Abstract
Context: Lawsone, lawsone methyl ether and 3,3′-methylelnebislawsone are the main active compounds of Impatiens balsamina L. (Balsaminaceae). These compounds possess various pharmacological activities that have been shown to assist with the treatment of skin diseases.
Objective: This work focused on increased naphthoquinone production in I. basamina root cultures using methionine feeding.
Materials and methods: I. balsamina root cultures were maintained in liquid Gamborg’s B5 medium supplemented with 0.1 mg/L α-naphthalene acetic acid, 0.1 mg/L kinetin, 1.0 mg/L 6-benzyladenine and 20 g/L sucrose. The effect of methionine concentration (50, 100, 300, 500 and 1000 mg/L) on naphthoquinone production of I. basamina root cultures was determined. Isolation of secondary metabolites from I. balsamina root cultures was also carried out.
Results and discussion: Feeding of 300 mg/L methionine to the root cultures at the beginning of the growth cycle increased the production of 3,3′-methylelnebislawsone almost two-fold (0.63 mg/g dry weight, compared to the control group 0.32 mg/g dry weight). Optimization of the feeding conditions showed that adding 500 mg/L methionine to a 21-day old root cultures increased production of lawsone methyl ether and 3,3′-methylenebislawsone up to 2.6- and 3.1-fold higher, respectively, compared to the controls. In addition, various pharmacologically interesting secondary metabolites were isolated from I. balsamina root cultures, such as a flavonoid, luteolin, a naphthoquinone, 2,3-dihydroxy-1,4-naphthoquinone, and a triterpenoid, echinocystic acid. This is the first report of the occurrence of these compounds in this plant.
Introduction
Precursor feeding has been an obvious and popular approach to increase secondary metabolite production in plant cell cultures. The concept is based on the idea that any compound which is an intermediate, in or at the beginning of a secondary metabolite biosynthetic route, stands a good chance of increasing the yield of the final product (CitationDiCosmo & Misawa, 1995). Attempts to induce or increase the production of plant secondary metabolites, by supplying precursor or intermediate compounds, have been effective in many cases (CitationRao & Ravishankar, 2002). For example, phenylalanine is a precursor of the N-benzoylphenylisoserine side chain of paclitaxel. Thus, supplementation of Taxus cuspidata Siebold & Zucc. (Taxaceae) cultures with phenylalanine resulted in increased yields of taxol (CitationFett-Neto et al., 1994).
Impatiens balsamina L. (Balsaminaceae) is a medicinal plant that has been traditionally used for the treatment of thorn or glass-puncture wounds, abscesses, ingrown nails, and chronic ulcers caused by allergic reactions to detergents (CitationFarnsworth & Bunyapraphatsara, 1992). The naphthoquinones, lawsone, lawsone methyl ether and 3,3′-methylenebislawsone () are the active constituents that possess various pharmacological activities, including antimicrobial (CitationTripathi et al., 1978; CitationYang et al., 2001), anti-anaphylaxis (CitationIshiguro et al., 1994), anti-allergic (CitationReanmongkol et al., 2003), antipruritic (CitationOku et al., 2002) and anti-inflammatory (CitationOku & Ishiguro, 2002) activities. I. balsamina root cultures have previously been established from leaf explants in our laboratory (Sakunphueak & Panichayupakaranant, Citation2010a). However, these root cultures produced very low levels of lawsone methyl ether, which is the most active antimicrobial constituent (CitationSakunphueak & Panichayupakaranant, 2012). Lawsone methyl ether is biosynthesized from lawsone by methylation at the hydroxyl group of lawsone. A study on the enzymic methylation of lawsone clarified that lawsone is methylated at the hydroxyl group by O-methyltransferase and uses S-adenosylmethionine (SAM) as a methyl group donor for the enzymatic reaction (CitationPanichayupakaranant & De-eknamkul, 1992). Feeding of methionine as a precursor of SAM might improve the metabolic flux to increase lawsone methyl ether. The aims of this study were therefore to use precursor feeding as a technique to increase naphthoquinone production in I. balsamina root cultures. In addition, a phytochemical study of the root cultures was carried out to make a chemical library of these plant cultures.
Materials and methods
Establishment of I. balsamina root cultures
I. balsamina root cultures were initiated from leaf explants of the I. balsamina, pink flower plant, in solid Gamborg’s B5 medium supplemented with 0.1 mg/L α-naphthalene acetic acid, 0.1 mg/L kinetin, 1.0 mg/L 6-benzyladenine, 20 g/L sucrose and 8 g/L agar. The root cultures were transferred into 250mL Erlenmeyer flasks containing 50 mL of liquid B5 medium supplemented with the same hormonal composition. The cultures were incubated on a rotary shaker (80 rpm), at 25 ± 2°C in with 16 h light/8 h dark cycle. Maintenance of the cultures was carried out by periodic subculture at three week intervals (Sakunphueak & Panichayupakaranant, Citation2010a).
Study on effect of methionine feeding on naphthoquinone production
The culture roots of I. balsamina (3-week old) were transferred to fresh B5 liquid medium with various concentrations of methionine added to the root cultures at the beginning of the growth cycle (day 0) to produce final concentrations of 50, 100, 300, 500 and 1000 mg/L, respectively. After 30 days of treatment, the cultured roots were harvested, extracted and extracts analyzed by HPLC analysis. The experiments were performed in triplicate.
High concentrations of methionine (300, 500 and 1000 mg/L) were added to 21-day old root cultures. After 9 days of treatment, the cultured roots were harvested, extracted and extracts analyzed by HPLC analysis. The culture media were also collected and extracted as described below prior to determining the naphthoquinone content by HPLC.
Extraction of naphthoquinones
The root cultures were dried at 50°C and ground to a fine powder by mortar and pestle. The root powder (200 mg) was extracted with 50% chloroform in methanol (20 mL) under reflux conditions for 1 h and then filtered. The filtrates were evaporated to dryness under reduced pressure (40°C). The residues were reconstituted in methanol and the volume adjusted to 10 mL (Sakunphueak & Panichayupakaranant, Citation2010c). The culture medium (50 mL) was acidified with 1N HCl to pH 2.0, and then partitioned with ethyl acetate (20 mL × 3). The pooled ethyl acetate fractions were then evaporated to dryness under reduced pressure (40°C). The residue was reconstituted in methanol and the volume adjusted to 10 mL (Sakunphueak & Panichayupakaranant, Citation2010c).
Quantitative HPLC analysis of naphthoquinones
Quantitative analysis of the naphthoquinone content using HPLC was carried out as previously reported (Sakunphueak & Panichayupakaranant, Citation2010c). HPLC analysis was carried out using an Agilent 1100 series equipped with a photodiode-array detector and autosampler. Separation was achieved at 25°C on a Supelco® C18 column (5 µm, 150 mm × 4.6 mm i.d.). The mobile phase consisted of 2% aqueous acetic acid (A) and methanol (B) with step gradient elution as follows: 0–10 min, A:B 75:25 % v/v; 10–20 min, A:B 68:32 % v/v; 20–35 min, A:B 45:55% v/v. The mobile phase flow rate was 1 mL/min. Sample injection volumes were 20 μL, and detection was by UV at a wavelength of 280 nm. The calibration curves of lawsone, lawsone methyl ether and 3,3′-methylenebis-lawsone were established using authentic standards (concentration range was 3.12 to 50 µg/mL). Lawsone, lawsone methyl ether and 3,3′-methylenebislawsone exhibited linearity over the evaluated ranges, with the linear equations of Y = 79.239X−60.38 (r2 = 0.9995), Y = 79.678X−54.18 (r2 = 0.9998) and Y = 73.712X−75.96 (r2 = 0.9998), respectively. Each calibration point was carried out in triplicate.
General experimental procedures for phytochemical studies
1H NMR (400 MHz) and 13C NMR (100MHz) spectra were recorded on a JEOL JNM-α 400 instrument with chemical shifts in δ (ppm) using tetramethylsilane (TMS) as an internal standard, and the coupling constants, J, are in Hertz. HPLC was carried out with a JASCO model PU-2080 pump and an 2075-UV variable-wavelength detector with reversed phase columns (TSK-GEL ODS, 5 μm, 2 × 25 cm, Tosoh Chemicals Co., Ltd at 9 mL/min with detection at 205 nm; Capcell Pak ODS, 5 μM, 2 × 25 cm, Shiseido Fine Chemicals Co. Ltd., at 9 mL/min with detection at 205 nm; TSKgel-ODS, 5 μm, 6 × 60 cm × 2, Tosoh Chemicals Co., Ltd., at 45 mL/min with detection at 205 nm; and Ascentis Phenyl, 5 μm, 2.2 × 25 cm, Supelco at 9 mL/min with detection at 205 nm).
Isolation of secondary metabolites from I. balsamina root cultures
Dried I. balsamina root cultures (150 g dry weight) were extracted with 50% chloroform in methanol (2 L × 3) under reflux conditions for 1 h. The extracts were combined and concentrated under reduced pressure to afford a crude extract (15.4 g). The crude extract was then partitioned between ethyl acetate and 10% NaOH solution. The pooled fractions of the ethyl acetate were evaporated to produce fraction E-1 (7.2 g). The aqueous phase was subsequently adjusted to pH 2.0 with conc. HCl, and again partitioned with ethyl acetate to produce fraction E-2 (6.0 g). Fraction E-1 was fractionated by a preparative HPLC [column: Tosoh, TSK gel ODS, 5 µm, 6 × 60 cm × 2; solvent: methanol-0.05% aqueous trifluoroacetic acid (TFA) (40:60→60:40); detector: UV 205 nm] to afford 15 fractions (I – XV). Fraction IX (12.6 mg) was then further separated using a semi-preparative HPLC [column: Tosoh TSK gel ODS 100V, 2 × 25 cm; solvent: methanol-0.05% TFA in water 35:65; detector: 205 nm] to afford a pure compound 1 (2 mg; tR 37 min). Fraction VI (50 mg) was further separated using a semi-preparative HPLC [column: Supelco, Ascentis Phenyl, 2.2 × 25 cm; solvent: acetronitrile-water 10:90; detector: 205 nm] to yield pure compounds 2 (5 mg; tR 144 min) and 3 (4 mg; tR 163 min). Fraction XV (838 mg) was rechromatographed on a silica gel column and eluted using a hexane-[chloroform:methanol (95:5)] step gradient solvent system (9:1, 7:3, 1:1, 1:4, 1 L each). Fractions were collected (50 mL for each fraction) and pooled to afford 7 fractions (A-G). Fraction A (120 mg) was further separated using a semi-preparative HPLC [column: Shiseido Capcell Pak ODS, 5 µm, 2 × 25 cm; solvent: acetronitrile-water 90:10; detector: 205 nm] to give a pure compound 4 (28 mg; tR 28 min). Fraction E (85 mg) was recrystallized using chloroform and methanol to obtain a pure compound 5 (13 mg).
Fraction E-2 (6 g) was fractionated using a Diaion® HP-20 column chromatography using 25, 50, 75, and 100% methanol and ethyl acetate (2 L each) as eluents, respectively, to yield five fractions (1–5). Fraction 1 (100 mg) was further separated using a semi-preparative HPLC [column: Supelco, Ascentis Phenyl, 2.2 × 25 cm; solvent: acetronitrile-0.05% TFA in water 10:90; detector: 205 nm] to give a pure compound 6 (7 mg; tR 26 min). Fraction 2 (500 mg) was further separated using a semi-preparative HPLC [column: Tosoh, TSK gel ODS 100V, 2 × 25 cm; detector: 205 nm] with various solvent systems to afford pure compounds as follows: a mixture of methanol-0.05% TFA in water (20:80) gave pure compounds 7 (8 mg; tR 36 min) and 8 (10 mg; tR 42 min); a mixture of acetronitrile-0.05% TFA in water (10:90) yielded a pure compound 9 (15 mg tR 37 min). Fraction 4 (500 mg) was further separated using a semi-preparative HPLC [column: Shiseido Capcell Pak ODS, 5 µm, 2 × 25 cm; solvent: acetronitrile-water 85:15; detector: 205 nm] to afford a pure compound 10 (19 mg; tR 49 min).
Statistical analysis
The data [mean ± standard error (SE)] obtained were statistically analysed by SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Data were submitted to analysis of variance, and mean values were then compared with one-way ANOVA, using Tukey’s test for multiple comparison. The term significant has been used to denote the differences for which p < 0.05.
Results and discussion
Effect of methionine feeding on naphthoquinone production
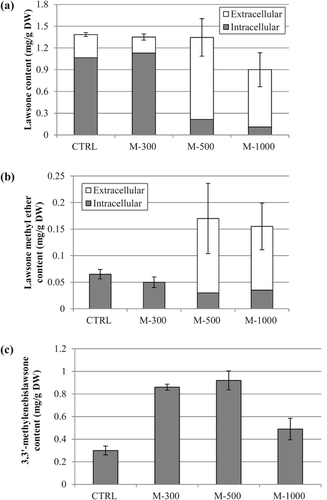
Addition of methionine to I. balsamina root cultures at the beginning of the growth cycle at the low concentrations of methionine (50, 100 and 300 mg/L) significantly decreased the growth of the root cultures, when compared to the untreated group. In addition, high concentrations of methionine (500 and 1000 mg/L) completely inhibited the growth and caused cell death. Only methionine at concentrations of 100 and 300 mg/L significantly increased 3,3′-methylenebislawsone production but did not affect lawsone and lawsone methyl ether production (). Methionine at 300 mg/L gave the highest level of 3,3′-methylenebislawsone production up to 0.63 mg/g dry weight, which was 1.9-fold higher compared to the level for the control. This implies that methionine plays an important role for 3,3′-methylenebislawsone production, and may act as a precursor of the methylene bridge in the structure of 3,3′-methylenebislawsone through SAM or as an elicitor.
Table 1. Naphthoquinone production in I. balsamina root cultures after adding methionine at various concentrations to the culture medium at day 0.
The result also indicated that methionine concentrations higher than 500 mg/L were toxic to I. balsamina root cultures during the lag phase of the growth cycle. Thus, the lag phase of the growth cycle may be not suitable for the methionine feeding experiment. Methionine feeding at a later linear phase of the growth cycle (day 21) was therefore examined. Methionine feeding at concentrations of 500 and 1000 mg/L increased lawsone and lawsone methyl ether secretion into the liquid media but not 3,3′-methylenebislawsone. This observation is in agreement with a previous report on the increased production of naphthoquinone by I. balsamina root cultures treated with 300 µM methyl jasmonate (Sakunphueak & Panichayupakaranant, Citation2010b). It is of interest that feeding methionine at 500 mg/L did increase production of both lawsone methyl ether and 3,3′-methylenebislawsone up to 0.17 and 0.92 mg/g dry weight, respectively which were 2.6- and 3.1-fold higher than the levels in the controls (). The appropriate age of root cultures for maximum production of lawsone methyl ether and 3,3′-methylenebislawsone by methionine feeding seems to be 21 days. This may be due to the biosynthesis of both compounds being more active at the late linear growth phase (Sakunphueak & Panichayupakaranant, Citation2010a). This result is in agreement with a previous report on the increased naphthoquinone production in I. balsamina root cultures treated with methyl jasmonate (CitationSakunphueak & Panichayupakaranant, 2012).
Figure 2. Intracellular and extracellular content of (a) lawsone, (b) lawsone methyl ether and (c) 3,3′-methylenebislawsone after feeding with various concentrations of methionine to 21-day old root cultures. Data are mean value ± SE of n = 5 (M-300, M-500 and M-1000 = methionine concentrations at 300, 500 and 1000 mg/L, respectively).
Isolation of secondary metabolites in I. balsamina root cultures
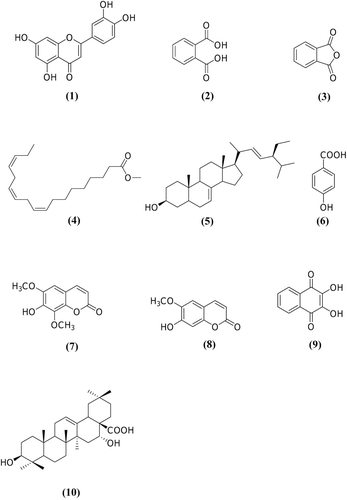
Dried cultured roots of I. balsamina were extracted in order to study the present of secondary metabolites. Ten known compounds were isolated and identified as luteolin (1) (CitationMiyazawa & Hisama, 2003), phthalic acid (2), phthalic anhydride (3), methyl linolenate (4) (CitationGunstone, 1990), spinasterol (5) (CitationJeon et al., 2005), p-hydroxybenzoic acid (6) (Dhakal et al., Citation2009), isofraxidin (7) (CitationPanichayupakaranant et al., 1995), scopoletin (8) (CitationKumar et al., 1994), 2,3-dihydroxy-1,4-naphthoquinone (9) (CitationKhandagale et al., 2005), and echinocystic acid (10) (CitationKhajuria et al., 2007) (), by comparison of their NMR data with the reported values in the literature. Isofraxidin and scopoletin are coumarins previously found in I. balsamina root cultures (CitationPanichayupakaranant et al., 1995). Small molecules such as phthalic acid, phthalic anhydride and p-hydroxybenzoic acid were also isolated. This is the first report on an isolation of these compounds from the plant in the genus Impatiens. These compounds might be involved in naphthoquinone biosynthesis via the shikimate pathway (CitationBentley & Meganathan, 1982). Although phthalic acid and phthalate anhydride have never been reported as part of the shikimate pathway, phthalic anhydride had been used as a precursor in a chemical synthesis of o-succinylbenzoate (CitationShaw et al., 1982). Thus, phthalic acid and phthalic anhydride might be new intermediates in the shikimate pathway. Shikonin, a naphthoquinone found in Lithospermum erythrorhizon Siebold & Zuccarini (Boraginaceae), is biosynthesized through the prenylation of p-hydroxybenzoic acid derived from l-phenylalanine with geranylpyrophosphate by p-hydroxybenzoic geranyltransferase (CitationTabata, 1996). The finding of p-hydroxybenzoic acid in the root cultures system indicated that other types of naphthoquinones that were derived from p-hydroxybenzoic acid might be found in I. balsamina root cultures.
Figure 3. Structures of (1) luteolin, (2) phthalic acid, (3) phthalic anhydride, (4) methyl linolenate, (5) spinasterol, (6) p-hydroxybenzoic acid, (7) isofraxidin, (8) scopoletin, (9) 2,3-dihydroxy-1,4-naphthoquinone and (10) echinocystic acid.
Luteolin has been reported to be an anti-mutagenic agent. It had suppressive effects on expression of the umu gene involved in the SOS response against many anti-mutagens. Luteolin also possesses good antioxidant activity (CitationPan et al., 2010). This compound has been isolated from the flowers of Impatiens textori Miq. and exhibited anti-allergic property in mice (CitationIwaoka et al., 2010). Normally, the flavonoids found in I. balsamina are kaempferol, quercetin and myriticin. This is the first report of luteolin in I. balsamina.
2,3-Dihydroxy-1,4-naphthoquinone was purified as a red crystalline compound. This compound has not been previously reported in I. balsamina but it has been obtained by chemical synthesis and is known to form a complex with lanthanide (CitationKhandagale et al., 2005). Thus, I. balsamina root cultures could be considered as a new source of this compound although it would be required to find a method to increase its yield. Echinocystic acid was found as a white amorphous powder. Echinocystic acid has been isolated from the rhizomes of Impatiens pritzellii var. hupehensis Hook. f. and exhibited inhibitory activities against interleukin-18 (CitationZhou et al., 2009). This compound also possesses many pharmacological activities, such as anti-HIV activity, anti-inflammatory activity and cytotoxicity against HL-60 cells through an ROS-independent mitochondrial dysfunction pathway (CitationTong et al., 2004).
Conclusions
Our findings suggest that feeding of methionine (500 mg/L) to a 21-day old root cultures increased production of lawsone methyl ether and 3,3′-methylenebislawsone up to 2.6- and 3.1-fold higher, respectively, compared to the controls. Moreover, addition of methionine at high concentrations to I. balsamina root cultures in the lag phase of the growth cycle is not recommended due to its toxicity. This is sthe first report of the occurrence of luteolin, 2,3-dihydroxy-1,4-naphthoquinone, and echinocystic acid in this plant.
Declaration of interest
The authors wish to thank Prince of Songkla University for a financial support in this research. The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bentley R, Meganathan R. (1982). Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev, 46, 241–280.
- Dhakal R, Rajbhandari M, Kalauni SK, Awale S, Gewali MB. (2009). Phytochemical constituents of the bark of Vitex negundo L. J Nep Chem Soc, 23, 89–92.
- DiCosmo F, Misawa M. (1995). Plant cell and tissue culture: alternatives for metabolite production. Biotechnol Adv, 13, 425–453.
- Farnsworth NR, Bunyapraphatsara N (1992). Thai medicinal plant, recommended for primary health care system Bangkok, Prachachon pp. 167–168.
- Fett-Neto AG, Melanson SJ, Nicholson SA, Pennington JJ, Dicosmo F. (1994). Improved taxol yield by aromatic carboxylic acid and amino acid feeding to cell cultures of Taxus cuspidata. Biotechnol Bioeng, 44, 967–971.
- Gunstone FD. (1990). The 13C-NMR spectra of oils containing [γ]-linolenic acid. Chem Phys Lipids, 56, 201–207.
- Ishiguro K, Fukumoto H, Osada S, Isoi K, Semma M. (1994). A practical, reproducible measure of murine anaphylaxis: Blood pressure monitoring of anaphylaxis and effect of Impatiens balsamina. Phytother Res, 8, 301–304.
- Iwaoka E, Oku H, Iinuma M, Ishiguro K. (2010). Allergy-preventive effects of the flowers of Impatiens textori. Biol Pharm Bull, 33, 714–716.
- Jeon GC, Park MS, Yoon DY, Shin CH, Sin HS, Um SJ. (2005). Antitumor activity of spinasterol isolated from Pueraria roots. Exp Mol Med, 37, 111–120.
- Khajuria A, Gupta A, Garai S, Wakhloo BP. (2007). Immunomodulatory effects of two sapogenins 1 and 2 isolated from Luffa cylindrica in Balb/C mice. Bioorg Med Chem Lett, 17, 1608–1612.
- Khandagale P, Chikate R, Joshi SB, Kulkarni BA. (2005). Binuclear lanthanide complexes of 2,3-dihydroxy-1,4-naphthoquinone. J Alloys Compd, 392, 112–119.
- Kumar V, Bulumulla HNK, Wimalasiri WR, Reisch J (1994). Coumarins and an indole alkaloid from Pamburus missionis. Phytochemistry, 36, 879–881.
- Miyazawa M, Hisama M. (2003). Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Biosci Biotechnol Biochem, 67, 2091–2099.
- Oku H, Ishiguro K. (2002). Cyclooxygenase-2 inhibitory 1,4-naphthoquinones from Impatiens balsamina L. Biol Pharm Bull, 25, 658–660.
- Oku H, Kato T, Ishiguro K. (2002). Antipruritic effects of 1,4-naphthoquinones and related compounds. Biol Pharm Bull, 25, 137–139.
- Pan Y, He C, Wang H, Ji X, Wang K, Liu P. (2010). Antioxidant activity of microwave-assisted extract of Buddleia officinalis and its major active component. Food Chem, 121, 497–502.
- Panichayupakaranant P, De-Eknamkul W. (1992). Enzymic methylation of lawsone to form 2-methoxy-1,4-naphthoquinone in Impatiens balsamina leaves. Thai J Pharm Sci, 16, 287–289.
- Panichayupakaranant P, Noguchi H, De-Eknamkul W, Sankawa U (1995). Naphthoquinones and coumarins from Impatiens balsamina root cultures. Phytochemistry, 40, 1141–1143.
- Rao SR, Ravishankar GA. (2002). Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol Adv, 20, 101–153.
- Reanmongkol W, Subhadhirasakul S, Panichayupakaranant P, Kim K-M. (2003). Anti-allergic and antioxidative activities of some compounds from Thai medicinal plants. Pharm Biol, 41, 592–598.
- Sakunphueak A, Panichayupakaranant P. (2010a). Effects of donor plants and plant growth regulators on naphthoquinone production in root cultures of Impatiens balsamina. Plant Cell Tissue Organ Cult, 102, 9–15.
- Sakunphueak A, Panichayupakaranant P. (2010b). Increased production of naphthoquinones in Impatiens balsamina root cultures by elicitation with methyl jasmonate. Bioresour Technol, 101, 8777–8783.
- Sakunphueak A, Panichayupakaranant P. (2010c). Simultaneous determination of three naphthoquinones in the leaves of Impatiens balsamina L. by reversed-phase high-performance liquid chromatography. Phytochem Anal, 21, 444–450.
- Sakunphueak A, Panichayupakaranant P. (2012). Comparison of antimicrobial activities of naphthoquinones from Impatiens balsamina. Nat Prod Res, 26, 1119–1124.
- Shaw DJ, Guest JR, Meganathan R, Bentley R. (1982). Characterization of Escherichia coli men mutants defective in conversion of o-succinylbenzoate to 1,4-dihydroxy-2-naphthoate. J Bacteriol, 152, 1132–1137.
- Tabata M. (1996). The mechanism of shikonin biosynthesis in Lithospermum cell cultures. Plant Tissue Cult Lett, 13, 117–125.
- Tong X, Lin S, Fujii M, Hou DX. (2004). Echinocystic acid induces apoptosis in HL-60 cells through mitochondria-mediated death pathway. Cancer Lett, 212, 21–32.
- Tripathi RD, Srivastava HS, Dixit SN. (1978). A fungitoxic principle from the leaves of Lawsonia inermis lam. Experientia, 34, 51–52.
- Yang X, Summerhurst DK, Koval SF, Ficker C, Smith ML, Bernards MA. (2001). Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fractionation. Phytother Res, 15, 676–680.
- Zhou XF, Tang L, Zhang P, Zhaod XY, Pi HF, Zhang YH, Ruan HL, Liu Y, Wu JZ. (2009). The interleukin-18 inhibitory activities of echinocystic acid and its saponins from Impatiens pritzellii var. hupehensis. Z Naturforsch, C, J Biosci, 64, 369–372.