Abstract
Context: Moringa oleifera Lam. (Moringaceae) is a rich source of essential minerals and antioxidants; it has been used in human and animal nutrition. The leaves and flowers are being used by the population with great dietary importance.
Objective: The present study was to investigate the therapeutic effects of the hydroethanolic extract of Moringa oleifera (MO) leaves and flowers against hepatotoxicity induced by acetaminophen (APAP) in rats.
Materials and methods: In the hepatoprotective study, either flowers or leaves of hydroethanolic extract (200 or 400 mg/kg bw through IP injection) were administered an hour after APAP administration. N-Acetylcysteine (NAC) was used as the positive control for this study. Liver and kidney function tests including lipid peroxidation levels were analyzed and histopathological changes of liver and kidney were also observed.
Results: Acetaminophen-induced hepatotoxicity increased the activities of liver marker enzymes. Histologically, the liver was observed to have inflammation and bridging necrosis. Liver marker enzymes were significantly reduced when treated with flower and leaf extracts of MO in animals with APAP induced toxicity. In addition, there were no significant changes observed in clinical markers of kidney function. Histological observation on liver tissue from the rats treated with MO flower and leaf extract showed reduction in the severity of the liver damage.
Discussion and conclusion: These results indicated the possible therapeutic action of flower and leaf extract from MO in protecting liver damage in rats given an over dosage of APAP.
Introduction
Liver diseases are one of the most serious health problems worldwide, and are caused by drugs, chemicals, and alcohol. The liver is the second largest organ of the body, located on the right side of the body in the upper abdomen. The liver plays an important role in metabolism of drugs and xenobiotics, and in maintaining biological equilibrium (CitationUng et al., 2011). The major role of this organ is to remove toxic substances from the portal circulation, placing it in the first line and prone to be attacked by foreign materials (CitationKuriakose & Kurup, 2011). Liver diseases still have extremely poor prognosis and high mortality because of the lack of effective therapy (CitationRiedemann et al., 2003).
Acetaminophen (APAP) is a widely used safe analgesic and antipyretic drug. However, acetaminophen is the most common cause of drug-induced liver diseases and acute liver failure in humans and experimental animals (CitationRamaiah, 2007). At toxic doses, the normal metabolic pathways become saturated (CitationDahlin et al., 1984), causing both more N-acetyl-p-benzoquinone imine (NAPQI) to be formed and hepatic glutathione to be rapidly depleted. Recent evidence shows that reactive oxygen species production (CitationHinson et al., 2010; CitationChandrasekaran et al., 2008), lipid peroxidation (CitationAgarwal et al., 2012) and mitochondrial dysfunction (CitationChandrasekaran et al., 2011) are all involved in acetaminophen-induced hepatotoxicity.
At present, no specific therapy is available especially for acute liver failure without any adverse effects. Therefore, the need is urgent for effective therapeutic agents from natural products for the treatment of liver diseases. Natural drugs are frequently considered to be less toxic and freer from side effects than synthetic drugs. Recently, the search for effective crude drugs of plant origin through nutritional aspects with strong antioxidant nature has become a central focus for the study of hepatoprotection (CitationKuriakose & Kurup, 2011).
Moringa oleifera Lam. (MO) is one of the best known naturalized species of the Moringaceae family (CitationAnwar et al., 2007). Different types of important nutritional and medicinal merits have been attributed to its roots, bark, leaves, flowers, fruits, and seeds (CitationAnwar et al., 2007; CitationKumar et al., 2010). The leaves, flowers and immature pods of this tree are used as a highly nutritive vegetable in many countries particularly in India, Pakistan, Philippines, Hawaii, and many parts of Africa (CitationAnwar et al., 2007). Medicinally important active compound analysis of MO edible parts show it rich in bioactive compounds containing simple sugar, rhamnose and fairly unique group of compounds called glucosinolates and isothiocyanates (CitationFahey et al., 2001). The flowers of MO contain various essential amino acids, sucrose, d-galactose, traces of alkaloids, flavonoids as well as known antioxidants vitamins (CitationAslam et al., 2005; CitationManguro & Lemmen, 2007; CitationAmaglo et al., 2010; CitationGowrishankar et al., 2010; CitationSiddhuraju & Becker, 2003).
Moringa leaves act as a good natural source of antioxidant vitamins/compounds such as ascorbic acid, flavonoids, phenolics and carotenoids. The higher concentration of ascorbic acid, estrogenic substances, and β-sitosterol, vitamins, and particular essential amino acids such as methionine, cysteine, tryptophan and lysine present in Moringa leaves and pods make it virtually an ideal nutritional supplement (CitationAdejumo et al., 2012). Therapeutic use of Moringa edible parts in many regions of Africa as well as Asia, it is widely consumed for self-medication by patients affected by diabetes, hypertension, or HIV/AIDS (CitationDièye et al., 2008; CitationKasolo et al., 2010). It possesses various biomedical properties such as anti-inflammatory, antioxidant, antimicrobial, antifertility, anticancer, antihepatotoxic, and antiulcer activities (CitationGoyal et al., 2007).
Evidently, in spite of the widely held “belief” in the beneficial health effects of MO, the valuable interest of the biomedical society in the medicinal prospective of this nutritional plant has been rather tepid. Therefore, we selected this present investigation on hydroethanolic extracts of MO flowers and leaves to carry out extensive medicinal as well as toxicological evaluation against APAP-induced hepatotoxicity.
In this present study, we investigated the therapeutic action of the MO leaves and flowers extract against APAP-induced hepatotoxicity in experimental rats. The results from present investigation were compared with positive control, N-acetylcysteine (NAC), an amino acid with antioxidant properties.
Materials and methods
Drugs and chemicals
Acetaminophen (APAP) was purchased from Sigma-Aldrich Co., (France). Eosin and haematoxylin solutions and DPX mountant were procured from BDH (UK). Paraffin, xylene and absolute ethanol were purchased from Fisher Scientific (USA). The diagnostic kits for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were obtained from the Roche Diagnostics (Germany). All other chemicals used were of analytical grade (AR).
Plant material and preparation of extract
The plant materials were identified and harvested in Taman Pertanian Universiti (TPU), Malaysia and confirmed by a plant taxonomist, Mr. Shamsul Khamis, Institute of Bioscience (IBS), Universiti Putra Malaysia, and voucher specimen (SK 1561/08) has been deposited at IBS Herbarium unit for future reference. Samples were soaked and occasional agitated in 80% ethanol for 6 days. All samples were then filtered with a double layer of gauze before using filter paper. The samples were then concentrated and lyophilized and kept in −20°C for further analysis (CitationFakurazi et al., 2008). Samples were stored as aliquots with 0.5% Tween 80 for further experiments.
Animals
Male Sprague-Dawley rats were acclimatized for 1 week in an animal house (26 ± 2°C) with 12 h light and dark cycles. They were fed with standard rodent pellets and water was provided ad libitum. All animal experiments were approved (Approval no: UPM/FPSK/PADS/BR-UUH/00356) and conducted by IACUC (Institutional Animal Care and Use Committee), Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Malaysia.
Dose-dependent in vivo studies of hepatotoxin
A preliminary study was conducted to determine the dosage of APAP in order to obtain significant hepatocellular damage. Necrotic hepatocytes in the liver histological studies have been accepted as a hallmark of APAP induced hepatocellular damage. Male rats (200–250 g) were placed in cages and randomly divided into six groups of 6 animals (N = 6). Group I was given only normal saline and Groups II, III, IV were orally treated with a single dose of 2 g APAP/kg body weight, 4 g APAP/kg body weight and 6 g APAP/kg body weight, respectively. The powder form of APAP was dissolved in preheated normal saline. Meanwhile, animals in Groups V and VI were given 400 mg flower extract/kg body weight and 400 mg leaf extract/body weight, respectively. Extract was dissolved in 0.5% (v/v) Tween 80 and injected intraperitoneally.
Diet was withdrawn from the animals 16 h before sacrifice. The animals were sacrificed 24 h after the treatment using diethyl ether. Blood was collected via cardiac puncture for biochemical analysis. The median lobe of the liver and kidney was fixed in 10% (v/v) normal buffered formalin for histological analysis.
Therapeutic effect of MO extracts against APAP intoxication
Male rats (200–250 g) were divided into the seven groups of six animals (N = 6). Acetaminophen was delivered orally while NAC (positive control) and the plant crude extracts were injected i.p. an hour after APAP intoxication. NAC dissolved in 0.5% (v/v) Tween 80 and the extracts were.
Experimental design
Group I: Animal received 0.5% Tween 80 only (vehicle control)
Group II: Animals received 7 g APAP/kg bw and 0.5% Tween 80
Group III: Animals received 7 g APAP/kg bw and 7.35 mmol NAC/kg bw (CitationTerneus et al., 2007) (positive control)
Group IV: Animals received 7 g APAP/kg bw and 200 mg flower extract/kg bw
Group V: Animals received 7 g APAP/kg bw and 400 mg flower extract/kg bw
Group VI: Animals received 7 g APAP/kg bw and 200 mg leaf extract /kg bw
Group VII: Animals received 7g APAP/kg bw and 400 mg leaf extract /kg bw
Diet was withdrawn from the animals 16 h before sacrifice. Animals were weighed and sacrificed 24 h after the treatment using diethyl ether. Blood was collected via cardiac puncture for biochemistry tests. Liver was removed and weighed. The median lobe of the liver and kidney tissues were fixed in 10% (v/v) normal buffered formalin for histological analysis. The rest of the serum and liver samples were stored at −80°C until analysis.
Determination of liver and kidney markers
Liver and kidney function markers including ALT, AST, BUN and creatinine were measured by commercial kits from Roche Diagnostics (Germany) according to the standard assay procedure.
Histopathological examination of liver and kidney tissues
Liver and kidney tissues for histopathological studies were cut and fixed in 10% formalin buffer. After the fixation, the tissues were washed and processed by standard histology procedures, and embedded in paraffin. Tissue sections were stained with haematoxylin and eosin (H&E). The stained slides were viewed under a light microscope (Olympus BX-51, Japan).
Determination of malondialdehyde (MDA) level
Lipid peroxidation was determined by measuring thiobarbituric acid reactive substances (TBARS) by a spectrophotometric assay according to a previous report (CitationOhkawa et al., 1979). Briefly, the reaction mixture containing 0.5 mL homogenate, 0.5 mL TCA and 0.5 mL TBA was incubated in boiling water for 15 min. A pink color chromogen was formed and then extracted with 2.0 mL of butanol solution. The mixture was centrifuged at 3000 rpm for 10 min. The supernatant was read at 532 nm.
Statistical analysis
All the data are presented as mean ± SEM. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 16.0. Statistical significance of the differences between the treatments groups in experiments were analyzed by post test Tukey’s multiple comparison tests. p < 0.05 was mentioned in the results as statistically significant.
Results
Dose-dependent effect of acetaminophen on liver, kidney markers and histological changes
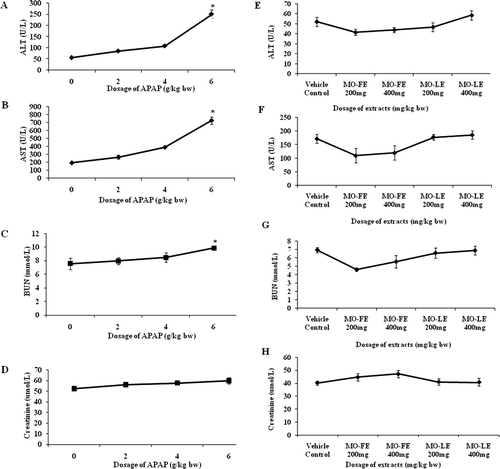
The highest dose of APAP (6 g/kg bw) significantly (p < 0.05) increased the activities of ALT and AST in experimental rats ( and ). The increased level in ALT and AST in rats suggested that there was damage of hepatocytes. Similar findings were observed in the kidney function test where animals given 6 g APAP/kg bw also showed the highest increase of BUN and creatinine, as important marker for kidney function ( and ).
Figure 1. Dose-dependent effect of APAP-induced liver injury and MO extracts. Values are presented as the means ± SEM of six rats per group. Statistical significance between the groups (*p < 0.05) compared with normal group.
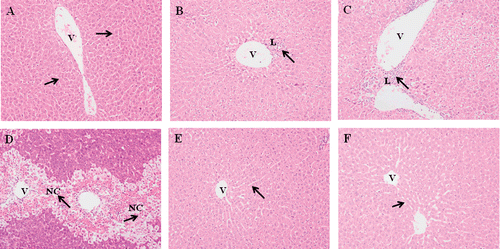
We observed the histopathological features of liver tissue to determine the various doses of APAP-induced liver injury. Under a light microscopic evaluation, severe hepatocyte damage around the perivenular area as well as bridging necrosis was evidenced after 6 g APAP/kg bw was given (, , , and 2D). Fatty changes and inflammation around the perivenular area were observed when administered at a dosage of 4 g APAP/kg bw. At 2 g APAP/kg bw, microscopic evaluation shows the presence of focal inflammation, but results were not significant. These results showed that the effect of APAP toxicity was dose-dependent.
Figure 2. Effect of different dosage of APAP and MO extracts on the histological changes in the liver tissues. Representative photographs are showing the histological changes in liver tissues. Rats were sacrificed 24 h posttreatment and all tissue sections were stained with hematoxylin and eosin (×200). a) Normal group: normal central vein and hepatic parenchyma; b) APAP-treated group (2 g/kg bw); c) APAP-treated group (4 g/kg bw); d) APAP-treated group (6 g/kg bw); e) MO leaves extract treated group (400 mg/kg bw); f) MO flowers extract treated group (400 mg/kg bw). The arrows indicate normal and necrotic areas. NC, necrotic hepatocyte; L, leucocytes; V, central vein.
The effect of MO flower and leaf extract on normal liver at a dosage of 200 and 400 mg/kg bw was analyzed by serum marker enzymes ( and ), kidney function test ( and ) and histological studies ( and ). We found that the activities serum marker enzymes of ALT, AST, and kidney marker levels BUN and creatinine were not significantly changed when compared with control animals, indicating the nontoxic nature of MO flower and leaf extracts. These dosages did not induce any hepatocellular damage based on the histopathological studies ( and ).
Therapeutic effect of MO flower and leaf extracts
Effect of MO extracts on changes in liver weight
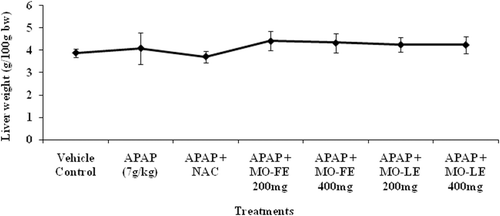
shows the changes of liver weight ratio in control and APAP-induced as well as treatment groups. A significant increase in the liver weight was observed when administered the high dose of APAP. After the administration of extracts, there was a moderate reduction of liver weight when compared to control rats. The result showed that MO extracts at a dosage of 200 and 400 mg/kg bw effectively protect the liver from hepatic toxicity.
Effect of MO extracts on liver and kidney markers
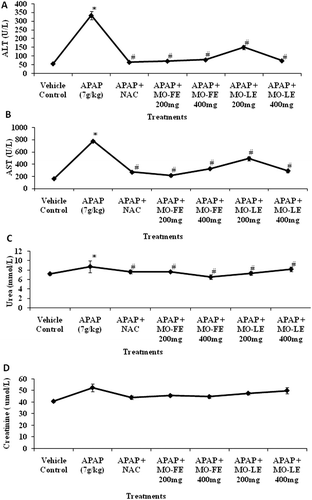
The activities of hepatic enzymes ALT and AST are used as important diagnostic markers for evaluation of early hepatic injury. These enzymes activities were significantly increased in APAP-treated groups when compared to control groups ( and ). In groups that were treated with MO flower and leaf extract, the activities of liver enzymes were significantly reduced. Animals treated with 200 mg/kg flower extract were effectively reduced ALT to 69.65 ± 2.90 U/L and AST to 213.48 ± 23.26 U/L when compared to other treated groups. The level of enzymes in animal receiving 400 mg/kg of flower extract showed low levels of ALT (76.61 ± 2.16 U/L) and AST (324.43 ± 15.56 U/L). and shows the concentration of kidney markers BUN and creatinine in serum of rats. There were no significant changes between control groups and treatment groups that suggested there was no untoward effect on kidney.
Figure 4. Effect of MO flower and leaf extracts on liver and kidney function markers in APAP-induced hepatotoxicity in rats. Values are presented as the means ± SEM of six rats per group. Statistical significance between the groups (*p < 0.05; #p < 0.05) compared with vehicle control and APAP-treated groups, respectively.
Effect of MO extracts on histological changes
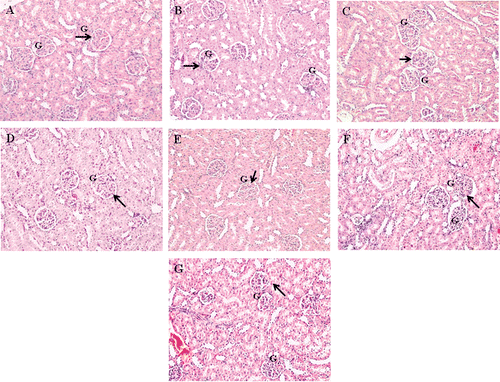
Our preliminary findings have indicated that 6 g APAP/kg bw significantly induced activities of enzymes ALT and AST along with hepatocellular damage and bridging necrosis. However, no significant changes in kidney histology studies lead to increase of dose to 7 g/kg body weight. A dose of 7 g APAP/kg bw resulted in sinusoidal congestion, hemorrhages, confluent necrosis and massive inflammatory cell infiltration around perivenular area (, , , , E, 5F, and 5G). Animals that received NAC treatment after APAP intoxication showed the presence of fatty changes. In animal groups treated with 200 and 400 mg/kg of the hydroethanol extract of flowers, there was a significant reduction in the severity of hepatocellular damage induced by hepatotoxicity. Meanwhile, administration of 200 mg/kg leaf extract did not reduce severity of liver injury after APAP intoxication. However, 400 mg/kg of the leaf extract moderately reduced the necrotic hepatocyte, indicating the reduction of hepatocellular damage. Therefore, 200 and 400 mg/kg flower extract as well as 400 mg/kg leaf extract were able to suppress the progression of hepatocyte damage even with high dose of APAP. All animals in different treated and control groups showed normal kidney structure despite being intoxicated with a high dose of APAP (, , , , E, 6F, and 6G).
Figure 5. Effect of different dosage MO flower and leaf extract as well as NAC on the liver histological changes in APAP-induced hepatotoxicity in rats. Representative photographs show the histological changes in liver tissues. Rats were sacrificed 24 h posttreatment, and all tissue sections were stained with hematoxylin and eosin (×200). a) Normal group: normal central vein and hepatic parenchyma; b) APAP-treated group (7 g/kg bw); c) APAP-treated group (7 g/kg bw) + NAC; d) APAP-treated group (7 g/kg bw) + MO flower extract (200 mg/kg bw); e) APAP-treated group (7 g/kg bw) + MO flower extract (400 mg/kg bw); f) APAP-treated group (7 g/kg bw) + MO leaf extract (200 mg/kg bw); g) APAP-treated group (7 g/kg bw) + MO leaf extract (400 mg/kg bw). The arrows indicate normal and necrotic areas. FC, fatty change; NC, necrotic hepatocyte; L, leucocytes; V, central vein.
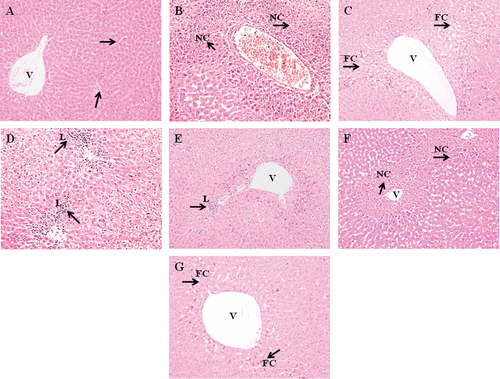
Figure 6. Effect of different dosage MO flower and leaf extract as well as NAC on the kidney histological changes in APAP-induced hepatotoxicity in rats. Representative photographs show the histological changes in kidney tissues. Rats were sacrificed 24 h posttreatment, and all tissue sections were stained with hematoxylin and eosin (×200). a) Normal group; b) APAP-treated group (7 g/kg bw); c) APAP-treated group (7 g/kg bw) + NAC; d) APAP-treated group (7 g/kg bw) + MO flowers extract (200 mg/kg bw); e) APAP-treated group (7 g/kg bw) + MO flowers extract (400 mg/kg bw); f) APAP-treated group (7 g/kg bw) + MO leaves extract (200 mg/kg bw); g) APAP-treated group (7 g/kg bw) + MO leaves extract (400 mg/kg bw). The arrows indicate normal kidney architecture. G, glomeruli.
Effect of MO extracts on malondialdehyde (MDA) levels
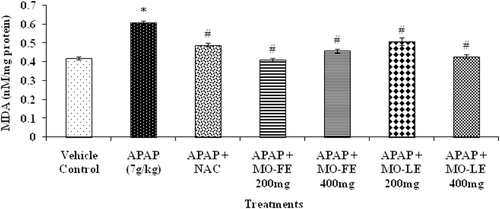
Acetaminophen intoxication showed significant adverse effect on the redox status of the liver which was evidenced by specific markers. Administration of APAP to test animals showed an increase in MDA levels compared to control group (). Animals treated with NAC showed a significant (p < 0.05) reduction of MDA level. Groups treated with either 200 or 400 mg/kg flower extract showed a significantly (p < 0.05) reduced MDA level. While in the group treated with MO leaf extract (dosage of 200 and 400 mg/kg bw), there was also a reduction of MDA levels when compared with the APAP-induced hepatotoxicity group.
Figure 7. Effect of MO flower and leaf extracts on hepatic lipid peroxidation (MDA) level in APAP-induced hepatotoxicity in rats. Values are presented as the means ± SEM of six rats per group. Statistical significance between the groups (*p < 0.05; #p < 0.05) compared with vehicle control and APAP-treated groups, respectively.
Discussion
In the present study, we investigated the therapeutic effect of hydroethanol extracts of MO against acute liver injury, induced by a hepatotoxin. As it is well known, the liver appears to be, due to its unique metabolic function, the most common target organ of any toxicity. APAP is a one of the chemical hepatotoxin known for inducing characteristic features similar to those of acute hepatitis in humans.
Many hepatoxins (CitationZhang et al., 2003; CitationMalhi et al., 2010) could induce liver injury through oxidative stress, inflammation, fibrogenesis and liver necrosis. Acute injury to hepatocytes alters their transport function and membrane permeability, leading to leakage of marker enzymes from the cells (CitationYang et al., 2008). The intracellular mechanisms of injury due to APAP in hepatocytes are the formation of reactive metabolites, depletion of glutathione and alkylation of proteins, especially the mitochondrial proteins (CitationNelson & Bruschi, 2003). These initiating events trigger mitochondrial membrane permeability transition. The breakdown of the mitochondrial membrane permeability transition precedes the plasma membrane failure with cell swelling and leakage of cell content, i.e., cell death through oncotic necrosis (CitationKon et al., 2004).
Assessment of liver injury was determined by serum concentrations of ALT and AST, one of the most commonly indicated markers for liver toxicity. When the liver cell plasma membrane is damaged, a variety of enzymes normally located in the cytosol are released into the bloodstream. Their amount in the serum is a useful quantitative marker of the extent and type of hepatocellular damage. Increased activities of serum enzymes were observed after APAP intoxication compared to normal rats indicating considerable hepatocellular damage ( and ). The increase in ALT is the most intense during the first 12 h (CitationChanda et al., 1995). Our experimental model of hepatotoxicity induced by 6 g APAP/kg bw demonstrated a significant rise of ALT and AST in the first 24 h. The present study correlates with other studies showing significant increases of ALT and AST values after administration of hepatotoxicants like APAP (CitationLiu et al., 2012; CitationLee et al., 2012; CitationAnwar et al., 2007).The normalization of the above enzymes activities seen in rats treated with the MO extracts indicates inhibition of liver cell injury and reduction of leakage of these enzymes into the blood. These results suggested that MO extracts reduced the toxicity due to elimination of the toxic products of APAP in rats.
Urea is the major excretory product of protein metabolism. Urea is carried by plasma to the kidney, where it is filtered from the plasma by the glomerulus. Creatinine is a nitrogenous product which is produced from the metabolism of creatinine in the skeleton muscles. Creatinine concentration not only assesses impairment of kidney function but also serves as a clinical chemistry endpoint to detect treatment related toxic effects of extracts/compounds on the kidney in experimental models. Although nephrotoxicity is a less common than hepatotoxicity in APAP overdose, kidney tubular damage and acute kidney failure can occur in the absence of liver injury (Mayo Foundation for Medical Education and Research, 2009). In the present investigation, there were no changes of BUN and creatinine levels after APAP intoxication ( and ). These results revealed the nontoxic nature of MO extracts in experimental system.
Necrosis of hepatocyte was the important experimental feature in the hepatotoxicity studies. The extensive cell necrosis was the development of an inflammatory response with the recruitment of neutrophils and mononuclear cells into the liver (CitationJaeschke, 1990). CitationSingh and Handa (1995) reported the presence of gross necrosis of centrilobular hepatocytes characterized by nuclear pyknosis, karyolysis, and eosinophilic infiltration in animals given an overdose of APAP. Our histopathology studies confirmed that the dosage of 6 g APAP/kg bw induced the features of hepatototoxicity with the presence of necrotic hepatocyte (, , , and 2D). In the hepatoprotective study, the dose was increased to 7 g APAP/kg bw to observe severe bridging necrosis in liver as well as kidney ( and ).
In a previous study, administration of 800 mg/kg leaves extract for 15 consecutive days showed no toxic effect on rats (CitationUma et al., 2010). In fact, another finding (CitationMazumder et al., 1999) has shown the LD50 of MO crude extract is about 2.8 g/kg intraperitoneally. This plant has been used traditionally for medicinal purposes (CitationFaizi et al., 1994) and sometimes prescribed for anemia (CitationSiddhuraju & Becker, 2003). Therefore, the plant is safe for human consumption without any undesirable effects. We have observed that the plant extracts have successfully reduced the extent of hepatotoxicity with a dose of 7 g APAP/kg bw. The liver marker enzymes, ALT and AST were restored. The biochemical findings also concurred with that of histopathological observations. Results were comparable with positive the control, NAC treated experimental animals ().
NAC is well known drug of choice for emergency treatment of APAP poisoning and related toxicity (CitationTokgoz et al., 2011). NAC allows the generation of sufficient level of glutathione to react with toxic intermediates NAPQI and prevent hepatic necrosis. NAC administration also has side effects such as diarrhoea and vomiting (CitationKao et al., 2003). The most important adverse effects of intravenous NAC have been anaphylactic reactions (CitationWalton et al., 1979) occurring in 2–10% of treated patients (CitationBateman et al., 1984). In 68 reaction reported, the common features were rash (81%), often urticarial, angio-edema (25%), bronchospasm (19%), hypotension (13%) and tachycardia (10%).
In our findings, the increment of liver weight was observed in each group (). In many cases, hypertrophy might be caused by the activity of the hepatic microsomal drug metabolizing enzymes. NAC treatment on rats shows minimal changes and the significant result was observed in animal receiving both APAP and flowers extract. Administration of positive control, 7.35 mmol NAC/kg bw, resulted in the best protection of liver weight. Increased in liver weight is associated with centrilobular hypertrophy. Hypertophy is an increase in the cell size and subsequently an increase in the size of the organ. It is frequently associated with adaptive enzyme induction activities and proliferation of smooth endoplasmic reticulum (CitationGreaves, 2007).
Histopathological investigation of the liver and kidney tissues were supported with liver and kidney marker parameters of hepatoprotective studies (, , , , E, 5F, and 5G). The extracts of flowers and leaves from MO effectively prevented the progression of hepatocellular damage observed in histological studies. The presence of necrotic hepatocytes and inflammatory cells infiltration were considerably decreased following the extract treatments. This result showed that there was a reduction of APAP toxicity despite being given 7 g APAP/kg bw and therefore inhibits the progression of liver damage. This result was consistent with the observation in the liver sections from animals treated with NAC where it showed the presence of microvesicular steatosis or fatty change which is reversible injury in the liver. These findings suggest that tissue protective effects of MO extracts against chemical induced toxicity in rats.
A previous study (CitationFakurazi et al., 2008) also showed that MO extract accelerated recovery of hepatic cells after intoxicated with 3 g APAP/kg bw. It was evidenced from the histopathological observation; the ability of MO to reverse the hepatic lesions was comparable to the treatment with silymarin. A study (CitationBlakely & McDonald, 1995) reveals that animals pretreated with MO were able to prevent further damage by APAP intoxication. The presence of focal infiltration of lymphocytes were observed within 24 h and after 48 h the damage was reduced to only focal hydropic degeneration.
Malondialdehyde (MDA) is one of the important end products of polyunsaturated fatty acid peroxidation and its level can reflect the degree of lipid peroxidation in tissues especially hepatocytes (CitationSaoudi & El Feki, 2012) Elevated MDA product coincides with hepatotoxicity seen with administration of APAP. It has also been reported in previous studies (CitationFakurazi et al., 2008; CitationUma et al., 2010), that high dosage of APAP enhances lipid peroxidation. After hydroethanol extract of flowers and leaves were given to animals, the level was significantly reduced. Hydroethanol extract was thought to contain high phenolic content that may reduce the oxidative stress in liver tissue (CitationFerguson, 2001). Certain phenolic compounds may also have induced phase II drug metabolism enzymes that will enhance the excretion of oxidizing species and inhibit cytochrome P450 activities. Depletion of antioxidant enzyme and production of reactive metabolite is associated with the mechanism of APAP overdose. Therefore, after animals were treated with hydroethanol extract, there was reduction in MDA level in rats (). Less inflammation around the centrilobular area where reactive metabolite is being produced was observed. However, little is known whether MO extracts affects other toxic products; further detailed investigations are needed to clarify the precise molecular action(s) by which active fraction(s) can modulate/eliminate the toxic metabolites in liver.
Conclusions
Our findings have suggested that MO crude extracts, especially from flowers, has a potential role in therapeutic properties against chemically induced acute liver injuries in rats. The therapeutic/hepatoprotective effects of MO extracts are probably due to inhibition of lipid peroxidation by-product as well as enhanced antioxidant enzymes (data not down). Hydroethanol extracts of MO flowers and leaves at dosages of 200 and 400 mg/kg bw showed beneficial effects. These findings confirmed that MO crude extracts are useful in reducing APAP induced acute liver toxicity, and were comparable with NAC. Further investigation is underway to determine the specific bioactive compounds responsible for its hepatoprotective and therapeutic properties. Additional molecular parameters should be added in further hepatotoxicity and hepatoprotective studies including the expression of mitochondrial proteins and immune inflammatory pathways which are more related to the hepatotoxicity and hepatoprotective pathways.
Declaration of interest
This research work was supported by E-Science research grant 02-01-04-SF1144 from the Ministry of Science, Technology and Innovation of Malaysia.
References
- Adejumo OE, Kolapo AL, Folarin AO. (2012). Moringa oleifera Lam. (Moringaceae) grown in Nigeria: In vitro antisickling activity on deoxygenated erythrocyte cells. J Pharm Bioallied Sci, 4, 118–122.
- Agarwal R, Hennings L, Rafferty TM, Letzig LG, McCullough S, James LP, MacMillan-Crow LA, Hinson JA. (2012). Acetaminophen-induced hepatotoxicity and protein nitration in neuronal nitric-oxide synthase knockout mice. J Pharmacol Exp Ther, 340, 134–142.
- Amaglo NK, Bennett RN, Lo Curto RB, Rosa EAS, Lo Turco V, Giuffrid A, Lo Curto A, Crea F, Timpo GM. (2010). Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem, 122, 1047–1054.
- Anwar F, Latif S, Ashraf M, Gilani AH. (2007). Moringa oleifera: A food plant with multiple medicinal uses. Phytother Res, 21, 17–25.
- Aslam M, Anwar F, Nadeem R, Rashid U, Kazi TG, Nadeem M. (2005). Mineral composition of Moringa oleifera leaves and pods from different regions of Punjab. Pakistan Asian J Plant Sci, 4, 417–421.
- Bateman DN, Woodhouse KW, Rawlins MD. (1984). Adverse reactions to N-acetylcysteine. Hum Toxicol, 3, 393–398.
- Blakely P, McDonald BR. (1995). Acute renal failure due to acetaminophen ingestion: A case report and review of the literature. J Am Soc Nephrol, 6, 48–53.
- Chanda S, Mangipudy RS, Warbritton A, Bucci TJ, Mehendale HM. (1995). Stimulated hepatic tissue repair underlies heteroprotection by thioacetamide against acetaminophen-induced lethality. Hepatology, 21, 477–486.
- Chandrasekaran VR, Chien SP, Hsu DZ, Liu MY. (2011). Anti-hepatotoxic effects of 3,4-methylenedioxyphenol and N-acetylcysteine in acutely acetaminophen-overdosed mice. Hum Exp Toxicol, 30, 1609–1615.
- Chandrasekaran VR, Wan CH, Liu LL, Hsu DZ, Liu MY. (2008). Effect of sesame oil against acetaminophen-induced acute oxidative hepatic damage in rats. Shock, 30, 217–221.
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. (1984). N-Acetyl-p-benzoquinone imine: A cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA, 81, 1327–1331.
- Dièye AM, Sarr A, Diop SN, Ndiaye M, Sy GY, Diarra M, Rajraji Gaffary I, Ndiaye Sy A, Faye B. (2008). Medicinal plants and the treatment of diabetes in Senegal: survey with patients. Fundam Clin Pharmacol, 22, 211–216.
- Fahey JW, Zalcmann AT, Talalay P. (2001). The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry, 56, 5–51.
- Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani AH. (1994). Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J Nat Prod, 57, 1256–1261.
- Fakurazi S, Hairuszah I, Nanthini U. (2008). Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem Toxicol, 46, 2611–2615.
- Ferguson LR. (2001). Role of plant polyphenols in genomic stability. Mutat Res, 475, 89–111.
- Gowrishankar R, Kumar M, Menon V, Divi SM, Saravanan M, Magudapathy P, Panigrahi BK, Nair KG, Venkataramaniah K. (2010). Trace element studies on Tinospora cordifolia (Menispermaceae), Ocimum sanctum (Lamiaceae), Moringa oleifera (Moringaceae), and Phyllanthus niruri (Euphorbiaceae) using PIXE. Biol Trace Elem Res, 133, 357–363.
- Goyal BR, Agrawal BB, Goyal RK. (2007). Phyto-pharmacology of Moringa oleifera Lam: An overview. Nat Prod Rad, 6, 34753.
- Greaves P. (2007). Liver and pancreas. In: Greaves P, ed. Histopathology of Pre-clinical Toxicity Studies. Amsterdam: Elsevier Ltd, 457–469.
- Hinson JA, Roberts DW, James LP. (2010). Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol, 196, 369–405.
- Jaeschke H. (1990). Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: The protective effect of allopurinol. J Pharmacol Exp Ther, 255, 935–941.
- Kao LW, Kirk MA, Furbee RB, Mehta NH, Skinner JR, Brizendine EJ. (2003). What is the rate of adverse events after oral N-acetylcysteine administered by the intravenous route to patients with suspected acetaminophen poisoning? Ann Emerg Med, 42, 741–750.
- Kasolo JN, Bimenya GS, Ojok L, Ochleng J, Ogwal-Okeng JW. (2010). Phyochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J Med Plant Res, 4, 753–757.
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. (2004). Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology, 40, 1170–1179.
- Kumar PS, Mishra D, Ghosh G, Panda GS. (2010). Medicinal uses and pharmacological properties of Moringa oleifera. Int J Phytomed 2, 210–216.
- Kuriakose GC, Kurup MG. (2011). Antioxidant and antihepatotoxic effect of Spirulina laxissima against carbon tetrachloride induced hepatotoxicity in rats. Food Funct, 2, 190–196.
- Liu WX, Jia FL, He YY, Zhang BX. (2012). Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol, 18, 2197–2202.
- Mazumder UK, Gupta M, Chakrabarti S, Pal D. (1999). Evaluation of hematological and hepatorenal functions of methanolic extract of Moringa oleifera Lam. root treated mice. Indian J Exp Biol, 37, 612–614.
- Malhi H, Guicciardi ME, Gores GJ. (2010). Hepatocyte death: A clear and present danger. Physiol Rev, 90, 1165–1194.
- Manguro LO, Lemmen P. (2007). Phenolics of Moringa oleifera leaves. Nat Prod Res, 21, 56–68.
- Mayo Foundation for Medical Education and Research (MFMER). (2009). http://www.All-MayoClinic.com DS 00961.
- Lee NH, Seo CS, Lee HY, Jung DY, Lee JK, Lee JA, Song KY, Shin HK, Lee MY, Seo YB, Kim H, Ha H. (2012). Hepatoprotective and antioxidative activities of Cornus officinalis against acetaminophen-induced hepatotoxicity in mice. Evid Based Complement Alternat Med 2012, 804924.
- Nelson SD, Bruschi SA. (2003). Mechanisms of acetaminophen induced liver disease. In: Kaplowitz N, DeLeve LD, eds. Drug-Induced Liver Disease. New York: Marcel Dekker, 287–325.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Ramaiah SK. (2007). A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol, 45, 1551–1557.
- Riedemann NC, Guo RF, Ward PA. (2003). The enigma of sepsis. J Clin Invest, 112, 460–467.
- Saoudi M, El Feki A. (2012). Protective role of Ficus carica stem extract against hepatic oxidative damage induced by methanol in male wistar rats. Evid Based Complement Alternat Med, 2012, 150458.
- Siddhuraju P, Becker K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem, 51, 2144–2155.
- Singh A, Handa SS. (1995). Hepatoprotective activity of Apium graveolens and Hygrophila auriculata against paracetamol and thioacetamide intoxication in rats. J Ethnopharmacol, 49, 119–126.
- Terneus MV, Kiningham KK, Carpenter AB, Sullivan SB, Valentovic MA. (2007). Comparison of S-adenosyl-l-methionine and N-acetylcysteine protective effects on acetaminophen hepatic toxicity. J Pharmacol Exp Ther, 320, 99–107.
- Tokgoz B, Ucar C, Kocyigit I, Somdas M, Unal A, Vural A, Sipahioglu M, Oymak O, Utas C. (2011). Protective effect of N-acetylcysteine from drug-induced ototoxicity in uraemic patients with CAPD peritonitis. Nephrol Dial Transplant, 26, 4073–4078.
- Uma N Jr, Fakurazi S, Hairuszah I. (2010). Moringa oleifera enhances liver antioxidant status via elevation of antioxidant enzymes activity and counteracts paracetamol-induced hepatotoxicity. Malays J Nutr, 16, 293–307.
- Ung CY, Lam SH, Zhang X, Li H, Ma J, Zhang L, Li B, Gong Z. (2011). Existence of inverted profile in chemically responsive molecular pathways in the zebrafish liver. PLoS ONE, 6, e27819.
- Walton NG, Mann TA, Shaw KM. (1979). Anaphylactoid reaction to N-acetylcysteine. Lancet, 2, 1298.
- Yang YS, Ahn TH, Lee JC, Moon CJ, Kim SH, Jun W, Park SC, Kim HC, Kim JC. (2008). Protective effects of pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats. Food Chem Toxicol, 46, 380–387.
- Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. (2003). Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem, 278, 1248–1258.