Abstract
Context: Roots of Combretum mucronatum Schumach. & Thonn. (Combretaceae) and Capparis thonningii Schum. (Capparaceae) are used in southwest Nigeria in the treatment of inflammatory disorders and mental illness.
Objective: This study evaluated the antidementic effect of the methanol root extracts of C. mucronatum and C. thonningii on scopolamine (3 mg/kg, i.p.) induced memory impairment in mice.
Materials and methods: The effect of C. mucronatum and C. thonningii (50–200 mg/kg) administered orally for 3 days on memory impairments induced in mice by scopolamine was assessed in the passive avoidance and Morris water maze test and compared with that of tacrine (5 mg/kg, i.p.). The activities of acetylcholinesterase (AchE) and antioxidant enzymes were estimated in the brain after the completion of behavioral studies.
Results: C. mucronatum and C. thonningii root extracts (50–200 mg/kg) reversed scopolamine-induced memory deficit with significant (p < 0.05) increase in transfer latency in passive avoidance test. Similarly, the extracts (200 mg/kg) ameliorated memory deficit as a result of significant (p < 0.001) decrease in escape latency and path length in Morris water maze test. The increased AChE activity induced by scopolamine was significantly (p < 0.05) inhibited by C. mucronatum and C. thonningii (100 and 200 mg/kg) treatment which was similar to the effect of tacrine. Both extracts significantly (p < 0.05) attenuated scopolamine-induced increase in oxidative stress parameters as well as restoration of glutathione activity.
Discussion and conclusion: C. mucronatum and C. thonningii extracts possess significant anticholinesterase, antioxidant and antidementic properties, which may be useful in the management of Alzheimer’s disease.
Introduction
Alzheimer’s disease (AD) is the most common cause of progressive decline of cognitive function in aged humans. People over the age of 65 are most frequently affected by this disease (CitationFrancis et al., 1999). Dementia is a collective name for conditions in which progressive degeneration of the brain affects memory, thinking, behavior and emotion. The disease is characterized neuropathologically by the presence of numerous senile plaques and neurofibrillary tangles accompanied by neuronal loss (CitationYamada et al., 1999). The mechanism of AD is complex but increasing evidence demonstrates that cholinergic deficit is correlated closely with the severity of cognitive dysfunction and memory loss in AD patients (CitationBartus et al., 1982; CitationGiovannini et al., 1997; CitationTerry & Buccafusco, 2003).
Blockade of central muscarinic acetylcholine receptors disrupts learning and memory functions in rodents (CitationAgrawal et al., 2009), nonhuman primates (CitationTaffe et al., 1999) and humans (CitationRiedel et al., 1995). Scopolamine, a muscarinic receptor antagonist, interferes with memory in animals and humans, particularly the processes of learning acquisition and short-term memory (CitationSharma et al., 2010; CitationKulkarni et al., 2010).
It is well known that scopolamine-induced memory impairment is mainly due to blockade of cholinergic function in brain. However, few studies have also shown elevated oxidative stress in brain following scopolamine administration (CitationSharma et al., 2010; CitationPachauri et al., 2012)
Scopolamine significantly increases acetylcholinesterase (AChE) and malondialdehyde (MDA) levels in the cortex and hippocampus (CitationJones et al., 1991; CitationJeong et al., 2008). Therefore, elevation of acetylcholine (Ach) by inhibition of its metabolizing enzyme, AChE, represents the cornerstone of therapy for AD (CitationBartus, 2000).
In the present study, we found a significant elevation of malondialdehyde, a marker of lipid peroxidation in mice brain indicating oxidative stress. Glutathione (endogenous antioxidant) and nitrite, an indicator of NO generation, in mice brain following scopolamine treatment (CitationTota et al., 2012).
In addition, recent studies have pointed out that AD is associated with inflammatory processes. Reactive oxidative species (ROS) are able to damage cellular constituents and act as secondary messenger in inflammation. The use of antioxidants may be useful in the treatment of AD (CitationGilgun-Sherki et al., 2002). An effective antioxidant treatment regimen could potentially buffer the effects of in vivo ROS, such that cellular damage remains minimal. Randomized trials have shown reduced risk of AD in users of antioxidant vitamin supplements (CitationZandi et al., 2004). Additionally, epidemiological study demonstrated that vitamin E and ascorbic acid (vitamin C) were associated with reduced prevalence and incidence of AD (CitationZandi et al., 2004).
Before the development of allopathic medicines, people relied on a large arsenal of natural remedies for the treatment of central nervous system (CNS) related maladies.
Combretum mucronatum Schumach & Thonn. (Combretaceae) (OGA) is a scandent shrub of secondary forest, occurring from the Gambia to southwest Nigeria and on to Zaire. The roots, cut small, are boiled with capsicum peppers (Solanaceae) or wood ash and the concoction is drunk for chest pains, gonorrhea and nervous disorders (CitationBurkhill, 1985).
Also, Capparis thonningii Schum (Capparaceae) (AWO) is a climbing or scrambling pricky shrubs of the savannah woodland indigenous to west Africa. In folk medicine, the root is used to reduce swelling, to cure cough, enhance memory in children and blood spitting in Ghana (CitationBurkhill, 1985). The root is used in Senegal to treat vaginal discharge and syphilis (CitationAjaiyeoba & Sama, 2006).
C. mucronatum and C. thonningii have wide margin of safety as there were no death recorded up to 4000 mg/kg following oral administration in acute toxicity study. Preliminary study in our laboratory has shown that C. mucronatum and C. thonningii are rich in polyphenol (using quercetin as standard). Quercetin and rutin are natural compounds widely found in the diet, have been studied for a long time and shown to have wide physiological effects (CitationSingh et al., 2008), including antidementic effects in zebrafish (CitationRichetti et al., 2011). The effects of a flavonoid-rich diet on cognitive function have been linked to the ability of flavonoids to interact with the cellular and molecular framework involved in learning and memory, including synaptic potentiation and plasticity (CitationHarvsteen, 2002). Flavonoids also have known antioxidant abilities, effectively protecting neurons against neurotoxins, suppressing neuroinflammation, and enhancing neuronal function (CitationVafeiadou et al., 2007; CitationWard et al., 2002), stimulating neuronal regeneration and revascularization (CitationHarvsteen, 2002; CitationSingh et al., 2008). Flavonoids have also been reported to act as an AChE inhibitor (CitationAhmed & Gilani, 2009).
However, the effects of C. mucronatum and C. thonningii on memory function and central cholinergic system have not been explored so far. Therefore, the present study assessed the effects of C. mucronatum and C. thonningii against memory impairments induced in mice by single intraperitoneal injection of scopolamine in the Morris water maze and passive avoidances. Their effects were compared with those of tacrine in relation to the degree of cholinesterase inhibition in the whole brain.
This study also showed the effect of C. mucronatum and C. thonningii on biochemical markers of oxidative stress, nitrite, MDA and glutathione (GSH) in the brains of scopolamine-induced dementic mice.
Materials and methods
Plants material
Plant materials were collected in March, 2009 based on traditional healers’ information. Fresh roots of C. mucronatum and C. thonningii were collected from Obantoko, Abeokuta, Ogun state, Nigeria.
They were identified and authenticated by Mr. T.K. Odewo (formerly Senior Superintendent, Forestry Research Institute of Nigeria (FRIN), now in the Department of Botany and Microbiology, University of Lagos, Nigeria. The herbarium specimen vouchers were also deposited in the same University department. The herbarium voucher specimen numbers are LUH 2889 and 2881 for C. mucronatum and C. thonningii, respectively.
Extraction
Air-dried roots were ground with a Hammer mill into a fine powder. About 2000 g each of the different ground roots were successively extracted in 9600 mL redistilled methanol by maceration at room temperature (28°C) five times within 15 days. Filtrates obtained were pooled together and concentrated under Buchi Rotavapor at 40°C. They were further dried with vacuum pump to remove moisture.
Laboratory animal
Eight-week-old Swiss albino mice (20–30 g, 55 mice) were obtained from the Laboratory Animal Services Division of Central Drug Research Institute, Lucknow, India. The animals were kept in polyacrylic cages [38 × 23 × 10 cm as in Das et al. (2002)] with five mice per cage and maintained under standard housing conditions (room temperature 24–27°C and humidity 60–65%) with a 12 h light and dark cycle. Food, in the form of dry pellets, and water were available ad libitum but food was not allowed from 2 h prior to till completion of the experiment. Experiments were performed according to international ethical standards and approved by the research ethics committee of Central Drug Research Institute [Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)]. Adequate measures were taken to minimize pain or discomfort with animal experimental procedures.
Healthy mice were screened on the basis of the swimming ability and normal behavior. Effects of the extracts on muscle coordination were evaluated using rotarod test in mice “before and after administration of the drug”. Mice, showing the normal fall time of 2 min from rotating rod at 20 rpm speed were selected for memory evaluation. Animal showing abnormal muscle coordination before or after drug administration were excluded from the study.
Chemicals
Tacrine, scopolamine, sodium hydroxide, Triton X-100, acetylthiocholine iodide, sodium chloride (NaCl), sodium nitrate (NaNO2), sulphanilamide, napthaylamine diamine dihydrochloric, bovine serum albumin (BSA), 5,5′-dithiobis(2-nitro-benzoic acid) (DTNB), Folin-Ciocalteu’s, hydrochloric acid, trichloroacetic acid and 2-thiobabituric acid (TBA) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Drug treatment schedule during Morris water test and passive avoidance
Mice (Group I-control) were administered normal saline (10 mL/kg/bw, p.o.) 1 h prior to training session on day 1 only.
Group II (scopolamine 3 mg/kg, i.p.) 5 min before the commencement of day 1 trial Group III–VIII were pretreated with (OGA: 50–200 mg/kg/bw; AWO: 50–200 mg/kg/bw, p.o.), respectively, for 3 days and 1 h before intraperitoneal injection of scopolamine (3 mg/kg/bw) 5 min before the first trial on day 1 to assess antiamnesic effect of the extract on scopolamine-induced amnesia. Group IX mice were pretreated with tacrine (5 mg/kg/bw, i.p.) 1 h before intraperitoneal injection of scopolamine (3 mg/kg), 5 min before the first trial as standard anti-amnesic agent.
For oral and intraperitoneal administration, drugs were given in a volume of 0.1 mL/10 g. Animals were randomly divided into groups of 6–8 mice each.
Tests employed for learning and memory functions
Morris water maze
Morris water maze (CitationMorris, 1984) was used to assess learning and memory in experimental mice. The apparatus for Morris water maze including a video tracking device were purchased from Columbus instruments (Ohio, USA). We followed the methodology of Morris water maze described by CitationTota et al. (2009). Briefly, the acquisition and retention of a spatial navigation task was examined using a Morris water maze. It consists of a circular water tank (120 cm diameter and 50 cm height) located in a darkened test room, filled with opaque water (26 ± 2°C) to a depth of 30 cm. Four equally spaced points around the edge of the pool were designed as N (North), E (East), S (South) and W (West). A black colored round platform of 8 cm diameter was placed 1 cm below the surface of water in a constant position in the middle of the NE quadrant in the pool; the time taken for the mouse to escape from the water onto the platform was measured.
The water was colored with non-toxic black dye to hide the location of the submerged platform. The position of platform was kept unaltered throughout the trials. The animal was released into the pool from the SW quadrant in all the trials. The mice were given a maximum time of 60 s (cut-off time) to find the hidden platform and were allowed to stay on it for 30 s. The time taken for the mouse to find the escape platform was measured by the video tracking system.
In the event, the animal was unable to locate the hidden platform within 60 s, it was gently guided to it. Each animal was subjected to a daily session of three trials per day for five consecutive days. Escape latency time (ELT) to locate the hidden platform in water maze was noted as an index of learning.
Mean Escape latency time of all three trials are shown in the results. A significant decrease in latency time from that of the first session was considered as a successful learning.
Passive avoidance test
A step-through type of passive avoidance task was used. The apparatus for the step through passive avoidance test consists of a rectangular box divided into two compartments; A and B (Columbus Instrument, Ohio, USA) with electrifiable grid floor. A is dark (base side; 50 × 50 cm, height 35 cm) and B is illuminated (base side; 50 × 50 cm, height 35 cm) compartment.
These two compartments were divided by a wall that has a guillotine door (2.5 cm in diameter) connecting them. A lamp (20 W, positioned above the apparatus) is used to illuminate the side of the light compartment.
This test uses normal behavior of mice, the animals avoid bright light and prefer dim illumination when placed into brightly illuminated space connected to a dark enclosure, they rapidly enter the dark compartment and remain there.
In this study, mice were subjected to the passive avoidance test as described by CitationTota et al. (2009). Briefly, on the 1st day, in the acquisition trial, the mice were gently placed into the illuminated compartment (light intensity of 8) [scale from 0 to 10 (brightest)] in a computerized shuttle box provided with a software program PACS 30 (Columbus Instruments, Ohio, USA)], facing away from the dark compartment. After an acclimatization period of 30 s, the guillotine door opened and the mouse was allowed to step with all four paws into the dark compartment, after which the door was immediately closed automatically.
The animal received a low-intensity foot shock (0.5 mA; 10 s) through the grid floor of the dark compartment. Infrared sensors monitored the transfer of the animal from one compartment to another, which was recorded as transfer latency time (TLT) in seconds. The latency to step-through was recorded, and the mice were removed from the dark compartment after the electric shock was delivered and returned to its home cage. Only those mice that entered the dark compartment within 90 s in the acquisition trial were used in subsequent experiments. Retention test was performed 24 h after the acquisition trial. The mice were again placed in the illuminated compartment and allowed to step into the dark compartment but no shock was delivered; the latency of step-through was recorded. The maximum cut-off time for the step-through latency was 270 s.
The criterion for learning was taken as an increase in the TLT on retention (2nd) trials as compared to acquisition (1st) trial.
Rotarod test for muscle coordination
Mice were subjected to the rotarod test to evaluate the possible non-specific sedative or muscle incoordination effects of extract or compounds. The rotarod apparatus (Rotamex, Columbus, OH, USA) consisted of a bar with a diameter of 3 cm, and subdivided into four compartments. The animals were trained on the rotarod at a fixed speed of 20 rpm until they could remain on the apparatus for 120 s without falling 24 h before the experiments. On the day of the experiments, 30 min after the treatment with extract (200 mg/kg, p.o.) or vehicle (10 mL/kg, p.o.), the animals were placed on the rotarod apparatus (20 rpm). The latency of falling was measured for 120 s at 5, 10 and 15 min. The average time for the mice to stay on the rotarod in each group was expressed as result. (Animals that either fall often or always jump out of the rod were excluded.)
Spontaneous locomotor activity
After a period of 15 min for acclimatization in the Optovarimex activity meter (Columbus, OH, USA), infrared sensors monitored the locomotor activity of each animal for 2 min at 15 min interval for 1 h. Results were expressed as mean counts/2 min.
Estimation of biochemical parameters
Acetylcholinesterase and biochemical parameters of oxidative stress, MDA, GSH and nitrite were measured in the brain on the 5th day after scopolamine injection in Morris water maze.
Brain tissue preparation
The mice were decapitated under ether anaesthesia. The skull was cut open and the brain was exposed from its dorsal side. The whole brain was quickly removed and cleaned with chilled normal saline on ice. A 10% (w/v) homogenate of brain sample (0.03 M sodium phosphate buffer, pH: 7.4) was prepared by using an Ultra-Turrax T25 (USA) homogenizer at a speed of 9500 rpm. The homogenized tissue preparation was used to measure AChE, MDA and GSH.
Acetylcholinesterase assay in brain
The brain homogenate in volume of 500 μL was mixed with 1% Triton X-100 (1% w/v in 0.03 M sodium phosphate buffer, pH: 7). Both Triton X-100-treated and non-Triton X-100-treated brain homogenate samples were centrifuged at 100,000g at 4°C in a Beckman Ultracentrifuge (LE 80, Sanford, ME, USA), using a fixed angle rotor (80 ti) for 60 min. Supernatant of Triton X-100-treated samples was collected and stored at 4°C for AChE estimation by the method of CitationEllman et al. (1961). The kinetic profile of enzyme activity was measured spectrophotometrically (Shimadzu, Alexandria, VA, USA) at 412 nm with an interval of 15 s. One unit of AChE activity was defined as the number of micromoles (μmol) of acetylthiocholine iodide hydrolyzed per minute (min) per milligram (mg) of protein. The specific activity of AChE is expressed in micromoles/min/mg of protein.
Measurement of MDA
MDA, which is a measure of lipid peroxidation, was measured spectrophotometrically by the method of CitationColado et al. (1997) using 1,1,3,3-tetraethoxypropane as standard. MDA is expressed as n moles per mg protein. To 500 μL of tissue homogenate in phosphate buffer (pH: 7.4), 300 μL of 30% trichloroacetic acid (TCA), 150 μL of 5 N HCl and 300 μL of 2% w/v 2-thiobarbituric acid (TBA) were added and then the mixture was heated for 15 min at 90°C. The mixture was centrifuged at 12,000g for 10 min. Pink colored supernatant was obtained, which was measured spectrophotometrically at 532 nm.
Measurement of GSH
GSH (μg/mg protein) was determined by its reaction with 5,5′-dithiobis (2-nitrobenzoic acid) to yield a yellow chromophore which was measured spectrophotometrically (CitationEllman, 1959). The brain homogenate was mixed with an equal amount of 10% TCA and centrifuged (Remi cold centrifuge) at 2000g for 10 min at 4°C. The supernatant was used for GSH estimation. To 0.1 mL of processed tissue sample, 2 mL of phosphate buffer (pH: 8.4), 0.5 ml of 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) and 0.4 mL of double-distilled water was added and the mixture was shaken vigorously on vortex. The absorbance was read at 412 nm within 15 min.
Protein estimation
Protein was measured in all brain samples for GSH and MDA activity by the method of CitationLowry et al. (1951) and for AChE activity by the method of CitationWang and Smith (1975). BSA (1 mg/mL) was used as standard and measured in the range of 0.01–0.1 mg/mL.
Nitrite estimation
Nitrite was estimated in mouse brain using the Greiss reagent and served as an indicator of nitric oxide production. Greiss reagent (100 μL) (1:1 solution of 1% sulphanilamide in 5% phosphoric acid and 0.1% napthaylamine diamine dihydrochloric acid in water) was added to 100 μL of post-mitochondrial supernatant and absorbance was measured at 542 nm (CitationGreen et al., 1982). Nitrite concentration was calculated using a standard curve for sodium nitrite. Nitrite levels were expressed as percentage of control.
Statistical analysis
The results are expressed as mean ± standard error (SE) of means. Significance levels (p < 0.01, 0.001 or 0.05) in passive avoidance, Morris water maze and biochemical values were determined by one-way analysis of variance (ANOVA) followed by Tukey post hoc test with the aid of Graphpad version 5 software.
Results
The effect of C. mucronatum and C. thonningii on scopolamine-induced memory impairment in mice (Passive avoidance)
Control mice showed clear retention as indicated by the significant increase (p > 0.05) in TLT of retention trial in comparison to acquisition trial while administration of scopolamine (i.p.) 5 min before acquisition trial caused no significant change in TLT of retention trial when compared with the acquisition trial (). However, C. mucronatum (50, 100, and 200 mg/kg, p.o.) had no significant effect (p > 0.05) on TLT in acquisition trial but caused a marked and significant increase (p < 0.05) in TLT in the retention test in a dose-dependent manner, with peak effect observed at 100 mg/kg () suggesting antagonism of scopolamine-induced amnesia.
Figure 1. Effect of the methanolic root extract of C. mucronatum and C. thonningii on scopolamine-induced amnesia in mice. Transfer latency time (TLT) of control, Scopolamine (i.p.), Tacrine (i.p.), OGA and AWO (p.o.) in passive avoidance task on the 1st trial (acquisition) and 2nd retention trials. Data values are expressed as mean TLT (s) ± SEM. *Significant increase (*p < 0.05, **p < 0.01 and ***p < 0.001) versus acquisition trial.
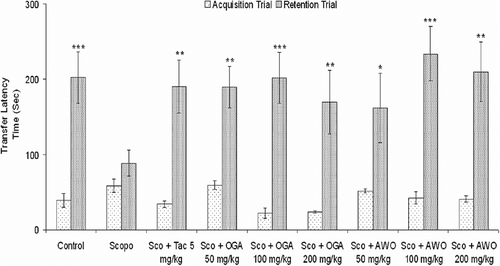
Similarly, 3 days oral administration of C. thonningii (50–200 mg/kg, p.o.) before intraperitoneal injection of scopolamine (3 mg/kg, i.p.) had no significant effect on TLT in acquisition trial but caused a significant increase in TLT in the retention test in a dose-dependent manner which was comparable to the effect of tacrine (5 mg/kg). There was no significant difference (p > 0.05) in TLT in the first trial (acquisition) among the different groups ().
Effects of C. mucronatum and C. thonningii on scopolamine-induced memory impairment in Morris water maze
Saline-treated mice quickly acquired the spatial task, as indicated by a gradual, session dependent decrease in escape latency and path length. The escape latency times and path length in the 3rd, 4th, and 5th retention sessions were significantly lower than in the 1st session [F(4,29) = 46.79, p < 0.001]. This indicates normal acquisition and retention in control untreated mice ().
Figure 2. Effect of the methanolic root extract of C. mucronatum and C. thonningii on scopolamine-induced amnesia in Morris water maze test. Comparison of latency time to reach hidden platform. Data values are expressed as mean escape latency time (ELT) (s) ± SEM. *Significant decrease (*p < 0.05, **p < 0.01 and ***p < 0.001) versus session 1.
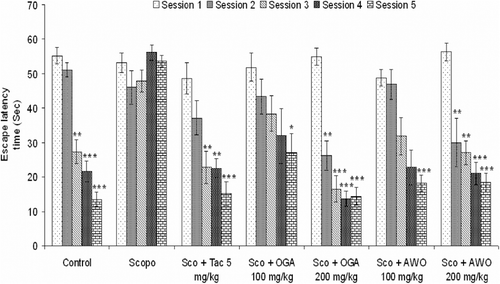
When normal saline was administered orally followed by intraperitoneal injection of scopolamine 3 mg/kg/bw. There was no significant change in latency to reach the platform in scopolamine (3 mg/kg, i.p.) treated mice in retention sessions (2nd–5th) in comparison to the 1st session [F(4, 29) = 1.86, p > 0.05] which is a sign of memory deficit in mice. Intraperitoneal administration of tacrine (5 mg/kg, i.p.) followed by scopolamine (3 mg/kg, i.p.) 5 min before the spatial learning session on day 1 produced a significant decrease in escape latency time when compared to the 1st session. Tacrine significantly reduced the mean escape latency time from the third session onward [F(4, 29) = 10.59, p < 0.05] (). C. thonningii (100 mg/kg) significantly decreased mean escape latency time from the 4th–5th session [F(4, 29) = 11.22, p < 0.05, p < 0.01] when compared to the 1st session, while 200 mg/kg. C. thonningii reduced the mean escape latency time significantly from the 2nd session onward [F(4, 29) = 13.44, p < 0.05, p < 0.01, p < 0.001] ().
Investigation of the path length revealed that scopolamine-treated animals had a significantly longer (p < 0.05) path length as compared to control group (). The C. thonningii treated group had a significantly (p < 0.05) reduced path length compared to the scopolamine injected mice. Oral administration of C. mucronatum ameliorated scopolamine-induced memory deficit in mice. C. mucronatum 100 mg/kg significantly decreased mean escape latency time at the 5th session [F(4, 29) = 2.93, p < 0.05] when compared to the 1st session, while 200 mg/kg C. mucronatum reduced the mean escape latency time significantly from the 2nd session onward [F(4, 29) = 30.16, p < 0.01, p < 0.001] ().
Figure 3. (A) Effect of the methanolic root extract of C. mucronatum and C. thonningii on scopolamine-induced amnesia in the Morris water maze test (Comparison of path length). Data values are expressed as mean path length (cm) ± SEM. *Significant decrease (*p < 0.05, **p < 0.01 and ***p < 0.001) versus session 1. (B) Correlation between mean latency time and path length.
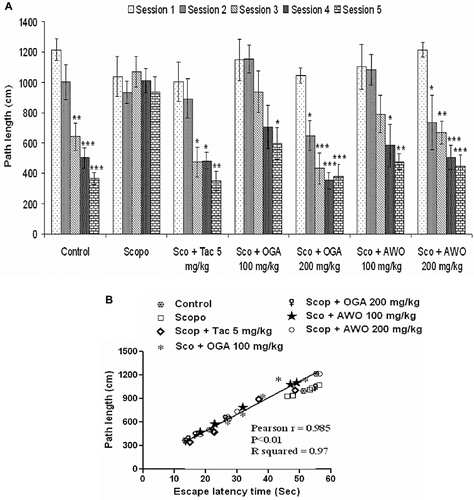
Investigation of the path length revealed that scopolamine-treated animals had a significantly longer (p < 0.05) path length as compared to the control group (). The C. mucronatum treated group had a significantly (p < 0.05) reduced path length compared with the scopolamine injected mice. Further, statistical analysis showed a significant correlation (Pearson r = 0.985; R2 = 0.97 and p < 0.01) between mean latency time and mean path length of all the groups in all sessions ().
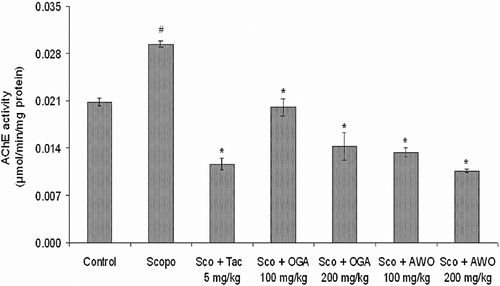
Acetylcholinesterase activity
To investigate the underlying mechanism of C. mucronatum and C. thonningii in attenuating memory impairment induced by scopolamine, activities of AChE enzymes were determined following the water maze test.
AChE activity (micromole/min/mg protein) was estimated on the day 5 after the first dose of scopolamine. The scopolamine-treated group had the highest AChE activity (p < 0.05) () when compared to the control group. AChE-specific activity was significantly (p < 0.05) reduced in 100 and 200 mg/kg C. mucronatum-treated groups (0.0132 ± 0.0006 and 0.0058 ± 0.0015 U/min/mg protein), respectively, when compared to the scopolamine-treated (0.0294 ± 0.0004 U/min/mg protein) group. Similarly, C. thonningii (100 and 200 mg/kg, p.o) significantly reduced AChE-specific activity (0.00824 ± 0.00073 and 0.00788 ± 0.00076 U/min/mg protein) (p < 0.05), respectively, when compared to scopolamine treated only group ().
Effect of C. mucronatum and C. thonningii on oxidative and nitrosative stress in scopolamine-treated mice
In order to further elucidate the mechanism of anti-amnesic activities of C. mucronatum and C. thonningii; lipid peroxidation, nitric oxide production and the activities of antioxidant enzyme in the brain were measured following the Morris water maze test.
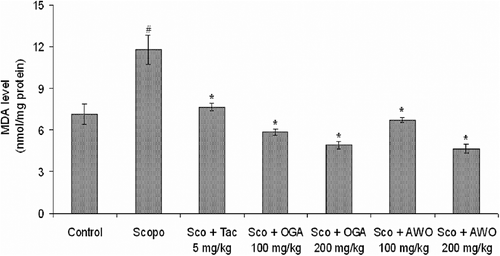
Malondialdehyde (MDA) level
The level of MDA was significantly (p < 0.05) higher in scopolamine (i.p.) treated mice as compared to control (7.13 ± 0.71 nmol/mg protein). However, tacrine treatment significantly (p < 0.05) attenuated the scopolamine-induced increase in MDA level (7.633 ± 0.26 nmol/mg protein). There were significant reductions in the level of MDA in C. thonningii (100 and 200 mg/kg) treated group (6.73 ± 0.17 and 4.66 ± 0.31 nmol/mg protein), respectively (). Similarly, C. mucronatum (100 and 200 mg/kg, p.o.) ameliorated scopolamine-induced lipid peroxidation as shown by a significant (p < 0.05) decrease in MDA level (5.86 ± 0.21 and 4.90 ± 0.26 nmol/mg protein), respectively, in mouse brain.
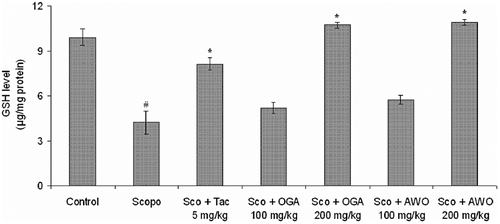
Glutathione (GSH) level
GSH (μg/mg protein) was estimated on day 5 after the first dose of scopolamine. A significant decrease in the levels of GSH was observed in the scopolamine (alone) treated group when compared to control group. Tacrine significantly (p < 0.05) () reversed the scopolamine-induced reduction in GSH level. Similarly, oral administration of C. mucronatum and C. thonningii produced significant (p < 0.05) () increase in GSH level in comparison to the scopolamine-treated group, indicating amelioration of scopolamine-induced oxidative stress.
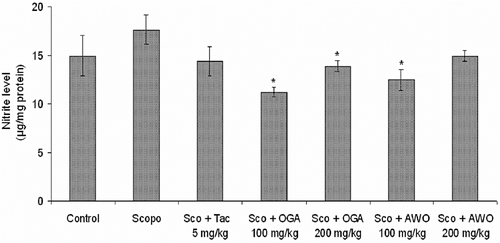
Effect of extract on scopolamine-induced nitrosative stress
As shown in , there was no significant (p > 0.05) difference in the level of nitric oxide production between the scopolamine-treated group and control as well as the tacrine treated group. However, C. mucronatum and C. thonningii produced a significant (p < 0.05) () attenuation of the scopolamine-induced increase in nitrite level in mouse brain.
Discussion
In this study, we evaluated the cognitive-enhancing effect of C. mucronatum and C. thonningii in preventing and treating learning and memory deficits such as those seen in AD. We examined the antidementic effect of C. mucronatum and C. thonningii using the passive avoidance and Morris water maze tests. C. mucronatum and C. thonningii reversed the memory deficits induced by scopolamine and inhibited AChE activities ex vivo, suggesting that C. mucronatum and C. thonningii affect cholinergic signalling to influence cognitive dysfunction.
Scopolamine interferes with memory and cognitive function in humans (CitationRiedel et al., 1995) and experimental animals (CitationSharma et al., 2010; CitationKulkarni et al., 2010) by blocking muscarinic receptors. Acute and systemic administration of scopolamine in animals provides the appropriate memory deficits related with the cholinergic deficit in AD or senile CNS dysfunction. The scopolamine-induced amnesic model has been widely used to provide a pharmacological model of memory dysfunction for screening potential cognition enhancing agents (CitationEbert & Kirch, 1998).
The step-through avoidance test is a passive avoidance response which can be used to evaluate the learning and memory capabilities as well as maturation of the inhibitory process (CitationHermans et al., 1992). In the present study, scopolamine significantly shortened the TLT in the retention trial, demonstrating that the central cholinergic neuronal system plays an important role in learning acquisition.
Combretum mucronatum and Capparis thonningii dose-dependently produce a significant increase in TLT in comparison to the scopolamine-treated group, with no differences in latency during the acquisition trial, indicating a lack of effect on the general behavior in mice. These results suggested that the anti-amnesic effect of C. mucronatum and C. thonningii on scopolamine-induced memory deficit may be related to mediation of the cholinergic nervous system.
To further confirm the effect of C. mucronatum and C. thonningii on cognition enhancement, we carried out the Morris water maze test on spatial learning. Scopolamine-treated mice had prolonged escape latency and longer path length when compared to control, C. mucronatum and C. thonningii treated groups had significantly reduced escape latency as well as shorter path length which was comparable to tacrine standard group. There was a strong correlation between escape latency and path length. The results of this study demonstrated that C. mucronatum and C. thonningii possess an ameliorating effect on the impairment of both memory registration and retrieval induced by scopolamine.
Scopolamine-induced memory impairment has been associated with cholinergic hypofunction and oxidative stress in the brain (CitationJones et al., 1991; CitationJeong et al., 2008; CitationBartus, 2000). To understand the possible mechanism of action of the extracts, anticholinesterase and antioxidants effects were investigated.
Scopolamine caused a significant increase in AChE activity in mouse brain which was ameliorated by C. mucronatum and C. thonningii in a dose-dependent manner. The inhibition of AChE by C. mucronatum and C. thonningii could lead to improved ACh level in brain which could be responsible for their antiamnesic effect in the scopolamine model. Therefore, cholinesterase inhibition may compensate for reduced ACh levels in brains with AD disease.
This study further evaluated whether scopolamine-induced memory deficit is associated with altered oxidative stress indices. Oxidative stress results from a marked imbalance between free radical production and elimination by antioxidant systems. Various studies have reported the strong positive correlation that memory impairments in the scopolamine-induced amnesic mice show similar patterns of oxidative damage in patients with amnesic mild cognitive impairment (CitationEl-Sherbiny et al., 2003; CitationFan et al., 2005). Moreover, many clinical studies have reported that oxidative stress is closely involved in the pathogenesis of AD (CitationMarcus et al., 1998).
MDA and GSH were used as indicators of lipid peroxidation and endogenous antioxidant, respectively. An elevated level of MDA suggests neuronal degeneration. GSH is the principal intracellular non-protein thiol and plays a major role in the maintenance of the intracellular redox state. The level of GSH diminishes with an increase in the generation of free radicals (CitationDringen et al., 2000). In the scopolamine model of dementia, MDA and GSH were estimated on the 5th day after the 1st injection of scopolamine. Scopolamine-treated mice showed a significant increase in MDA and decrease in GSH level in the brain from control values, indicating elevated oxidative stress. In addition, C. mucronatum and C. thonningii significantly attenuated the scopolamine-induced increase in nitrite level dose-dependently.
These findings provide important insights into the development of potential treatment regimens and even allude to the possibility of a preventative cure. These observations suggest that C. thonningii and C. mucronatum produced significant antioxidant activity against scopolamine-induced oxidative stress. Similarly, C. thonningii and C. mucronatum dose-dependently attenuated the increase in nitrite level.
Scopolamine administration resulted in a significant increase in MDA level, an important marker for lipid peroxidation, an increase in nitrite level, indicative of nitric oxide production, and in a reduction in GSH level, in the brain homogenate of amnesic mice.
The administration of C. mucronatum and C. thonningii produced a significant fall in MDA, nitrite level, and restored the activities of GSH in mice brain.
We suggest the restoration of the activities of GSH by C. mucronatum and C. thonningii might promote scavenging of free radicals by GSH. The present study demonstrated that C. mucronatum and C. thonningii both possess potent antioxidant activities. They scavenge ROS and exert a protective effect against oxidative damage induced by scopolamine by diminishing the reduction in the activities of GSH. The effect of C. mucronatum and C. thonningii on antioxidant enzyme was significant. The potent anti-amnesic effect of C. mucronatum and C. thonningii might result, in part, from the reduction in oxidative and nitrosative stress and, more importantly, inhibition of AChE activity which is the cornerstone of AD management.
Preliminary studies have shown that C. mucronatum and C. thonningii contain alkaloids, cardiac glycosides, saponins glycosides, steroidal nucleus, flavonoids and tannins. The ability of methanol root extract of C. mucronatum and C. thonningii to reverse scopolamine-induced memory deficit could probably result from the activities of their polyphenolics constituents (CitationRichetti et al., 2011). The effects of a flavonoid-rich diet on cognitive function have been linked to the ability of flavonoids to interact with the cellular and molecular framework involved in learning and memory, including synaptic potentiation and plasticity (CitationHarvsteen, 2002). Some studies already reported a possible relationship between polyphenolic ingestion and the prevention of AD (CitationSingh et al., 2008; CitationJi & Zhang, 2006). One or a combination of these phytoconstituents may be responsible for the observed antidementic activity of C. mucronatum and C. thonningii in this study.
In conclusion, C. mucronatum and C. thonningii showed potent cognitive-enhancing activities by inhibition of acetylcholinesterase activity, and by the regulation of the antioxidant system. As such, C. mucronatum and C. thonningii might offer a useful therapeutic choice in either the prevention or the treatment of Alzheimer’s disease.
These results also suggest that C. mucronatum and C. thonningii may provide the basis for a clinical strategy for the treatment of age-related memory impairment and dementia. Hence, we have commenced a bioactivity guided assay to isolate and identify the active principle(s) responsible for the cognitive-enhancing effects.
Acknowledgements
We acknowledge the provision of research facilities by Central Drug Research Institute (CDRI), India.
Declaration of interest
We acknowledge the Third World Academy of Science (TWAS) and Council for Scientific and Industrial Research (CSIR) for the postgraduate fellowship given to Ishola, Ismail O. The authors have declared that there is no conflict of interest.
References
- Agrawal R, Tyagi E, Saxena G, Nath C. (2009). Cholinergic influence on memory stages: A study on scopolamine amnesic mice. Indian J Pharmacol, 41, 192–196.
- Ahmed T, Gilani AH. (2009). Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol Biochem Behav, 91, 554–559.
- Ajaiyeoba EO, Sama W. (2006). Phytochemical and antimicrobial studies of C. thonningii and C. tormentosa. Pharmacog Mag, 2, 118–122.
- Bartus RT. (2000). On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol, 163, 495–529.
- Bartus RT, Dean RL, 3rdBeer B, Lippa AS. (1982). The cholinergic hypothesis of geriatric memory dysfunction. Science, 217, 408–414.
- Burkhill HM. (1985). The Useful Plants of West Tropical Africa, Vol.1. 2nd edition. UK: White Friars Press.
- Colado MI, O’Shea E, Granados R, Misra A, Murray TK, Green AR. (1997). A study of the neurotoxic effect of MDMA (‘ecstasy’) on 5-HT neurones in the brains of mothers and neonates following administration of the drug during pregnancy. Br J Pharmacol, 121, 827–833.
- Dringen R, Gutterer JM, Hirrlinger J. (2000). Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem, 267, 4912–4916.
- Ebert U, Kirch W. (1998). Scopolamine model of dementia: Electroencephalogram findings and cognitive performance. Eur J Clin Invest, 28, 944–949.
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys, 82, 70–77.
- Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol, 7, 88–95.
- El-Sherbiny DA, Khalifa AE, Attia AS, Eldenshary Eel-D. (2003). Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol Biochem Behav, 76, 525–533.
- Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M. (2005). Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett, 374, 222–226.
- Francis PT, Palmer AM, Snape M, Wilcock GK. (1999). The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J Neurol Neurosurg Psychiatry, 66, 137–147.
- Gilgun-Sherki Y, Melamed E, Offen D. (2002). Antioxidant treatment in Alzheimer’s disease: Current state. J Mol Neurosci, 21, 1–11.
- Giovannini MG, Casamenti F, Bartolini L, Pepeu G. (1997). The brain cholinergic system as a target of cognition enhancers. Behav Brain Res, 83, 1–5.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem, 126, 131–138.
- Harvsteen BH. (2002). The biochemistry and medical significance of the flavonoids. Pharmacol Ther, 96, 67–202.
- Hermans RH, Hunter DE, McGivern RF, Cain CD, Longo LD. (1992). Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol Teratol, 14, 119–129.
- Jeong EJ, Lee KY, Kim SH, Sung SH, Kim YC. (2008). Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated mice. Eur J Pharmacol, 588, 78–84.
- Ji HF, Zhang HY. (2006). Theoretical evaluation of flavonoids as multipotent agents to combat Alzheimer’s disease. J Mol Struct, 767, 3–9.
- Jones RW, Wesnes KA, Kirby J. (1991). Effects of NMDA modulation in scopolamine dementia. Ann N Y Acad Sci, 640, 241–244.
- Kulkarni KS, Kasture SB, Mengi SA. (2010). Efficacy study of Prunus amygdalus (almond) nuts in scopolamine-induced amnesia in rats. Indian J Pharmacol, 42, 168–173.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Marcus DL, Thomas C, Rodriquez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML. (1998). Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp Neurol, 150, 40–44.
- Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods, 11, 47–60.
- Pachauri SD, Tota S, Khandelwal K, Verma PR, Nath C, Hanif K, Shukla R, Saxena JK, Dwivedi AK. (2012) Protective effect of fruits of Morinda citrifolia L. on scopolamine induced memory impairment in mice: a behavioral, biochemical and cerebral blood flow study. J Ethnopharmacol, 139, 34–41.
- Richetti SK, Blank M, Capiotti KM, Piato AL, Bogo MR, Vianna MR, Bonan CD. (2011). Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav Brain Res, 217, 10–15.
- Riedel W, Hogervorst E, Leboux R, Verhey F, van Praag H, Jolles J. (1995). Caffeine attenuates scopolamine-induced memory impairment in humans. Psychopharmacology (Berl), 122, 158–168.
- Sharma D, Puri M, Tiwary AK, Singh N, Jaggi AS. (2010). Antiamnesic effect of stevioside in scopolamine-treated rats. Indian J Pharmacol, 42, 164–167.
- Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. (2008). Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem, 56, 4855–4873.
- Taffe MA, Weed MR, Gold LH. (1999). Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res, 8, 203–212.
- Terry AV Jr, Buccafusco JJ. (2003). The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J Pharmacol Exp Ther, 306, 821–827.
- Tota S, Kamat PK, Awasthi H, Singh N, Raghubir R, Nath C, Hanif K. (2009). Candesartan improves memory decline in mice: involvement of AT1 receptors in memory deficit induced by intracerebral streptozotocin. Behav Brain Res, 199, 235–240.
- Tota S, Nath C, Najmi AK, Shukla R, Hanif K. (2012). Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: Role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res, 232, 66–76.
- Vafeiadou K, Vauzour D, Spencer JP. (2007). Neuroinflammation and its modulation by flavonoids. Endocr Metab Immune Disord Drug Targets, 7, 211–224.
- Ward CP, Redd K, Williams BM, Caler JR, Luo Y, McCoy JG. (2002). Ginkgo biloba extract: Cognitive enhancer or antistress buffer. Pharmacol Biochem Behav, 72, 913–922.
- Wang C, Smith RL. (1975). Lowry determination of protein in the presence of Triton X-100. Anal Biochem, 63, 414–417.
- Yamada K, Komori Y, Tanaka T, Senzaki K, Nikai T, Sugihara H, Kameyama T, Nabeshima T. (1999). Brain dysfunction associated with an induction of nitric oxide synthase following an intracerebral injection of lipopolysaccharide in rats. Neuroscience, 88, 281–294.
- Zandi PP, Anthony JC, Khachaturian AS, Stone SV, Gustafson D, Tschanz JT, Norton MC, Welsh-Bohmer KA, Breitner JC. (2004). Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County study. Arch Neurol, 61, 82–88.