since ancient times numerous formulae have been developed for the treatment of RA, and the pain-relieving, anti-inflammation and/or immunomodulatory effects of these formulae have been confirmed in many basic and clinical studies (CitationSetty & Sigal, 2005). Clematichinenoside (AR-6) is a triterpene saponin from an anti-arthritic herbal formula Wei-Ling-Xian in Chinese, which is a commonly used herb with a long clinical use history in Asia. The Chinese Pharmacopoeia (2010 edition) defines the Chinese medicine Wei-Ling-Xian as an herbal medicine derived from the dried root and rhizome of Clematis chinensis Osbeck, C. hexapetala Pall., or C. manshurica Rupr. (Ranunculaceae). Clematis chinensis has been frequently used to cure RA and dermatitis glandularis erythematosa in ancient China ().
Previous investigations in our laboratory indicated that oral administration of AR-6 to arthritic rats resulted in a clear decrease of clinical signs compared to untreated controls in chronic rat adjuvant-induced arthritis (AA) and collagen-induced arthritis (CIA). AR-6 significantly inhibited NO and TNF-α produced by peritoneal macrophages, decreased the proliferation of synoviocyte. In addition, the anti-inflammatory effects of AR-6 may be associated with NF-κB, TNF-α and COX-2 (CitationSun et al., 2010; CitationPeng et al., 2011).
In the present study, CIA, as a most commonly used animal model of RA, was induced to verify the possible mechanism of AR-6 derived cytokines and signaling pathways. For that purpose, the CIA model was built to examine the modulating effect of AR-6 on rheumatoid arthritis. Since the transcription of TNF-α has been known to be induced by NF-κB in RA (CitationFishman et al., 2006), immunohistochemistry of TNF-α, PI3K and the phosphorylation of Akt (p-Akt) in synovium was evaluated to identify their correlation upon AR-6 treatment. In order to investigate further, we also have explored the synovial expression of TNF-α mRNA, PI3K mRNA and p-Akt mRNA by using RT-PCR analysis.
Materials and methods
Experimental animal
Male Wistar rats, weighing 110–130 g were purchased from Suzhou Aiermaite Technology Co. Ltd. They were kept under standard laboratory conditions, in a temperature-controlled environment (22 ± 2°C), 55 ± 5% relative humidity with a 12 h:12 h light–dark cycle, fed with standard chow and water, for at least 1 week before any manipulations. All experimental protocols were approved by the Animal Care and Use Committee of China Pharmaceutical University, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Reagents and assay kits
AR-6 (95.3% purity): prepared by School of Traditional Chinese Pharmacy, China Pharmaceutical University (CitationMa et al., 2009). Eighteen samples of Clematis chinensis and four samples of C. hexapetala were collected from 2008 to 2009 in different habitats of China. These samples were authenticated by one of the authors (Lifang Liu), and then all the collected samples were combined to make one batch. The voucher specimens were deposited in our department. The information of each sample is listed in detail in . AR-6 was suspended in distilled water to prepare 32, 16 and 8 mg/kg three doses before administration. Methotrexate (MTX), product of Shanghai Sine Pharmaceutical Co. Ltd, was dissolved in 0.5% CMC-Na suspension before use. Total glucosides of peony (TGP), product of Ningbo Lihua Pharmaceutical Co. Ltd, was dissolved in 0.5% CMC-Na suspension before use. Freund’s complete adjuvant, Collagen II and phosphate buffered saline (PBS) were purchased from Sigma Chemical Company. Anti-TNF-α, Anti-PI3K and Anti-phospho-Akt/PKB were purchased from Beijing Boisynthesis Biotechnology Co. Ltd. PrimeScript RT-PCR Kit was obtained from Takara Biotechnology (Dalian) Co. Ltd. Primers were synthesized by Shanghai Generay Biotech Co. Ltd. All other reagents were of analytical grade and commercially available.
Table 1. The samples of Wei-Ling-Xian collected from different habitats in the present study.
CIA induction and administration
Collagen II was dissolved in 0.1 M glacial acetic acid and Freund’s complete adjuvant was slowly added, and then thoroughly stirred into an emulsion. Wistar rats were divided separately into seven groups randomly: the normal control group, the model group, the positive control group of western medicine (MTX, 2.7 mg/kg), Chinese medicine positive control group (TGP, 180 mg/kg) and AR-6 high dose (AR6-H, 32 mg/kg), the middle dose (AR6-M, 16 mg/kg) and low dose (AR6-L, 8 mg/kg). Each rat was injected intradermally with 1 mL of the emulsion at five sites (one at tail base and four other on the back) under anesthetic, as the primary immunization. On day 7, a booster injection of the same amount of emulsion, as the secondary immunization, was given at the same five sites. Five days after booster injection, induction of arthritis, as indicated by redness and swelling of the paws was observed. Therefore, on day 19, AR-6 (32, 16, 8 mg/kg) and TGP were respectively given to each treatment group by intragastric administration once daily for 14 days, while MTX was administered once a week for 14 days. For model group and the normal control rats were given an equal volume of distilled water.
Body weight of rats was measured every 7 days from day 19, when rats were treated. Paw swelling was measured every 3 days from day 19, by toe volume measurement instrument, which was purchased from Jinan Yiyan Technical Development Co., Ltd. The swollen ratio was calculated by dividing the original at day 0 from the paw swelling. Arthritis index (AI) was recorded every 3 days according to the following criteria: score 0, no sign of inflammation; score 1, swelling and/or redness of paw or one digit; score 2, swelling in two or more joints; score 3, gross swelling of the paw with more than two joints involved; score 4, severe arthritis of the entire paw and digits. Maximum score of a single paw was 4 and a single rat was 16. All assessments were carried out without knowledge of the treatments applied. Moreover, organ indexes were assayed as well.
Histological observation – hematoxylin and eosin staining
The hind paws were harvested from rats after the serum collection, and then fixed in 10% neutral buffered formalin for 3 days, decalcified in 10% ethylene diamine tetraacetate (EDTA) for 30 days, and then embedded in paraffin. Serial paraffin sections (4 µm) were stained with hematoxylin and eosin. Histological analysis was carried out on the basis of inflammatory cells, synovial hyperplasia, pannus formation, cartilage degradation and bone erosion.
Immunohistochemistry
For immunohistochemistry, deparaffinized, hydrated tissue sections were used. Rinse and block endogenous peroxidase with 0.3% hydrogen peroxide in PBS for 10 min and subsequently incubate tissue sections with Anti-TNF-α, Anti-PI3K or anti-phospho-Akt/PKB antibody overnight at 4°C. After washing, sections were incubated for 30 min with biotinylated secondary antibody anti-rabbit or anti-goat IgG and Vectastain. Thereafter, sections were stained with 3,3-diaminobenzidine and counterstained with hematoxylin, embedded in Permount. The expressions of TNF-α, PI3K and p-Akt were measured by mean optical density (OD) value.
Reverse transcription-polymerase chain reaction
After 14 days of medication, the rats were killed by cervical dislocation and synovial tissue of knee joints was extracted, immediately followed by frozen in liquid nitrogen. Samples were ground into powder using a grinder, and then total RNA of synovium was estimated as directed.
Total RNA (2 µg) was reverse-transcribed to complementary DNA (cDNA) using oligo-dT primers, and 5 µL of cDNA from the RT reaction was then used for polymerase chain reaction (PCR) amplification.
Primers were as follows:

PCR was then subjected to 94°C for 5 min predenaturation, followed by 30 cycles of 94°C for 30 s denaturation, 55°C for 30 s annealing and 72°C for 1 min extension. After the last cycle, a final extension at 72°C for 5 min was done. Then 10 µL of PCR product was added to 2% agarose gel electrophoresis. After 30 min running of gel electrophoresis, the gel was photographed and analyzed by JEDA, which is a gel imaging analysis system. The relative content of each sample was computed and compared with internal consulting. The mean OD value of the strap of amplified products was determined.
Statistical analysis
The experimental results were expressed as the mean ± S.D. for each group. Data were assessed by one-way ANOVA followed by post hoc test, and a value of p < 0.05 was considered to be statistically significant.
Results
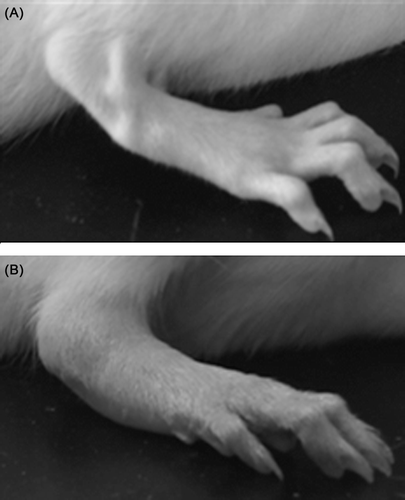
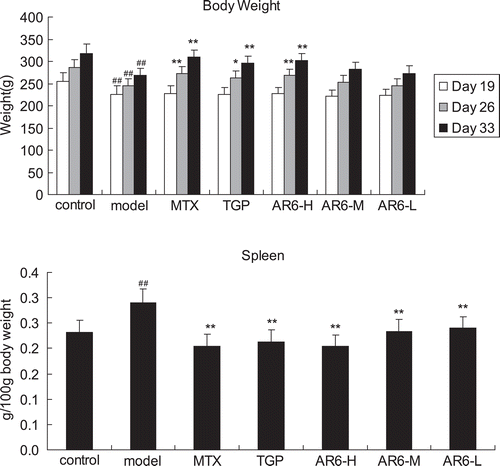
Functional impairment, nerve irritability and red swelling in the paws were observed, which indicates the building of CIA model was successful (). There were no particular behaviors, clinical or physiological signs observed during the process of drug administration in AR-6 all dose levels. Generally, weight gain in AR6-H-treated, MTX-treated and TGP-treated rats increased more than model animals (p < 0.01), while body weight of CIA rats treated with AR6-M and AR6-L, despite a trend towards efficacy, did not achieve a statistically significant difference from model animals (p > 0.05). Furthermore, organ index of spleen () in all treatment rats was significantly lower than model rats (p < 0.01).
Figure 3. The effect of AR-6 on body weight and organ index of spleen in CIA rats (n = 8, mean ± S.D.). ##p < 0.01 vs. control; *p < 0.05, **p < 0.01 vs. model group. AR-6 high dose (AR6-H), AR-6 middle dose (AR6-M), AR-6 low dose (AR6-L), methotrexate (MTX) and total glucosides of paeony (TGP).
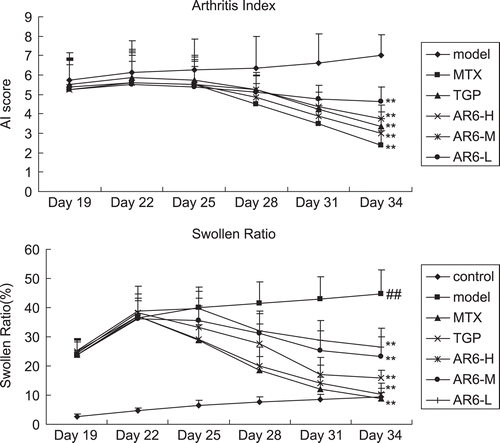
The progression of arthritis severity in the model group was shown by consecutive increases in swollen ratio. The ratios of treatment groups reached the peak at Day 22 following the first immunization (3 days following the drug administration), and AR-6 treated groups suppressed the exacerbation of collagen-induced chronic inflammatory paw swelling in a dose-dependent manner (). MTX-treated and TGP-treated groups also significantly reduced the paw swelling (p < 0.01). In addition, AI and swollen ratio changed synchronously. As is shown in , AI in treatment groups began to descend at day 25 following the first immunization (6 days following the drug administration), with a significant difference in comparison with model group (p < 0.01). AI then decreased continuously and thereafter kept at a low level.
Figure 4. AI and the effect of AR-6 on swollen ratio in CIA rats paw edema (n = 8, mean ± S.D.). ##p < 0.01 vs. control; **p < 0.01 vs. model group. AR-6 high dose (AR6-H), AR-6 middle dose (AR6-M), AR-6 low dose (AR6-L), methotrexate (MTX) and total glucosides of paeony (TGP).
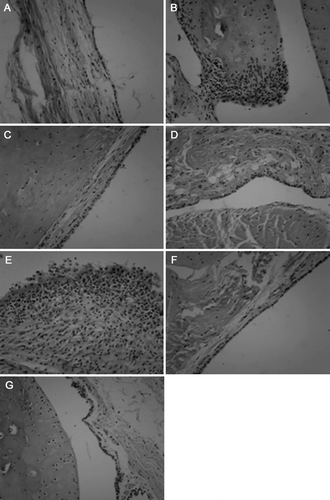
Representative histologic lesions in ankle joints were observed by histopathological examination as shown in . Infiltration of inflammatory cells, synovial hyperplasia, pannus formation, cartilage degradation and bone erosion were seen in hind ankle joints from model group CIA rats. MTX-treated, TGP-treated and AR6-H-treated groups significantly attenuated synovial hyperplasia, pannus formation and cartilage destruction. Meanwhile, infiltration of inflammatory cells was also reduced. By comparison, the effect of high dose level of AR-6-treated group was better than those of middle and low dose-treated groups.
Figure 5. The effect of AR-6 on histological changes in CIA rats. Representative histopathological lesions using hematoxylin and eosin stain of hind ankle joint are shown: (A) control group, (B) model group, (C) AR-6 high dose group, (D) AR-6 middle dose group, (E) AR-6 low dose group, (F) MTX group and (G) TGP group. Original magnifications, ×400.
Immunohistochemistry studies were then performed to localize TNF-α expression and investigate whether there was a reduction in the expression of molecules downstream of TNF-α, such as PI3K and p-Akt. In the model group, TNF-α, PI3K and p-Akt were apparently primarily detected in the synovial intimal lining on a large scale, although positive cells were also identified in the sublining region (). However, treatment groups with MTX, TGP and AR6-H significantly reduced the expression of TNF-α, PI3K and p-Akt in those areas (p < 0.01). In addition, expression in AR6-M and AR6-L-treated groups was relatively reduced in comparison with model group ().
Figure 6. Immunohistochemistry of p-Akt (A), PI3K (B) and TNF-α (C) in rat synovium. Representative serial sections from control, model and drugs treated rats are shown: (a) control group, (b) model group, (c) AR-6 high dose group, (d) AR-6 middle dose group, (e) AR-6 low dose group, (f) MTX group and (g) TGP group. Original magnifications, ×400.
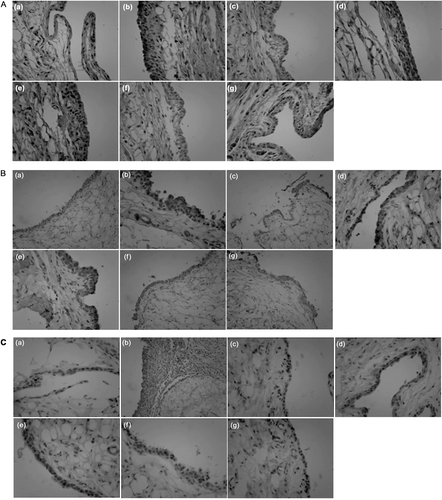
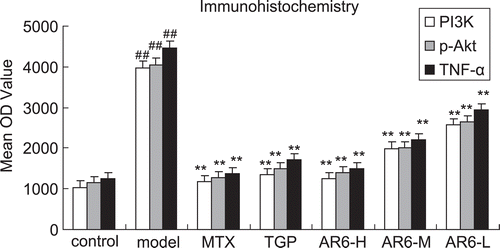
Figure 7. The effect of AR-6 on the expression of TNF-α, PI3K and p-Akt of CIA rats by immunohistochemistry (n = 8, mean ± S.D.). ##p < 0.01 vs. control; **p < 0.01 vs. model group. AR-6 high dose (AR6-H), AR-6 middle dose (AR6-M), AR-6 low dose (AR6-L), methotrexate (MTX) and total glucosides of paeony (TGP).
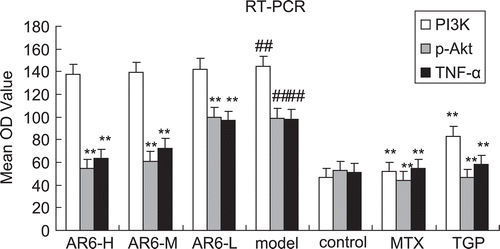
To elucidate the mechanism of the treatment, TNF-α, PI3K and p-Akt mRNA expression was determined by RT-PCR as shown in . Synovial TNF-α mRNA, PI3K mRNA and p-Akt mRNA expression in CIA rats was significantly elevated in the model group when compared with that of control. In AR-6-treated groups, marked decreases in these parameters were seen. Reduced mRNA level for the high dose of AR-6 was more impressive (). MTX and TGP also significantly diminished these values (p < 0.01).
Figure 8. Detection of TNF-α mRNA, PI3K mRNA, p-Akt mRNA and β-actin expression in CIA rats’ synovium. M: Marker, Lane 1: AR-6 high dose group, Lane 2: AR-6 middle dose group, Lane 3: AR-6 low dose group, Lane 4: model group, Lane 5: control group, Lane 6: MTX group, Lane 7: TGP group.
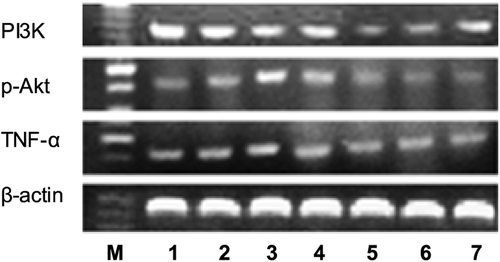
Figure 9. The effect of AR-6 on the expression of TNF-α mRNA, PI3K mRNA and p-Akt mRNA of CIA rats (n = 4, mean ± S.D.). ##p < 0.01 vs. control; **p < 0.01 vs. model group. AR-6 high dose (AR6-H), AR-6 middle dose (AR6-M), AR-6 low dose (AR6-L), methotrexate (MTX) and total glucosides of paeony (TGP).
Discussion
The current study examined the effects of AR-6 and its possible inflammatory and immunological mechanisms. The CIA model was developed in most rats in about 2 weeks following the primary immunization. Loss of weight, red swelling in the paws, paw edema, inflammatory cells infiltration, synovitis, cartilage and bone erosion, joint deformation were observed as hallmarks of CIA in model group rats. AI and swollen ratio began to decrease in about 5 days following the treatment, and kept at a low level after the peak of arthritis in all AR-6 treated groups. Meanwhile, histopathological observation also indicated that infiltration of inflammatory cells, synovial hyperplasia, cartilage and bone destruction, joint deformation were improved significantly, whereas, the model group rats experienced progressive cartilage degradation and bone erosion, thereby eventually causing serious anchylosis and joints deformation (–). As one of the most widely used models for the identification of mechanisms and genes involved in arthritis (CitationWilliams-Skipp et al., 2009), CIA shares a variety of pathological and immunological characteristics with RA patients, therefore, we can conclude that the anti-arthritic effect of AR-6 on CIA suggests its potential of clinical therapeutic interest and favorable biological activities.
TNF-α contributes to the RA pathogenesis as a pivotal cytokine (CitationPerera et al., 2011), its main source is macrophages of the synovium (CitationChoy & Panayi, 2001; CitationMatsuno et al., 2002). Several studies have shown that the levels of TNF-α correlated with arthritis severity, and that TNF-α neutralizing therapy using Anti-TNF-α monoclonal antibody and soluble TNF receptor improves clinical symptoms and inhibits (or even reverses) inflammatory bone destruction in RA (CitationLi et al., 2010). In synovial tissue of RA patients, a positive regulatory cycle is produced whereby NF-κB induces the transcription of pro-inflammatory cytokines such as TNF-α, and cell adhesion molecules (CitationCapria et al., 2010). Moreover, our previous study has demonstrated this upon treatment with AR-6 in CIA rats. According to our studies (–) AR-6 has functioned to inhibit TNF-α which also leads to ameliorate the symptoms and signs in RA, indicating that AR-6 may effectively involve in the regulation of the inflammatory process.
PI3K/Akt signaling pathway is essential for cytokine network triggering and amplifying (CitationGupta & Wagner, 2009). Akt is a serine/threonine protein kinase that prevents apoptosis and is activated by phosphorylation through upstream kinases in the PI3K cascade (CitationChang et al., 2003). It has been documented that PI3K/AKT pathway potentiated synovial overgrowth and joint destruction in RA and the translocation of NF-κB p65 into the nucleus was inhibited by a PI3K/AKT inhibitor, indicating that the activation of NF-κB in synovial cells was AKT-dependent (CitationHashiramoto et al., 2007). Akt, acting as a survival signal in the PI3K cascade, controls apoptosis via the modulation of downstream key signaling protein NF-κB by phosphorylating downstream proteins such as IKK and Iκ-B, which in turn release NF-κB from its complex (CitationFishman et al., 2002). Indeed, AR-6 treatment diminished the NF-κB protein expression levels (CitationPeng et al., 2011). In this study, downregulation of PI3K/AKT signaling pathway was observed including a decrease in the expression level of PI3K and p-Akt (–), suggesting that one of the mechanisms that can be attributed to this phenomenon is inhibition of apoptosis due to stimulation of the PI3K pathway, which leads to activation of Akt. This conclusion demonstrated that PI3K/Akt may be an upstream target for AR-6.
Since the mRNA and immunohistochemistry results essentially mirrored each other ( and ), the deregulation of PI3K/Akt–TNF-α signal transduction pathway takes place. One of the major mechanisms responsible for the development of arthritis is the upregulation of PI3K/Akt–NF-κB pathway that consequently contributes to the stimulatory effect of TNF-α by inhibiting apoptosis (CitationTong et al., 2011). Upon AR-6 treatment, the modulation of PI3K/Akt–NF-κB–TNF-α leads to amelioration of the inflammatory process. However, the current study is not enough to directly demonstrate the mechanism of AR-6, and further research on molecular mechanism of AR-6 is still required.
Conclusions
In summary, AR-6 exerts an immunomodulatory activity, which entails down-regulation of the PI3K/Akt–NF-κB pathway resulting in decreased levels of TNF-α, prevention of osteoclasts formation and expression manifested by marked improvement in the histology of the inflamed tissues (CitationOchaion et al., 2008). Overall, the deregulation of PI3K/Akt–TNF-α signaling pathway attributes to the anti-arthritic effect of AR-6. Taken together, the present study contributes to an understanding of modulating effects of AR-6 (32, 16 and 8 mg/kg) in clinical practice. These results supplement the evaluation of AR-6 in CIA rats and indicate that, especially the high dose level of AR-6 (32 mg/kg), showing the favorable effect on chronic inflammation of CIA, may be effectively applied to the therapeutic potential in patients with inactive RA. Compared to most current immunosuppressive agents, MTX (2.7 mg/kg) and TGP (180 mg/kg), satisfactory effect of this herbal drug displays its advantages to the future treatment for clinical rheumatoid arthritis.
Declaration of interest
This work was supported by the National Natural Science Foundation of China [fund number 30772770], High Technology Foundation Program of Jiangsu Province [fund number BG2007613] and Fund of Major Special for National New Drugs Creation [fund number 2009ZX09103-371].
References
- Arntz OJ, Geurts J, Veenbergen S, Bennink MB, van den Brand BT, Abdollahi-Roodsaz S, van den Berg WB, van de Loo FA. (2010). A crucial role for tumor necrosis factor receptor 1 in synovial lining cells and the reticuloendothelial system in mediating experimental arthritis. Arthritis Res Ther, 12, R61.
- Bingham CO 3rd. (2002). The pathogenesis of rheumatoid arthritis: Pivotal cytokines involved in bone degradation and inflammation. J Rheumatol Suppl, 65, 3–9.
- Capria A, De Nardo D, Baffetti FR, Barbini U, Violo A, Tondo T, Fontana L. (2010). Long-term anti-TNF-α treatments reverse the endothelial dysfunction in rheumatoid arthritis: The biological coherence between synovial and endothelial inflammation. Int J Immunopathol Pharmacol, 23, 255–262.
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. (2003). Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia, 17, 590–603.
- Choy EH, Panayi GS. (2001). Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med, 344, 907–916.
- Downward J. (2004). PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol, 15, 177–182.
- Fingar DC, Blenis J. (2004). Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene, 23, 3151–3171.
- Fishman P, Bar-Yehuda S, Madi L, Rath-Wolfson L, Ochaion A, Cohen S, Baharav E. (2006). The PI3K-NF-κB signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Res Ther, 8, R33.
- Fishman P, Madi L, Bar-Yehuda S, Barer F, Del Valle L, Khalili K. (2002). Evidence for involvement of Wnt signaling pathway in IB-MECA mediated suppression of melanoma cells. Oncogene, 21, 4060–4064.
- Goldring SR. (2003). Pathogenesis of bone and cartilage destruction in rheumatoid arthritis. Rheumatology (Oxford), 42, 11–16.
- Gupta V, Wagner BJ. (2009). Search for a functional glucocorticoid receptor in the mammalian lens. Exp Eye Res, 88, 248–256.
- Hashiramoto A, Sakai C, Yoshida K, Tsumiyama K, Miura Y, Shiozawa K, Nose M, Komai K, Shiozawa S. (2007). Angiopoietin 1 directly induces destruction of the rheumatoid joint by cooperative, but independent, signaling via ERK/MAPK and phosphatidylinositol 3-kinase/Akt. Arthritis Rheum, 56, 2170–2179.
- Herlaar E, Brown Z. (1999). p38 MAPK signalling cascades in inflammatory disease. Mol Med Today, 5, 439–447.
- Kim JE, Son JE, Jung SK, Kang NJ, Lee CY, Lee KW, Lee HJ. (2010). Cocoa polyphenols suppress TNF-α-induced vascular endothelial growth factor expression by inhibiting phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase kinase-1 (MEK1) activities in mouse epidermal cells. Br J Nutr, 104, 957–964.
- Li T, Zuo X, Zhou Y, Wang Y, Zhuang H, Zhang L, Zhang H, Xiao X. (2010). The vagus nerve and nicotinic receptors involve inhibition of HMGB1 release and early pro-inflammatory cytokines function in collagen-induced arthritis. J Clin Immunol, 30, 213–220.
- Ma X, Xie L, Liu L, Tang Q, Wan Z, Li Y. (2009). Simultaneous quantification of seven main triterpenoid saponins in Radix et Rhizoma Clematidis by LC–ELSD. Chromatographia, 69, 437-43.
- Matsuno H, Yudoh K, Katayama R, Nakazawa F, Uzuki M, Sawai T, Yonezawa T, Saeki Y, Panayi GS, Pitzalis C, Kimura T. (2002). The role of TNF-α in the pathogenesis of inflammation and joint destruction in rheumatoid arthritis (RA): A study using a human RA/SCID mouse chimera. Rheumatology (Oxford), 41, 329–337.
- Miossec P. (2004). An update on the cytokine network in rheumatoid arthritis. Curr Opin Rheumatol, 16, 218–222.
- Montalban AG, Boman E, Chang CD, Ceide SC, Dahl R, Dalesandro D, Delaet NG, Erb E, Ernst J, Gibbs A, Kahl J, Kessler L, Lundström J, Miller S, Nakanishi H, Roberts E, Saiah E, Sullivan R, Wang Z, Larson CJ. (2010). KR-003048, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. Eur J Pharmacol, 632, 93–102.
- O’Dell JR. (2004). Therapeutic strategies for rheumatoid arthritis. N Engl J Med, 350, 2591–2602.
- Ochaion A, Bar-Yehuda S, Cohen S, Amital H, Jacobson KA, Joshi BV, Gao ZG, Barer F, Patoka R, Del Valle L, Perez-Liz G, Fishman P. (2008). The A3 adenosine receptor agonist CF502 inhibits the PI3K, PKB/Akt and NF-κB signaling pathway in synoviocytes from rheumatoid arthritis patients and in adjuvant-induced arthritis rats. Biochem Pharmacol, 76, 482–494.
- Peng C, Perera PK, Li YM, Fang WR, Liu LF, Li FW. (2011). Anti-inflammatory effects of Clematis chinensis Osbeck extract(AR-6) may be associated with NF-κB, TNF-α, and COX-2 in collagen-induced arthritis in rat. Rheumatol Int. [Epub ahead of print].
- Perera PK, Peng C, Xue L, Li Y, Han C. (2011). Ex vivo and in vivo effect of Chinese herbal pill Yi Shen Juan Bi (YJB) on experimental arthritis. J Ethnopharmacol, 134, 171–175.
- Setty AR, Sigal LH. (2005). Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Semin Arthritis Rheum, 34, 773–784.
- Sun SX, Li YM, Fang WR, Cheng P, Liu L, Li F. (2010). Effect and mechanism of AR-6 in experimental rheumatoid arthritis. Clin Exp Med, 10, 113–121.
- Tong XM, Wang JC, Shen Y, Xie JJ, Zhang JY, Jin J. (2011). Inhibition of inflammatory mediators and related signaling pathways by macrophage-stimulating protein in rheumatoid arthritis synovial fibroblasts. Inflamm Res, 60, 823–829.
- Williams-Skipp C, Raman T, Valuck RJ, Watkins H, Palmer BE, Scheinman RI. (2009). Unmasking of a protective tumor necrosis factor receptor I-mediated signal in the collagen-induced arthritis model. Arthritis Rheum, 60, 408–418.
- Wu T, Mohan C. (2009). The AKT axis as a therapeutic target in autoimmune diseases. Endocr Metab Immune Disord Drug Targets, 9, 145–150.
- Zwerina J, Hayer S, Redlich K, Bobacz K, Kollias G, Smolen JS, Schett G. (2006). Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum, 54, 463–472.