Abstract
Context: Resveratrol (3,5,4′-trihydroxystilbene) is a phytoalexin synthesized by plants, most notably grapes, against microbial invasion or ultraviolet stimulation, and is known to exert antioxidant, anticancer, and antiobesity effects.
Objective: This study was conducted to find resveratrol derivatives with higher anti-obesity activity compared to resveratrol and to verify their mechanism of action.
Materials and methods: The inhibitory effect of resveratrol and its derivatives on adipocyte differentiation in 3T3-L1 cells was studied using Oil Red O staining, and the effects on the intracellular expression of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) were measured via Western blot analysis.
Results: A derivative of resveratrol, 4-[2-(3,5-dimethoxyphenyl)vinyl]pyridine (DPVP), exerted inhibitory effects against 3T3-L1 adipocyte differentiation (IC50 = 13.5 µM) and FAS expression. Notably, it displayed higher activity at concentrations lower than 25 µM compared to resveratrol.
Discussion and conclusion: DPVP is considered to have greater potential as an anti-obesity substance, as it exhibits excellent activity at low concentrations compared to resveratrol.
Introduction
Obesity is a major factor in heart disease, cancer, hypertension, diabetes, and degenerative arthritis (CitationChen, 2011; CitationWannamethee et al., 2011; CitationRhéaume et al., 2011; CitationAbete et al., 2011). According to the World Health Organization (WHO), there are one billion people who are overweight; at least 300 million of them are clinically obese (CitationClapham & Arch, 2011). It is expected that one-third of the world’s population will become obese (over BMI 30 kg/m2) within 20 years if the current trend of rising obesity continues. Therefore, the scale of the world’s anti-obesity materials market is expected to rapidly increase every year, due to the increase in the number of obese people. However, since the adverse effects of commonly used anti-obesity drugs, such as orlistat, sibutramine and sertraline, have been reported (CitationGhanizadeh, 2008; CitationTabak et al., 2009; CitationGarcía & Martín, 2011), studies involving the research and development of anti-obesity substances that can replace the above mentioned drugs are being actively conducted.
A triglyceride (TG, neutral fat) is made of fatty acids and glycerol. In the initial stage of cellular fatty acid synthesis, acetyl-CoA carboxylase (ACC), an enzyme that plays a critical role in the process, induces the carboxylation of acetyl-CoA and promotes the production of malonyl-CoA (CitationKreuz et al., 2009; CitationTong, 2005). Fatty acid synthesis is the process by which fatty acids are made from malonyl-CoA via the action of an enzyme called fatty acid synthase (FAS) with a molecular weight of 250 kDa (CitationWakil & Abu-Elheiga, 2009). Thus, it is expected that FAS and ACC inhibitors will be able to reduce fat production and suppress obesity.
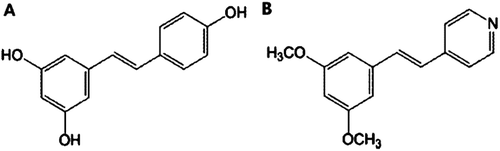
Resveratrol (3,5,4′-trihydroxystilbene) is a stilbenoid present in nature, as well as a phytoalexin produced naturally by several plants in response to attack by pathogens such as bacteria or fungi. Resveratrol is known to possess several useful physiological activities such as antioxidant, anticancer, and cardiovascular protective effects, and has also been reported to exert an anti-obesity effect (CitationAggarwal et al., 2004; CitationBaur et al., 2006; Citationde la Lastra & Villegas, 2007; CitationRayalam et al., 2008; CitationSzkudelska & Szkudelski, 2010; CitationBaile et al., 2011; CitationPetrovski et al., 2011). In this study, the anti-obesity activities of resveratrol derivatives were investigated using 3T3-L1 cells, among which 4-[2-(3,5-dimethoxyphenyl)vinyl]pyridine (DPVP) exerted profound inhibitory effects on 3T3-L1 cell differentiation and FAS expression at lower concentrations compared to resveratrol ().
Figure 1. Chemical structures of resveratrol and DPVP. (A) Resveratrol, (B) DPVP. The spectra data of DPVP are as follows: 1H-NMR (300 MHz, CDCl3): 8.51 (d, 2H, J = 5.7 Hz), 7.39–7.33 (m, 2H), 7.19 (d, 1H, J = 16.5 Hz), 6.93 (d, 1H, J = 16.2 Hz), 6.63 (d, 2H, J = 2.1 Hz), 6.40 (t, 1H, J = 2.1 Hz), 3.77 (s, 6H); MS (EI+) m/z 241 (M+, 77), 199 (100), 183 (97), 152 (50).
Materials and methods
Instrumentations and materials
The UV spectra were determined using a Molecular Devices E09090 microplate reader. The 1H-(300 MHz) spectra were run on a Gemini 2000 spectrometer. The FABMS spectra were measured on a Hewlett-Packard mass spectrometer. 3T3-L1 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Resveratrol, Oil Red O, insulin, 1-methyl-3-isobutyl xanthine (IBMX), and dexamethasone were purchased from Sigma (St Louis, MO, USA). Antibodies against FAS and acetyl CoA carboxylase were purchased from Cell Signaling Technology (Beverly, MA, USA).
Synthesis of DPVP
The synthesis of DPVP was conducted using the previously reported method (CitationKim et al., 2002).
Cell culture and differentiation
3T3-L1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum at 37°C in a 5% CO2 incubator. 3T3-L1 cells were maintained in 48-well culture plates. After 100% confluence was reached, the cells were treated for 48 h with a hormone mixture containing 10 µg/mL insulin, 0.5 µM dexamethasone, and 0.5 mM IBMX, and then exchanged with DMEM containing insulin. The cells were differentiated into adipocytes until day 8 in the presence or absence of stimuli.
Oil Red O staining and TG assay
After adipocyte differentiation was completed, the TG contents were evaluated via Oil Red O staining. The cells were washed twice in phosphate-buffered saline (PBS) and fixed with 3.7% formaldehyde in PBS. Adipocytes were incubated for 1 h with Oil Red O dye, which stained red. The lipid droplets were dissolved in isopropanol and measured at 510 nm.
Western blotting
The cells were washed twice in ice-cold PBS after stimulation and lysed in lysis buffer (50 mM Tris-HCl, 1% Triton X-100, 0.5% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, 1 mM NaF, and 0.2% protease inhibitor cocktail, pH 7.2). Collected proteins were centrifuged at 14 000 rpm for 5 min, and supernatant was collected in a new tube. The protein concentration was measured with the Bradford assay system (Bio-Rad, Hercules, CA, USA). Thirty µg protein were loaded on a 10% SDS-PAGE, and transferred to a nitrocellulose membrane. Western blot analysis was conducted with specific antibodies (ACC: 1:500; FAS: 1:1000; β-actin 1: 4000 dilutions).
Statistical analysis
The data are expressed as the means ± SD from three independent experiments. Statistical analysis was conducted via one-way ANOVA followed by Tukey’s test.
Results
Inhibitory effect on adipocyte differentiation
To confirm whether DPVP has anti-obesity effects, 3T3-L1 cells were treated for 8 days and the degree of inhibition of hormonal induced-adipocyte differentiation was measured by Oil Red O Staining. The results show that DPVP has a concentration-dependent inhibitory effect on adipocyte differentiation, evidencing a significantly higher inhibitory effect at a concentration of 12.5 µM, compared to resveratrol (). The IC50 of DPVP and resveratrol were 13.5 and 22.1 µM, respectively. The morphological changes of 3T3-L1 cells treated by resveratrol and DPVP at 12.5 µM are shown in .
Figure 2. Inhibitory effects on 3T3-L1 adipocyte differentiation. Hormone: hormone mixture (including insulin, dexamethasone, and IBMX), DPVP: 4-[2-(3,5-dimethoxyphenyl)vinyl]pyridine, Res: resveratrol. Three independent experiments were conducted; results are expressed as means ± SD of absorbance at 510 nm. **p < 0.01, and ***p < 0.001 represent significant differences when comparing the hormone treatment group.
![Figure 2. Inhibitory effects on 3T3-L1 adipocyte differentiation. Hormone: hormone mixture (including insulin, dexamethasone, and IBMX), DPVP: 4-[2-(3,5-dimethoxyphenyl)vinyl]pyridine, Res: resveratrol. Three independent experiments were conducted; results are expressed as means ± SD of absorbance at 510 nm. **p < 0.01, and ***p < 0.001 represent significant differences when comparing the hormone treatment group.](/cms/asset/589ae5b5-41f3-4f3d-be45-cc4b4cb13127/iphb_a_711841_f0002_b.gif)
Effects on the expression of ACC and FAS
ACC and FAS, which are major enzymes in fatty acid biosynthesis pathways, are known as important factors in the regulation of obesity. The possible inhibitory effect of DPVP on the intracellular expression of ACC and FAS was measured in 3T3-L1 cells induced by treatment with a hormone cocktail comprising insulin, dexamethasone, and IBMX. The results show that it does not affect ACC expression, but does reduce FAS expression, with a significantly higher inhibitory effect at a concentration of 12.5 µM, relative to resveratrol. This result is consistent with that of the inhibitory effect on adipocyte differentiation, showing excellent activity at a low concentration. Thus, the inhibitory effect of DPVP on adipocyte differentiation is considered to be mainly attributable to reduced FAS expression ().
Discussion
Resveratrol inhibits the adipogenesis of 3T3-L1 cells and increases the survival rate of mice on a high-fat diet by reducing the expression of some adipocyte-specific genes, including FAS (CitationRayalam et al., 2008). Resveratrol has been reported to have various physiological activities including an anti-obesity effect, but has the problem of low bioavailability (CitationWenzel & Somoza, 2005; CitationWalle, 2011). As such, many studies have been conducted to discover resveratrol derivatives with higher physiological activity and bioavailability; some resveratrol derivatives with considerably higher effects, compared to resveratrol, on cytochrome P450 inhibition, tyrosinase inhibition, and neuro-protective action have been reported, and are being derived as potential materials for the development of new drugs of natural origin (CitationShin et al., 1998; CitationKim et al., 2002; CitationChoi et al., 2007). In this context, DPVP, a nonpolar derivative of resveratrol, exerted inhibitory effects on 3T3-L1 adipocyte differentiation and FAS expression at concentrations lower than 25 µM, compared to resveratrol. Moreover, based on the relationship between the polarity and the excretion, DPVP is expected to have higher bioavailability than resveratrol. Although the degree of physiological activity and bioavailability in the body have yet to be confirmed, DPVP is considered to have significant potential as a potent anti-obesity substance at a low concentration. In future studies, the anti-obesity activity, mechanism and toxicity of DPVP in the body will be verified.
Declaration of interest
This work was supported by a grant from the Korea Food Research Institute (KFRI).
References
- Abete I, Goyenechea E, Zulet MA, Martínez JA. (2011). Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr Metab Cardiovasc Dis, 21 Suppl 2, B1–15.
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. (2004). Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res, 24, 2783–2840.
- Baile CA, Yang JY, Rayalam S, Hartzell DL, Lai CY, Andersen C, Della-Fera MA. (2011). Effect of resveratrol on fat mobilization. Ann N Y Acad Sci, 1215, 40–47.
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 444, 337–342.
- Chen J. (2011). Multiple signal pathways in obesity-associated cancer. Obes Rev, 12, 1063–1070.
- Choi SY, Kim S, Son D, Lee P, Lee J, Lee S, Kim DS, Park Y, Kim SY. (2007). Protective effect of (4-methoxybenzylidene)-(3-methoxynophenyl)amine against neuronal cell death induced by oxygen and glucose deprivation in rat organotypic hippocampal slice culture. Biol Pharm Bull, 30, 189–192.
- Clapham JC, Arch JR. (2011). Targeting thermogenesis and related pathways in anti-obesity drug discovery. Pharmacol Ther, 131, 295–308.
- de la Lastra CA, Villegas I. (2007). Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem Soc Trans, 35, 1156–1160.
- García Díaz E, Martín Folgueras T. (2011). Systematic review of the clinical efficacy of sibutramine and orlistat in weight loss, quality of life and its adverse effects in obese adolescents. Nutr Hosp, 26, 451–457.
- Ghanizadeh A. (2008). Sertraline-associated hair loss. J Drugs Dermatol, 7, 693–694.
- Kim S, Ko H, Park JE, Jung S, Lee SK, Chun YJ. (2002). Design, synthesis, and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem, 45, 160–164.
- Kreuz S, Schoelch C, Thomas L, Rist W, Rippmann JF, Neubauer H. (2009). Acetyl-CoA carboxylases 1 and 2 show distinct expression patterns in rats and humans and alterations in obesity and diabetes. Diabetes Metab Res Rev, 25, 577–586.
- Petrovski G, Gurusamy N, Das DK. (2011). Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci, 1215, 22–33.
- Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. (2008). Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res, 22, 1367–1371.
- Rhéaume C, Leblanc MÈ, Poirier P. (2011). Adiposity assessment: explaining the association between obesity, hypertension and stroke. Expert Rev Cardiovasc Ther, 9, 1557–1564.
- Shin NH, Ryu SY, Choi EJ, Kang SH, Chang IM, Min KR, Kim Y. (1998). Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem Biophys Res Commun, 243, 801–803.
- Szkudelska K, Szkudelski T. (2010). Resveratrol, obesity and diabetes. Eur J Pharmacol, 635, 1–8.
- Tabak F, Gunduz F, Tahan V, Tabak O, Ozaras R. (2009). Sertraline hepatotoxicity: Report of a case and review of the literature. Dig Dis Sci, 54, 1589–1591.
- Tong I. (2005). Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell Mol Life Sci, 62, 1784–1803.
- Wakil SJ, Abu-Elheiga LA. (2009). Fatty acid metabolism: Target for metabolic syndrome. J Lipid Res, 50 Suppl, S138–S143.
- Walle T. (2011). Bioavailability of resveratrol. Ann N Y Acad Sci, 1215, 9–15.
- Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. (2011). Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? J Am Coll Cardiol, 58, 1870–1877.
- Wenzel E, Somoza V. (2005). Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res, 49, 472–481.
![Figure 3. Photographs of 3T3-L1 adipocytes after Oil Red O staining. HM: hormone mixture (including insulin, dexamethasone, and IBMX), Res: 12.5 µM of resveratrol; DPVP: 12.5 µM of 4-[2-(3,5-dimethoxyphenyl)vinyl]pyridine.](/cms/asset/6e86b6bb-db1a-476c-9974-4cd3d3d28b41/iphb_a_711841_f0003_b.gif)
![Figure 4 . Effect on intracellular expression of ACC and FAS. N: normal; HM: hormone mixture; DPVP: 4-[2-(3,5-dimethoxyphenyl)vinyl]pyridine; Res: resveratrol.](/cms/asset/308b7a9a-05f5-487a-95ee-74343abf369e/iphb_a_711841_f0004_b.gif)