Abstract
Context: For 2000 years, traditional Chinese medicine has been used as a remedy for general health improvement, including the fight against aging. Pearl powder has recently been used as a health food that has antioxidant, antiaging, antiradioactive, and tonic activities for cells; it is also applied to cure aphthous ulcer, gastric ulcer, and duodenal ulcer on clinical therapy. In addition, the mother of pearl, nacre, could enhance the cell adhesion and tissue regeneration of skin fibroblasts.
Objective: Fibroblast is regarded as indispensable in the processes of wound healing. Therefore, the effect of pearl extract (PL) on fibroblasts is investigated in this study.
Materials and methods: PL is produced by a room temperature super extraction system (Taiwan patent no. I271 220). DMEM medium containing PL (300 μg/mL) was used to examine the effect of migration-promoting potential on human fibroblast cell line or human primary fibroblast cells in a wound healing model in vitro.
Results: Medium containing PL (300 μg/mL) demonstrated that the migratory cell numbers of fibroblasts were three times more than that without PL, and mRNA expression of collagen type III was higher than in collagen type I in fibroblasts. It revealed a migration-promoting potential of human fibroblasts in a wound healing model in vitro.
Discussion and conclusion: The present study found that the migration-promoting effect in PL, which could be a supplement in cell culture. These data suggest PL could be useful for enhancing the wound healing of fibroblasts.
Keywords::
Introduction
The process of wound healing is very complex involving various physiological responses. Wound healing must combine with different cells, growth factors, and extracellular matrix (ECM), and so forth. Previous study shows that there are four distinct processes in wound healing: hemostasis, inflammation, proliferation, and remolding (CitationLevin, 2002). In these processes, different cells are needed in different stages, such as the macrophage plays a main role of removing nonfunctional cells and bacteria in the inflammation stage (CitationDiPietro, 1995). Moreover, the fibroblast is regarded as indispensable in the processes of wound healing. It has been shown that fibroblasts can secrete growth factor, ECM to induce proliferation, differentiation, and migration of other cells or itself during the stages of wound healing (CitationPark et al., 2006; CitationClark, 2001), and it is also reported that increasing numbers of fibroblasts could improve wound healing in experimental models (CitationLamme et al., 2000).
For 2000 years, traditional Chinese medicine has been used as a remedy for general health improvement, including the fight against aging (CitationCheng et al., 2005). Chinese medicine is widely used for different diseases today. Previous studies show that Astragalus membranaceus Bunge (Leguminosae) (RA) and Rehmannia glutinosa Steud (Scrophulariaceae) (RR) were effective in enhancing the proliferation fibroblasts in wound healing (CitationLau et al., 2008, Citation2009). Ginsenoside from Panax ginseng C. A. Mey (Araliaceae) could act against UVB-induced growth inhibition and nucleolus damage of HaCaT cells (CitationLau et al., 2008). Other studies also show that sinomenine from Sinomenium acutum (Thunb.) Rehder et E. H. Wilson (Menisupermaceae) displays an anti-inflammatory activity by inhibition of TNF-α-induced phosphorylation, and the extract of edible bird’s nest (EBN) could promote the wound healing of corneal (CitationZainal Abidin et al., 2011).
Another Chinese medicine, pearl powder, has recently been used as health food. It has been shown that pearl powder has antioxidant, antiaging, antiradioactive, and tonic activities for cells (CitationDai et al., 2010; CitationXu et al., 2001). The pearl powder is also applied to cure aphthous ulcer, gastric ulcer, and duodenal ulcer on clinical therapy (CitationDai et al., 2010). In addition, the mother of pearl, nacre, could enhance the cell adhesion and tissue regeneration of skin fibroblasts (CitationLopez et al., 2000).
By these reports, it is reasonable to assume that pearl extraction (PL) has repair ability for wound healing. However, the extraction and enrichment of analytic sample of food or Chinese medicine are important, but general extraction methods are usually costly in terms of organic solvents, causing a loss or degradation of active compound with relative low-extraction efficiency (CitationDamm & Kappe, 2011; CitationXiao et al., 2012; CitationMolto-Puigmarti et al., 2011; CitationBeyer & Biziuk, 2008). Therefore, we utilized a low-temperature extraction to produce PL; thus, extract of pearl was used to be a supplement with a cultured fibroblast cell line (Hs68 cells) and human primary cells (human foreskin fibroblast cells, HFFs). This study investigated the effect of PL on fibroblasts. It was found that the migration-promoting potential of Hs68 cells and HFFs could be enhanced to perform wound healing when the cell culture medium contained PL. On the other hand, these cultured cells appeared to accelerate the rate of fibroblast migration. It is expected that PL, by promoting the migratory ability of the cell, may have a wound healing application.
Materials and methods
Extraction system
The extract ion system has been described in a previous study (CitationYang et al., 2011). Pearls, which had been harvested from freshwater pearl oyster, Hyriopsis cumingii Lea (Unionidae), were first cleaned by washing in deionized water and drying at 40°C. The pearls were ground into >10,000 mesh pearl powder by an air impacting grinder. Pearl powder (1 kg) was extracted with 10 L of water for 4 h with a room temperature super extraction system (RTSES, MesoPhase Technologies, Inc., Taiwan; Taiwan patent number: I271 220; Germany patent number: Nr.102 005 061 822). The powder–water mixture was centrifuged at 3000g for 10 min and the supernatant was collected as the water extract.
Cell culture
The protocols from previous studies were modified for cell culture (CitationLou et al., 2010; CitationChung et al., 2011). The cell line used in this study was a human newborn foreskin fibroblast cell line, Hs68 cell (BCRC number: 60 038). The culture medium in this study was Dulbecco’s Modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS, Biological Industries, Israel) in the presence or absence of 300 μg/mL PL (Mesophase Technolies, Taiwan, containing 10 μg/mL water-soluble protein, 90–140 ppm hydrolyzed amino acid, and 3200 ppb calcium ion, 339 ppb magnesium ion).
Primary culture of HFFs was obtained from the surgical specimen of excised redundant foreskin during major foreskin operations. The study protocol was approved by the Institutional Review Board of the National Taiwan University Hospital (number: 9 561 703 036). We conformed to the Helsinki Declaration with respect to human subjects in biomedical research. The subcutaneous tissues were cut into small pieces and then digested with dispase at 37°C with agitation for 2 h in Hank’s balanced salt solution. The dissociated tissues were incubated without shaking for 5 min at room temperature, followed by the separation of dermis cell-enriched supernatant to a new tube. The dermis fraction was centrifuged at 300 g for 5 min. The pellet was then resuspended in DMEM with 10% FBS in the presence or absence of 300 μg/mL PL, and the cells were seeded for subsequent experiments.
MTT assay
Cell survival was evaluated by cellular ability to reduce 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) (CitationYoung & Hu, 2003). Since mitochondrial dehydrogenases of viable cells cleave selectively at the tetrazolium ring, yielding blue/purple formazan crystals, the level of the reduction of MTT into formazan can reflect the level of cell metabolism (CitationDeckwerth & Johnson, 1993). For the MTT assay, the culture medium was removed after a predetermined culture time, and then cells were incubated with 0.1 mL of MTT (2 mg/mL in PBS) for 3 h at 37°C. After incubation, the medium was aspirated and the formazan reaction products were dissolved by dimethyl sulfoxide in PBS and shaken for 15 min. The optical density of the formazan solution was read on an ELISA plate reader (M2e, Molecular Devices) at 570 nm. Cell viability determined by the MTT assay was expressed as a percentage of control neurons.
Scratch assay
According to a protocol detailed previously, the scratch assay was prepared from cell-confluent wells (CitationNakajima et al., 2007; CitationLoeber & Runyan, 1990). Hs68 cells or HFFs were cultured in DMEM containing 10% FBS in the presence or absence of 300 μg/mL PL on TCPS and cultured until confluency. In order to create a rectangular scratch, the monolayer was gently scratched with a pipette tip. Then, culture medium and detached cells were removed. The scratched well was washed twice with PBS, and fresh medium in the presence or absence of PL was added. The cells were then incubated for 16 h. Furthermore, cell motility was measured by an inverse phase contrast time-lapse microscopic system (Lieca DMI600, Germany). All statistical analysis was performed using one-way ANOVA followed by Duncan’s test with p < 0.05 being considered significant.
Real-time quantitative polymerase chain reaction (PCR)
mRNA expression of Hs68 cells or HFFs was evaluated by real-time quantitative PCR (CitationShao et al., 2010). Total cellular RNA was extracted from Hs68 cells or HFFs using Mix Total RNA Extraction kit (Bio-Rad, CA, USA). RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystem, CA, USA). Expression of mRNA was detected using an ABI Prism 7900 HT Sequence Detection System (Applied Biosystem, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Assay ID: Hs99 999 905; Applied Biosystem, CA, USA) was used as the endogenous control. Data were collected with the Applied Biosystem SDS 2.1 software and analyzed using the threshold cycle number (Ct) relative quantification method. The TaqMan Gene Expression assays of target gene were purchased from Applied Biosystem (Collagen Type I Assay ID: Hs00 164 004 and Collagen Type III Assay ID: Hs00 164 103; Applied Biosystem, CA, USA). The results were presented as the ratio of mRNA expression of collagen type III relative to collagen type I in Hs68 cells. All statistical analysis was performed using one-way ANOVA followed by Duncan’s test with p < 0.05 being considered significant.
Results
Behavior of Hs68 cells cultured on TCPS in the presence or absence of PL
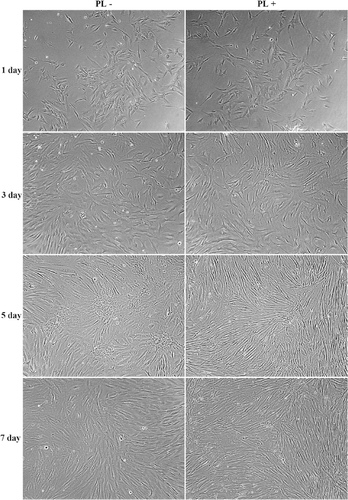
The effects of Hs68 cells cultured on TCPS in the presence or absence of 300 μg/mL PL were investigated. As shown in , the behaviors of Hs68 cells cultured on TCPS in the presence or absence of PL were compared. The results show that Hs68 cells cultured in medium containing PL started to attach on TCPS and the morphology of Hs68 cells displayed a flat and polygon shape at 1 day of incubation. After 3 days of incubation, a growth of Hs68 cell could be observed; it revealed that the cell number of Hs68 cell increased when Hs68 cells were cultured on TCPS in medium containing PL. Furthermore, it revealed that Hs68 cells could continuously grow in medium containing PL and reach confluent morphology. In addition, it was observed that most of the Hs68 cells displayed a spindle shape in confluent culture after 7 days of incubation. Similar to medium containing PL, Hs68 cells attached on TCPS in medium in the absence of PL and showed a polygon shape at 1 day of incubation. Thus, Hs68 cells were continuously cultured on TCPS in the absence of PL for 7 days of incubation, and Hs68 cells could also grow to confluence and the spindle shape morphology of most all of Hs68 cells could be observed. Based on these results, it is assumed that medium containing PL does not inhibit the growth and survival of Hs68 cells.
MTT assay
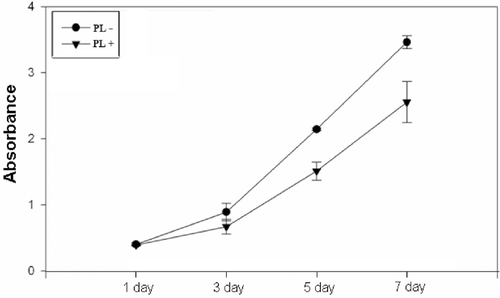
MTT assay relies on the ability of the viable cells to reduce a water-soluble yellow dye to a water-insoluble purple formazan product. shows the MTT conversion of Hs68 cells cultured on TCPS in the presence or absence of PL medium. Although it was observed that the MTT conversion of Hs68 cells in medium in the absence of PL was slightly higher than that in medium in the presence of PL at 5 and 7 days of incubation, the MTT reduction activity of cells in the presence or absence of PL exhibited a significantly stable increase at all time points, respectively. These results indicated that medium containing PL was favorable for the proliferation of Hs68 cells. This is consistent with the result of that the growth of Hs68 cells on TCPS was greatly increased with medium in the presence of PL. Therefore, it suggests that the cell number would greatly increase on TCPS with culture medium in the presence or absence of PL.
Scratch assay for Hs68 cells in the presence or absence of PL
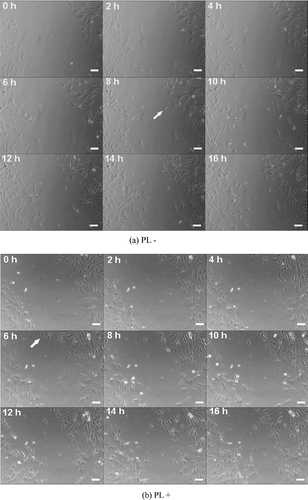
Furthermore, the wound healing ability of Hs68 cells was tested by the scratch assay. We characterize the movement of Hs68 cells on TCPS in the presence or absence of 300 μg/mL PL after the confluent culture was scratched. After scratching a confluent monolayer cells, it could be observed that the first cell of Hs68 cells in PL-free medium started to migrate into the scratched site at 8 h of incubation (, arrow); and the first migratory cell of Hs68 cells in PL medium in the scratched site at 6 h of incubation was also observed (, arrow). On other hand, the Hs68 cells would start to migrate in 8 h after scratching. Subsequently, the movement of Hs68 cells continued to be observed from 8 to 16 h, shows that there were few cells to be observed in scratched site at 16 h of incubation. Compared with PL-free medium, more cells in medium containing PL which continued to migrate were observed. This clearly revealed that more polygonal and spindly e-shaped Hs68 cells migrated from the edge of the scratched site to the center during 8 to 16 h of incubation. Furthermore, quantitative analysis shows the migratory cell number of Hs68 cells in the absence of PL was 8 ± 2, it revealed that Hs68 cells in PL-free medium could not be significantly increased. Dissimilar to the effect of PL-free medium, the migratory cell number of Hs68 cells in the presence of PL was 26 ± 2 which was three times more than that in the absence of PL, as shown in . Based on the results of scratch assay, it may be suggested that Hs68 cells cultured in medium containing PL could have better migratory ability than that in PL-free medium.
Table 1. Average numbers of migratory Hs68 cells were counted in the scratch site at 0 and 16 h of incubation.
mRNA expression of Hs68 cell cultured in the presence or absence of PL
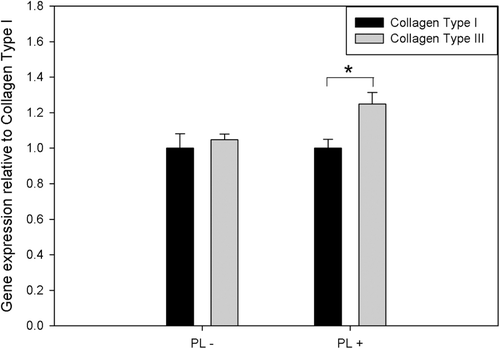
The mRNA expressions of collagen type I and collagen type III in Hs68 cells in the presence or absence of PL were assayed by real-time quantitative PCR after 1 day of incubation. In PL-free medium, shows no significant difference of mRNA expression between collagen type I and collagen type III in Hs68 cells could be observed. In contrast, when Hs68 cells culture medium containing PL, the mRNA expression of collagen type I and collagen type III were different from cells in PL-free medium. The trend of mRNA expression was increasing from collagen type I to collagen type III, indicating that mRNA expression of collagen type III was higher than that of collagen type I in PL medium (1.25 ± 0.06-fold, p < 0.05). Therefore, these results indicated that Hs68 cells cultured in PL medium could promote the mRNA expression of collagen type III.
Figure 4. The mRNA expression of Collagen Type I and Collagen Type III in Hs68 cells cultured in the absence or presence of PL on TCPS after 1 day of incubation. The results were presented as the ratio of gene expression of Collagen Type III relative to Collagen Type I. *Significant difference as determined by one-way ANOVA followed by the Duncan’s test (p < 0.05).
Scratch assay for HFFs in the presence or absence of PL
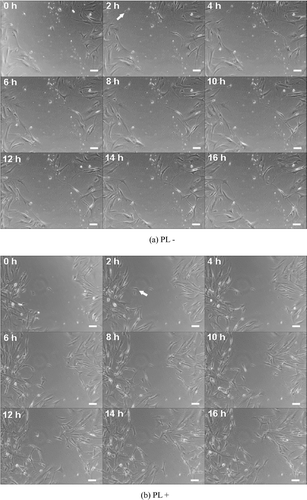
Finally, the wound healing ability of human primary cells, HFFs, cultured in the presence or absence of 300 μg/mL PL, was also tested by the scratch assay. Interestingly, it could be observed that the movements of HFFs in the presence or absence PL were similar to Hs68 cells. After scratching a confluent monolayer of cells, it could be observed that the first cell of HFFs in PL-free medium started to migrate into the scratched site at 2 h of incubation (, arrow). Subsequently, a few cells migrated into scratched site after 16 h of incubation, as shown in . Compared with PL-free medium, it could be observed that HFFs in the presence of PL continued to migrate. More polygonal and spindly HFFs migrated from the edge of the scratched site to the center in 16 h of incubation. Furthermore, quantitative analysis shows the migratory cell number of HFFs in PL-free medium was 7 ± 2, revealing that HFFs in PL-free medium could not be significantly increased. Dissimilar to the effect of PL-free medium, the migratory cell number of HFFs was 20 ± 2, which was more than that in PL-free medium (p < 0.05), as shown in . The results of the scratch assay suggest that medium containing PL also could promote the migratory ability of HFFs.
Table 2. Average numbers of migratory HFFs were counted in the scratch site at 0 and 16 h of incubation.
Discussion
It is well known that fibroblasts play an important role in wound healing in the human body. Proliferation and migration of fibroblasts are a critical stage in wound healing. In wound healing, fibroblasts would proliferate and migrate into the wound site to repair it. Therefore, promoting proliferation and migration of fibroblasts is a very important topic. Previous studies show that the behavior of fibroblasts could be regulated by different nutrition, growth factors, and cytokines. For example, the survival and growth of fibroblasts in vitro could be regulated by egg components (CitationCarrel & Ebeling, 1923). EGF could be a mitogenic factor for fibroblastic cells (CitationNakagawa et al., 1985) and TGF-α acts synergistically with TGF-β to induce the growth of fibroblasts (CitationAnzano et al., 1983). In addition, FGFs family such as bFGF also can enhance the mitosis and migration of fibroblasts (CitationEsch et al., 1985) and thrombin could simulate the migration of fibroblasts (CitationDawes et al., 1993). Furthermore, some Chinese medicines are considered to have an effect on wound healing for cells, such as enhancing viability of primary fibroblasts to improve the healing of a diabetic foot ulcer by the mixture of herbs RA and RR in diabetic patients (CitationZhang & Yao, 2011), or Genipin to mediate the proliferation and migration of subconjunctival fibroblasts (CitationKitano et al., 2006). Therefore, it is interesting to study whether the interaction of fibroblasts with particular components of pearl can direct cells to proliferation and migration or not. The major finding of this study was that a fibroblast cell line, Hs68 cell, could survive and promote migratory ability when culture medium was in the presence of PL, which was produced by a low-temperature extraction process.
Previous studies showed that bone marrow cells could be stimulated to differentiate and form bone by utilizing nacre as a substrate (CitationLamghari et al., 1999; CitationMoutahir-Belqasmi et al., 2001). Therefore, in the present study, extract of pearl was used to be a supplement to culture Hs68 cells. At 1 day of incubation, the morphology of most cells displayed a polygon when culture medium was in the presence of PL, and a confluent culture could be observed after 7 days of incubation. In addition, in 7 days of culture, the cell shape of Hs68 cell in confluent wells changed from polygon to spindle, meaning that active fibroblasts transferred into an inactive fibroblast phenotype (). Furthermore, according to the MTT assay, MTT reduction activity of cells in the presence of PL exhibited a significantly stable increase at all time points (); this result is consistent with the morphology of . Compared with PL medium, the results of Hs68 cells in the absence of PL were similar to that in the presence of PL. On the other hand, it is reasonable to suggest that Hs68 cells cultured in medium containing PL could survive and did not inhibit proliferation of cells.
According to previous studies, fibroblasts would proliferate and migrate into the wound site in the short term when there is a wound to be created (CitationYamaguchi & Yoshikawa, 2001; Falanga, 2001; CitationKoopmann, 1995). Therefore, the wound healing abilities of Hs68 cells and HFFs were analyzed by the scratch assay. After scratching a confluent monolayer, the first migratory cell of Hs68 cells in PL medium migrated into the scratched site at 6 h of incubation and it also could be observed that the cell morphology of scratched edge appeared as a polygonal shape. It is assumed the Hs68 cells transferred to an active fibroblast phenotype when they sensed the wound was created. Subsequently, in order to repair the wound, more Hs68 cells were observed to migrate into the scratched site after 16 h of incubation. Compared with medium containing PL, the rate of first migratory cell in medium in the absence of PL was later than that in the presence of PL. Thus, it also revealed that the cell number at the scratched site in PL-free medium (8 ± 2) was three times lower than in medium containing PL (26 ± 2), as shown in and . The results of the scratch assay suggest medium containing PL could be promoting migration of fibroblasts in a wound healing model.
Furthermore, previous studies show that fibroblasts would secrete ECM in migratory or repairing processes (CitationFalanga, 2001; CitationBreuing et al., 1997; Schultz, Citation1987). In addition, other studies show that the secretion of collagen I is less than that of collagen III in fibroblasts during the wound healing process (CitationShegogue & Trojanowska, 2004; CitationSinger & Clark, 1999; CitationRoss & Benditt, 1965). Thus, in this present study, the mRNA expression of collagen type I and III in Hs68 cells were assayed by real-time quantitative PCR. It was clearly observed that mRNA expression of collagen type III was higher than that of collagen type I on Hs68 cells in the presence of PL rather than in the absence of PL (). Compared with the timelapse results of the scratch assay ( and ), the trends of mRNA expression of Hs68 cells were consistent with the results of migratory abilities of Hs68 cells in the presence or absence of PL. This meant that the wound simulated Hs68 cells to express high gene expression of collagen type III than collagen type I after scratching; and morphology of Hs68 cells started to change from inactive fibroblast (spindly shape) to active fibroblast (polygonal shape). Consequently, it was observed that Hs68 cells migrated in the scratched site for repair. Therefore, this suggests that medium containing PL could mediate mRNA expression of collagen in Hs68 cells. In addition, since PL contained components with molecular weights less than 30 kDa (data not shown), it is a possibility that PL may contain some of the growth factors, cytokines, polyamines, vitamins and electrolytes responsible for the conjugated effect for fibroblasts. Besides, previous studies report that two peptides of the putative imaginal disc growth factor (IDGF) of Diaprepes abbreviatus Linnaeus (Curculionidae) in pearl extract which we used in this study had been identified (CitationYang et al., 2011); it is suspected that the pearl protein was identified with sequence homology to IDGF of D. abbreviatus may be involved to stimulate fibroblast mitosis and motility of insect imaginal disc cells (CitationDai et al., 2010; CitationKawamura et al., 1999). Therefore, this suggests that the synergistic effects of IDGF of D. abbreviatus and serum components to enhance the migratory fibroblast and mRNA expression of collagen type III were higher than collagen type I in fibroblasts.
Finally, because Hs68 is a cell line, we next investigated the migratory ability of human primary cells with culture medium in the presence or absence of PL. Interestingly, the trend of migratory ability of HFFs was similar to that of Hs68 cells. This revealed that the movement of HFFs in the presence of PL was faster than that in PL-free medium in 16 h of incubation, and the morphology of HFFs also transferred to active fibroblast (). In addition, it also appeared that the first HFFs migrated into the scratched area at 2 h of incubation. The rate of HFFs was three times faster than that of Hs68 cells (); it might be that the primary cells were more sensitive than the cell line. Results of the scratch assay for HFFs suggest that medium in the presence of PL could be used for human primary cells.
Conclusions
The present study demonstrated that a migration-promoting effect was present in an extract of pearl, which could be a supplement for culture. In addition, the extract of pearl which was produced by low-temperature extraction could promote gene expression of collagen type III and repair the wound when a wound was created. These results are very encouraging since this information should be useful for growth of strategies to enhance the healing of wounds.
Declaration of interest
The authors thank the National Science Council of Taiwan, the Republic of China and Southern Taiwan Science Park Administration for their financial support (Project number: 99RC02). The authors report no declarations of interest.
References
- Anzano MA, Roberts AB, Smith JM, Sporn MB, De Larco JE. (1983). Sarcoma growth factor from conditioned medium of virally transformed cells is composed of both type α and type β transforming growth factors. Proc Natl Acad Sci USA, 80, 6264–6268.
- Beyer A, Biziuk M. (2008). Applications of sample preparation techniques in the analysis of pesticides and PCBS in food. Food Chem, 108, 669–680.
- Breuing K, Andree C, Helo G, Slama J, Liu PY, Eriksson E. (1997). Growth factors in the repair of partial thickness porcine skin wounds. Plast Reconstr Surg, 100, 657–664.
- Carrel A, Ebeling AH. (1923). Survival and growth of fibroblasts in vitro. J Exp Med, 38, 487–497.
- Cheng Y, Shen LH, Zhang JT. (2005). Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin, 26, 143–149.
- Chung YC, Cheng TY, Young TH. (2011). The role of adenosine receptor and caveolae-mediated endocytosis in oligonucleotide-mediated gene transfer. Biomaterials, 32, 4471–4480.
- Clark RA. (2001). Fibrin and wound healing. Ann NY Acad Sci, 936, 355–367.
- Dai JP, Chen J, Bei YF, Han BX, Guo SB, Jiang LL. (2010). Effects of pearl powder extract and its fractions on fibroblast function relevant to wound repair. Pharm Biol, 48, 122–127.
- Damm M, Kappe CO. (2011). A high-throughput platform for low-volume high-temperature/pressure sealed vessel solvent extractions. Anal Chim Acta, 707, 76–83.
- Dawes KE, Gray AJ, Laurent GJ. (1993). Thrombin stimulates fibroblast chemotaxis and replication. Eur J Cell Biol, 61, 126–130.
- Deckwerth TL, Johnson EM Jr. (1993). Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol, 123, 1207–1222.
- DiPietro LA. (1995). Wound healing: the role of the macrophage and other immune cells. Shock, 4, 233–240.
- Esch F, Baird A, Ling N, Ueno N, Hill F, Denoroy L, Klepper R, Gospodarowicz D, Böhlen P, Guillemin R. (1985). Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci USA, 82, 6507–6511.
- Falanga V. (2001). Cutaneous Wound Healing. London:Martin Dunitz.
- Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ. (1999). A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development, 126, 211–219.
- Kitano A, Saika S, Yamanaka O, Ikeda K, Reinach PS, Nakajima Y, Okada Y, Shirai K, Ohnishi Y. (2006). Genipin suppresses subconjunctival fibroblast migration, proliferation and myofibroblast transdifferentiation. Ophthalmic Res, 38, 355–360.
- Koopmann CF Jr (1995). Cutaneous wound healing. An overview. Otolaryngol Clin North Am, 28, 835–845.
- Lamghari M, Almeida MJ, Berland S, Huet H, Laurent A, Milet C, Lopez E. (1999). Stimulation of bone marrow cells and bone formation by nacre: In vivo and in vitro studies. Bone, 25, 91S–94S.
- Lamme EN, Van Leeuwen RT, Brandsma K, Van Marle J, Middelkoop E. (2000). Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J Pathol, 190, 595–603.
- Lau TW, Lam FF, Lau KM, Chan YW, Lee KM, Sahota DS, Ho YY, Fung KP, Leung PC, Lau CB. (2009). Pharmacological investigation on the wound healing effects of Radix Rehmanniae in an animal model of diabetic foot ulcer. J Ethnopharmacol, 123, 155–162.
- Lau TW, Sahota DS, Lau CH, Chan CM, Lam FC, Ho YY, Fung KP, Lau CB, Leung PC. (2008). An in vivo investigation on the wound-healing effect of two medicinal herbs using an animal model with foot ulcer. Eur Surg Res, 41, 15–23.
- Levin ME. (2002). Management of the diabetic foot: Preventing amputation. South Med J, 95, 10–20.
- Loeber CP, Runyan RB. (1990). A comparison of fibronectin, laminin, and galactosyltransferase adhesion mechanisms during embryonic cardiac mesenchymal cell migration in vitro. Dev Biol, 140, 401–412.
- Lopez E, Le Faou A, Borzeix S, Berland S. (2000). Stimulation of rat cutaneous fibroblasts and their synthetic activity by implants of powdered nacre (mother of pearl). Tissue Cell, 32, 95–101.
- Lou PJ, Chiu MY, Chou CC, Liao BW, Young TH. (2010). The effect of poly (ethylene-co-vinyl alcohol) on senescence-associated alterations of human dermal fibroblasts. Biomaterials, 31, 1568–1577.
- Molto-Puigmarti C, Permanyer M, Castellote AI, Lopez-Sabater MC. (2011). Effects of pasteurisation and high-pressure processing on vitamin c, tocopherols and fatty acids in mature human milk. Food Chem, 124, 97–702.
- Moutahir-Belqasmi F, Balmain N, Lieberrher M, Borzeix S, Berland S, Barthelemy M, Peduzzi J, Milet C, Lopez E. (2001). Effect of water soluble extract of nacre (Pinctada maxima) on alkaline phosphatase activity and Bcl-2 expression in primary cultured osteoblasts from neonatal rat calvaria. J Mater Sci Mater Med, 12, 1–6.
- Nakagawa S, Yoshida S, Hirao Y, Kasuga S, Fuwa T. (1985). Biological effects of biosynthetic human EGF on the growth of mammalian cells in vitro. Differentiation, 29, 284–288.
- Nakajima M, Ishimuro T, Kato K, Ko IK, Hirata I, Arima Y, Iwata H. (2007). Combinatorial protein display for the cell-based screening of biomaterials that direct neural stem cell differentiation. Biomaterials, 28, 1048–1060.
- Park HJ, Zhang Y, Georgescu SP, Johnson KL, Kong D, Galper JB. (2006). Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev, 2, 93–102.
- Ross R, Benditt EP. (1965). Wound healing and collagen formation. V. Quantitative electron microscope radioautographic observations of proline-H3 utilization by fibroblasts. J Cell Biol, 27, 83–106.
- Schultz JS. 1987. Special issue on membranes in biotechnology. J Memb Sci, 30, 239–241.
- Shao HJ, Lee YT, Chen CS, Wang JH, Young TH. (2010). Modulation of gene expression and collagen production of anterior cruciate ligament cells through cell shape changes on polycaprolactone/chitosan blends. Biomaterials, 31, 4695–4705.
- Shegogue D, Trojanowska M. (2004). Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem, 279, 23166–23175.
- Singer AJ, Clark RA. (1999). Cutaneous wound healing. N Engl J Med, 341, 738–746.
- Xiao X, Song W, Wang J, Li G. (2012). Microwave-assisted extraction performed in low temperature and in vacuo for the extraction of labile compounds in food samples. Anal Chim Acta, 712, 85–93.
- Xu HB, Huang KX, Gao QH, Gao ZH, Han XX. (2001. A study on the prevention and treatment of myopia with nacre on chicks. Pharmacol Res, 44, 1–6.
- Yamaguchi Y, Yoshikawa K. (2001). Cutaneous wound healing: An update. J Dermatol, 28, 521–534.
- Yang SC, Kao WM, Wu CJ, Kuo YL, Shen GH, Shieh CJ, Yu SP, Chen JH. (2011). Detection and partial identification of proteins in pearls formed in Hyriopsis cumingii (Lea). Afr J Biotechnol, 82, 19,203–19,210.
- Young TH, Hu WW. (2003). Covalent bonding of lysine to EVAL membrane surface to improve survival of cultured cerebellar granule neurons. Biomaterials, 24, 1477–1486.
- Zainal Abidin F, Hui CK, Luan NS, Mohd Ramli ES, Hun LT, Abd Ghafar N. (2011). Effects of edible bird’s nest (EBN) on cultured rabbit corneal keratocytes. BMC Complement Altern Med, 11, 94.
- Zhang Y, Yao X. (2011). Suppressive effects of YiGanKang, a combination of Chinese herbs, on collagen synthesis in hepatic stellate cell. J Ethnopharmacol, 134, 949–952.